
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Gisondi | + 1471 word(s) | 1471 | 2020-12-10 09:05:17 | | | |
| 2 | Catherine Yang | Meta information modification | 1471 | 2020-12-31 04:30:21 | | |
Video Upload Options
There is no evidence to support the idea that patients receiving systemic therapy (i.e. immunosuppressive/ immunomodulating agents) should stop their treatment in order to prevent COVID-19 infection. People with psoriasis undergoing systemic therapy should be advised to follow current guidelines for hygiene and physical distancing as recommended in their respective area of residence. Chronic plaque psoriasis is an inflammatory skin disease affecting 2–3% of the general population. In case of moderate to severe psoriasis patients are candidate to systemic treatments that have immunosuppressive/immunomodulating properties.
1. Introduction
The pandemic of coronavirus disease (COVID-19), a viral pneumonia caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has raised serious concern among dermatologists and psoriatic patients receiving systemic immunomodulating/immunosuppressive treatments [1]. Plaque psoriasis is a chronic inflammatory skin disease affecting 2–3% of the general population, and approximately one-third of patients are candidates for systemic treatments because of disease severity, extensions and/or localization in sensitive or visible areas (Figure 1) [2]. Systemic treatments include synthetic or biologic disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, cyclosporine and TNF-α, IL-17, IL-12/23 and IL-23 inhibitors, which have been associated with increased risk of infection, including respiratory tract viral infection [3]. Moreover, patients with moderate-to-severe psoriasis are also frequently affected by cardio-metabolic comorbidities, such as obesity, diabetes mellitus and arterial hypertension, that have been associated with higher risk of hospitalization and fatal outcome of COVID-19 pneumonia [4]. The aim of this narrative review was to investigate whether there is an increased risk of COVID-19 infection or more severe infection outcomes in psoriasis patients on systemic treatments.
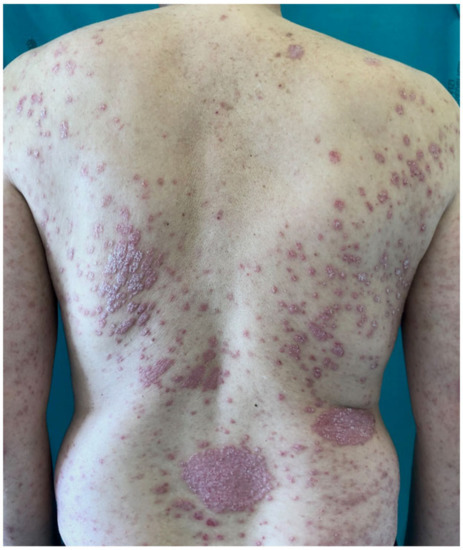
Figure 1. Man affected by severe psoriasis, a candidate for systemic treatment.
