
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | valérian dormoy | + 916 word(s) | 916 | 2021-07-14 04:17:37 | | | |
| 2 | valérian dormoy | Meta information modification | 916 | 2021-07-21 16:09:18 | | | | |
| 3 | Bruce Ren | + 9285 word(s) | 10201 | 2021-07-22 04:13:42 | | | | |
| 4 | Conner Chen | Meta information modification | 10201 | 2021-09-22 09:36:11 | | |
Video Upload Options
Lung cancer represents the first cause of death by cancer worldwide and remains a challenging public health issue. Hypoxia, as a relevant biomarker, has raised high expectations for clinical practice.
Introduction
2. Biological Features Associated with Hypoxia in NSCLC
2.1. Hypoxia-Inducible Factor Detection in Whole Tumour Tissues
2.2. Tumour-Initiating Cells and Hypoxic Conditions
2.2.1. Cancer Stem Cells Are Influenced by Hypoxia
2.2.2. Epithelial to Mesenchymal Polarization by Hypoxia
2.3. Tumour Microenvironment Features in Hypoxic Condition
2.3.1. Hypoxia-Driven Stroma Modifications
2.3.2. Inflammation Landscape in the Hypoxic Context
2.3.3. Immune Checkpoint Disruption by Hypoxia
2.4. Molecular Signature of Hypoxic Tumours
2.4.1. Metabolic Consequences of Hypoxia
2.4.2. Hypoxia Widely Impairs Cancer Gene Expression
2.4.3. Hypoxia Supports Molecular Alterations
2.4.4. EGFR and ALK Genes Are More Frequently Altered in Hypoxic NSCLC Tumours
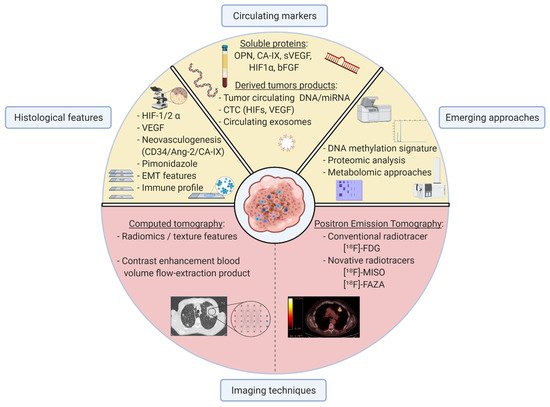
3. Available Tools to Detect Hypoxia in Clinical Practice
| Hypoxic Marker | Biological Material | Method of Assessment | Observation | Clinical Interest | Advantage | Limitation | Patients (n) | REF |
|---|---|---|---|---|---|---|---|---|
| HIF-1 α | Tumour cells | Histological | ↑ expression associated with lymph node metastasis | Staging | Reproducible | Variability on HIF-1α threshold of positivity and its intracellular localization | 1436 | [38] |
| Tumour cells | ↑ expression associated with ↓ OS | Prognosis | 1049 | [38] | ||||
| 1113 | [47] | |||||||
| 256 | [136] | |||||||
| Serum concentrations | Circulating blood marker | ↓ serum concentration during chemoradiotherapy course | Response monitoring | Mini-invasive procedure Reproducible Real-time monitoring |
No control group | 80 | [137] | |
| HIF-2 α | Tumour cells | Histological | ↑ expression associated with ↑ stages ↑ expression associated with ↓ OS |
Staging Prognosis |
Reproducible | Restricted to stages I to III No association with HIF-1 α expression |
140 | [50] |
| Ang-2 | Tumour cells | Histological and mRNA expression | ↑ expression associated with ↑stages ↑ expression associated with ↓ OS |
Staging Prognosis |
Reproducible | mRNA expression not currently transposable in clinical routine Significance restricted to AC subgroup |
1244 | [46] |
| Serum concentration | Circulating blood marker | ↑ concentration associated with lung cancer (Se 92.5 and Sp 97.5%) | Diagnosis | Mini-invasive procedure Reproducible Real-time monitoring |
High threshold | 228 | [43] | |
| ↑ expression associated with ↑ stages ↑ expression associated with ↓ OS |
Staging Prognosis |
- | 575 | [44] | ||||
| GLUT-1 | Tumour cells | Protein and mRNA expression | Co-expression with PD-L1 ↓ co- expression associated with ↑ OS |
Prognosis | Reproducible | No evaluation of ICI sensibility | 295 | [104] |
| CA-IX | Serum concentration | Circulating blood marker | ↑ concentrations associated with ↓ survival rates under radiotherapy | Prognosis | Mini-invasive procedure Reproducible Real-time monitoring |
Small effective | 55 | [138] |
| Flow extraction product | CT radiomic analysis | CT features | ↓ enhancement associated with lymph node metastasis Correlation with GLUT expression | Staging | Radiometabolic hypoxia-related markers | Small effective Non-standardised techniques of acquisition and analyses |
14 | [139] |
| Standardised Uptake Values (SUV) | PET radiomic analysis | Metabolic features | Correlation with histological HIF-1α expression | Not established | Non-invasive procedure Tumour heterogeneity consideration |
No investigation on prognosis | 288 | [140] |
| OPN | Serum concentration | Circulating blood marker | ↑ concentrations associated with ↓ OS | Predictive of ↓ outcomes with chemoradiotherapy Predictive of ↓ outcomes with radiotherapy Predictive of tumoural response and prognosis with chemoradiotherapy |
Non-invasive procedure Tumour heterogeneity consideration Reproducible Real-time monitoring |
Included in a multiparametric model with other proteins | 263 | [141] |
| Small effective | 44 | [142] | ||||||
| 81 | [143] | |||||||
| 55 | [138] | |||||||
| Significance restricted to SqCC subgroup | 337 | [144] | ||||||
| VEGF | Tumour cells | Histological and mRNA expression | ↑ concentration associated with ↓ OS | Monitoring of tumoural response under chemoradiotherapy | Reproducible | Variability on VEGF threshold of positivity and intracellular localization | 1549 | [145] |
| Serum concentration | Circulating blood marker | ↑ concentration associated with ↓ OS | Predictive of response and prognosis with immunotherapy | Non-invasive procedure Tumour heterogeneity consideration Reproducible Real-time monitoring |
Significance restricted to elderly (>75 y.o) or PS > 2. | 235 | [146] | |
| ↑ concentration associated with ↓ survival rates under radiotherapy | Predictive of ↓ outcomes with radiotherapy | Small effective | 55 | [138] | ||||
| bFGF | Serum concentration | Circulating blood marker | ↑ concentration associated with ↓ OS | Monitoring of tumoural response under chemoradiotherapy regimen | Non-invasive procedure Tumour heterogeneity consideration Reproducible Real-time monitoring |
Variability on bFGF threshold of positivity | 358 | [147] |
| miR-21, miR-128, miR-155, miR-181a | mi-RNA serum concentration | Circulating blood marker | Prediction of outcomes with first-line chemotherapy | Predictive of tumoural response Prognosis |
Non-invasive procedure Reproducible Real-time monitoring |
Not