2. Discussion
There is no increased susceptibility for SARS-CoV-2 infection or increased severity of the disease course of COVID 19 in patients with psoriasis receiving synthetic or biologic DMARDs. This finding may appear surprising because systemic treatments have been associated with higher risk of other infections, as cytokines inhibited by biologics are involved in the immune response against pathogens. The influence of biologics on respiratory tract infection susceptibility has recently been investigated. Higher risks of opportunistic infections and herpes zoster were reported among patients receiving immunosuppressive therapy to treat moderate-to-severe psoriasis [5]. TNF-α is expressed in the lung epithelial cells in response to respiratory viruses, such as influenza, and it recruits lympho-monocytes in the site of infection. Nevertheless, no higher serum levels of TNF-α in severe acute respiratory syndrome (SARS) were found, compared to other respiratory illness [6]. A meta-estimate from phase 3 placebo-controlled clinical trials of anti-TNF-α in psoriasis found no evidence of an increased risk of respiratory tract infection (RTI) (OR 1.08; 95% CI 0.84–1.38) [7]. A similar meta-estimate on anti-IL-17 agents found an increased risk of RTI of any etiology (OR 1.56; CI 95% 1.04–2.33) [8]. A pooled analysis of clinical trials of secukinumab versus etanercept found a similar rate of RTI [9]. IL-17A in viral infections may contribute to secondary inflammatory injury recruiting neutrophils and lymphocytes, and its levels have been found to be elevated in patients with severe COVID-19 pneumonia [10]. A slightly reduced risk of respiratory infections among patients treated with ustekinumab versus secukinumab was reported in a Swedish population-based register-linked cohort study [11]. Data from pharmacovigilance indicate that anti-IL-23 biologics are not associated with additional risk of infection. Similar rates of infection were found in patients treated with guselkumab and risankizumab, respectively, compared to adalimumab [12][13]. The risk of infection due to conventional immunosuppressant drugs has been extensively investigated. Cyclosporin is well known to be associated with an increased risk of infection [14]; a multicenter prospective cohort study found a 58% higher risk compared with methotrexate (adjusted RR 1.58; 95% CI 1.17–2.15) [15]. Nevertheless, cyclosporin targets cyclophilin, which is required for viral replication, and it may inhibit influenza A virus, hepatitis C virus and coronavirus [16]. Methotrexate is also associated with increased risk of infection; a multicenter prospective cohort study reported a 40% higher risk of infection compared to acitretin [15]. Acitretin does not appear to cause immunosuppressive adverse events; a study from the BIODAVERM registry comparing the infection rates among different systemic drugs found that acitretin showed the lowest risk of infection [15].
Apremilast does not affect B cells, T cells, or IgG and IgM secretion, but it partially inhibits TNFα, INF-γ, IL-17 and IL-23. Given its immunomodulatory properties and its specific mechanism of action, refs. [17][18] apremilast does not favor either infection or cytokine storm, and it does not increase the risk of pulmonary fibrosis, one of COVID-19’s mortality factors.
In addition to the potential treatment-associated risk of infection, psoriatic patients show higher prevalence of cardio-metabolic comorbidities compared to the general population. Severe COVID-19 patients are older and affected by comorbidities such hypertension and diabetes mellitus [19]. Hypertension, diabetes mellitus and cardiovascular disease are more prevalent in patients with psoriasis and are associated with risk of hospitalization and fatal outcome of COVID-19 pneumonia [4]. The potential impact of co-morbidity status such as obesity, hypertension, diabetes and positive history of cardiovascular disease was addressed in the international study of Mahil et al. analyzing data from the PsoPROTECT registry. They found that risk factors including being older, male, non-white and having chronic lung disease were associated with higher hospitalization rates for COVID-19 [20]. Some hypotheses may explain why a higher incidence of fatal COVID-19 outcomes was not found in psoriasis patients on systemic treatments. Patients with psoriasis under systemic treatment may have more defensive behaviors (e.g., wearing a facial mask, social distancing) compared to the general population. Another hypothesis relies on a possible protective effect against COVID-19 provided by biologics. Inhibition of the COVID-19 immune response would be harmful in the early phase of infection, but it would be helpful in the progression to the severe form of the disease [8]. In COVID-19, inflammatory cytokines appear to play a double role: they may elicit the activation of the immune response in the first phase, but later they can mediate the development of an exaggerated systemic inflammation [8]. Because of the hyperactive inflammatory effects of SARS-CoV-2, agents that modulate the immune response are being explored as adjunctive treatments for the management of moderate to critical cases. There is sufficient evidence to support clinical trials of anti TNF-α therapy in patients with COVID-19 [21][22]. It was recently demonstrated that angiotensin-converting enzyme 2 (ACE2) is the main receptor employed by SARS-CoV-2. Because IL-17 mediates inflammation, increasing ACE2 expression in epithelia, IL-17 inhibitors may prove advantageous in patients with psoriasis at risk of infection [23]. In addition, given that COVID-19 pneumonia patients with a Th17 profile may show worse outcomes, a clinical trial investigating the use of anti-IL17 is currently ongoing [24][25]. Nonetheless, there is concern that patients on immunosuppressant biologic therapies might be at higher risk of being infected and a question as to whether they need to discontinue their treatment pre-emptively. Indeed, a reduction in prescribing of immunosuppressants and biologics has been a common practice [26][27]. Generally, the decision to discontinue systemic treatment has been reported to be autonomous, due to fear of a risk of infection, rather than shared with the general practitioner. As consequence, a significant percentage of patients experience worsening of the disease, mainly because of drug withdrawal [28]. A cross-sectional study among Chinese patients with psoriasis, conducted by a web questionnaire, demonstrated that nonadherence to treatment during the COVID-19 epidemic was associated with aggravation of psoriasis, perceived stress and symptoms of anxiety and depression [29]. If patients express concerns about the safety of systemic psoriasis therapy during the COVID-19 pandemic, shared decision-making is needed after informing the patient about current guideline recommendations and about the benefits and risks of treatment discontinuation [30][31].
Nonetheless, our findings appear unequivocal, and although additional studies may be helpful to confirm these results, existing studies appear sufficient to conclude that psoriatic patients receiving systemic treatment are unlikely to be at increased risk of SARS-CoV-2 infection or severe COVID-19. Moreover, patients affected by inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease, and treated with biologics show similar COVID-19 clinical outcomes to those of psoriatic patients [32]. However, these results should be confirmed by further studies comprising a longer follow-up period and including a control group. Moreover, other studies are needed to investigate the prevalence of Sars-CoV-2 infection in asymptomatic patients with psoriasis.
3. Conclusions
It is reasonable to interrupt systemic therapy in psoriasis patients that test positive for SARS-CoV-2 or with COVID-19 because potentially negative effects of the therapy cannot be fully excluded. Currently, however, there is no evidence to support the idea that patients receiving systemic therapy should stop their treatment. Rather, monitoring frequency could be increased in the beginning of newly started systemic therapy. To better understand the impact of SARS-CoV-2 infection, psoriasis patients that test positive should be followed carefully and entered into global registries, such as PsoPROTECT. People with psoriasis undergoing systemic therapy should be advised to follow current guidelines for hygiene and physical distancing as recommended in their respective area of residence. Given that this is a novel and rapidly changing situation, recommendations may be modified as more data become available [33].
References
- Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-2019). People Who Are at Higher Risk for Severe Illness. Available online: https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html (accessed on 1 November 2020).
- Nast, A.; Spuls, P.I.; van der Kraaij, G.; Gisondi, P.; Paul, C.; Ormerod, A.D.; Saiag, P.; Smith, C.H.; Dauden, E.; de Jong, E.M.; et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris—Update Apremilast and Secukinumab—EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1951–1963.
- Li, X.; Andersen, K.M.; Chang, H.-Y.; Curtis, J.R.; Alexander, G.C. Comparative risk of serious infections among real-world users of biologics for psoriasis or psoriatic arthritis. Ann. Rheum. Dis. 2020, 79, 285–291.
- Piaserico, S.; Gisondi, P.; Cazzaniga, S.; Naldi, L. Lack of Evidence for an Increased Risk of Severe COVID-19 in Psoriasis Patients on Biologics: A Cohort Study from Northeast Italy. Am. J. Clin. Dermatol. 2020, 21, 1–3.
- Ladda, M.; Lynde, C.W.; Fleming, P. Severe Acute Respiratory Syndrome Coronavirus 2 and the Use of Biologics in Patients with Psoriasis. J. Cutan. Med. Surg. 2020, 1203475420945234.
- Seo, S.H.; Webster, R.G. Tumor Necrosis Factor Alpha Exerts Powerful Anti-Influenza Virus Effects in Lung Epithelial Cells. J. Virol. 2002, 76, 1071–1076.
- Syed, M.N.; Shah, M.; Shin, D.B.; Wan, M.T.; Winthrop, K.L.; Gelfand, J.M. Effect of anti-tumor necrosis factor therapy on the risk of respiratory tract infections and related symptoms in psoriasis patients—A meta-estimate of pivotal phase 3 trials relevant to decision-making during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 2020.
- Wan, M.T.; Shin, D.B.; Winthrop, K.L.; Gelfand, J.M. The risk of respiratory tract infections and symptoms in psoriasis patients treated with interleukin 17 pathway-inhibiting biologics: A meta-estimate of pivotal trials relevant to decision making during the COVID-19 pandemic. J. Am. Acad. Dermatol. 2020, 83, 677–679.
- Van De Kerkhof, P.; Griffiths, C.E.; Reich, K.; Leonardi, C.L.; Blauvelt, A.; Tsai, T.-F.; Gong, Y.; Huang, J.; Papavassilis, C.; Fox, T. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J. Am. Acad. Dermatol. 2016, 75, 83–98.e4.
- Pacha, O.; Sallman, M.A.; Evans, S.E. COVID-19: A case for inhibiting IL-17? Nat. Rev. Immunol. 2020, 20, 345–346.
- Srinivas, C.; Odsbu, I.; Linder, M. Risk of common infections among individuals with psoriasis in Sweden: A nationwide cohort study comparing secukinumab to ustekinumab. Pharmacoepidemiol. Drug Saf. 2020.
- Reich, K.; Papp, K.; Armstrong, A.W.; Wasfi, Y.; Li, S.; Shen, Y.; Randazzo, B.; Song, M.; Kimball, A.B. Safety of guselkumab in patients with moderate-to-severe psoriasis treated through 100 weeks: A pooled analysis from the randomized VOYAGE 1 and VOYAGE 2 studies. Br. J. Dermatol. 2019, 180, 1039–1049.
- Reich, K.; Gooderham, M.; Thaçi, D. Risankizumab compared with adalimumab in patients with moderate-tosevere plaque psoriasis (IMMvent): A randomised, doubleblind, active-comparator-controlled phase 3 trial. Lancet 2019, 394, 576–586.
- Kaushik, S.B.; Lebwohl, M.G. Review of safety and efficacy of approved systemic psoriasis therapies. Int. J. Dermatol. 2019, 58, 649–658.
- Dávila-Seijo, P.; Dauden, E.; Descalzo, M.; Carretero, G.; Carrascosa, J.-M.; Vanaclocha, F.; Gómez-García, F.-J.; De La Cueva-Dobao, P.; Herrera-Ceballos, E.; Belinchón, I.; et al. Infections in Moderate to Severe Psoriasis Patients Treated with Biological Drugs Compared to Classic Systemic Drugs: Findings from the Biobadaderm Registry. J. Investig. Dermatol. 2017, 137, 313–321.
- Di Lernia, V.; Goldust, M.; Feliciani, C. Covid-19 infection in psoriasis patients treated with cyclosporin. Dermatol. Ther. 2020, 33.
- Mahil, S.K.; Dand, N.; Mason, K.J.; Yiu, Z.Z.; Tsakok, T.; Meynell, F.; Coker, B.; McAteer, H.; Moorhead, L.; MacKenzie, T.; et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—Insights from a global registry-based study. J. Allergy Clin. Immunol. 2020.
- Carugno, A.; Gambini, D.M.; Raponi, F.; Vezzoli, P.; Robustelli Test, E.; Arosio, M. Coronavirus disease 2019 (COVID-19) rash in a psoriatic patient treated with Secukinumab: Is there a role for Interleukin 17? Dermatol. Ther. 2020, e14011.
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374.
- Mahil, S.K.; Dand, N.; Mason, K.J.; Yiu, Z.Z.; Tsakok, T.; Meynell, F.; Coker, B.; McAteer, H.; Moorhead, L.; MacKenzie, T.; et al. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—Insights from a global registry-based study. J. Allergy Clin. Immunol. 2020.
- Feldmann, M.; Maini, R.N.; Woody, J.N.; Holgate, S.T.; Winter, G.; Rowland, M.; Richards, D.; Hussell, T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet 2020, 395, 1407–1409.
- A Randomized, Open-Label, Controlled Trial for the Efficacy and Safety of Adalimumab Injection in the Treatment of Patients with Severe Novel Coronavirus Pneumonia (COVID-19); ChiCTR2000030089; Chinese Clinical Trial Registry: Shanghai, China, 2020.
- Krueger, J.G.; Murrell, D.F.; Garcet, S.; Navrazhina, K.; Lee, P.C.; Muscianisi, E.; Blauvelt, A. Secukinumab lowers expression of ACE2 in affected skin of patients with psoriasis. J. Allergy Clin. Immunol. 2020.
- Faure, E.; Poissy, J.; Goffard, A. Distinct immune response in two MERS-CoV-infecred patients: Can we go from bench to bedside? PLoS ONE 2014, 9, e88716.
- A Randomized, Blinded, Controlled, Multicenter Clinical Trial to Evaluate the Efficacy and Safety of Ixekizumab Combined with Conventional Antiviral Drugs in Patients with Novel Coronavirus Pneumonia (COVID-19); CHICTR: Shanghai, China, 2020.
- Leis, M.; Fleming, P.; Lynde, C.W. Impacts of COVID-19 on Dermatologic Practice, Disease Presentation, and Immunomodulator Prescriptions. J. Cutan. Med. Surg. 2020, 2020.
- Georgakopoulos, J.R.; Mufti, A.; Vender, R.; Yeung, J. Treatment discontinuation and rate of disease transmission in psoriasis patients on biologic therapy during the COVID-19 pandemic—A Canadian multicenter retrospective study. J. Am. Acad. Dermatol. 2020.
- Pirro, F.; Caldarola, G.; Chiricozzi, A.; Tambone, S.; Mariani, M.; Calabrese, L.; D’Urso, D.F.; De Simone, C.; Peris, K. The impact of COVID-19 pandemic in a cohort of Italian psoriatic patients treated with biological therapies. J. Dermatol. Treat. 2020, 2020, 1–5.
- Wang, Q.; Luo, Y.; Lv, C.; Zheng, X.; Zhu, W.; Chen, X.; Shen, M.; Kuang, Y. Nonadherence to Treatment and Patient-Reported Outcomes of Psoriasis During the COVID-19 Epidemic: A Web-Based Survey. Patient Preference Adherence 2020, 14, 1403–1409.
- Centre of Evidence Based Dermatology. CEBD Coronavirus Dermatology Resource. Available online: https://www.nottingham.ac.uk/research/groups/cebd/resources/Coronavirus-resource/Coronavirushome.aspx (accessed on 1 November 2020).
- Talamonti, M.; Galluzzo, M.; Chiricozzi, A.; Quaglino, P.; Fabbrocini, G.; Gisondi, P.; Marzano, A.; Potenza, C.; Conti, A.; Parodi, A.; et al. Management of biological therapies for chronic plaque psoriasis during COVID-19 emergency in Italy. J. Eur. Acad. Dermatol. Venereol. 2020.
- Haberman, R.; Axelrad, J.; Chen, A.; Castillo, R.; Yan, D.; Izmirly, P.; Neimann, A.; Adhikari, S.; Hudesman, D.; Scher, J.U. Covid-19 in Immune-Mediated Inflammatory Diseases—Case Series from New York. N. Engl. J. Med. 2020, 383, 85–88.
- Gadarowski, M.B.; Balogh, E.A.; Bashyam, A.M.; Feldman, S.R. Examining recommendations for the use of biologics and other systemic therapies during COVID-19: A review and comparison of available dermatology guidelines and patient registries. J. Dermatol. Treat. 2020, 2020, 1–5.




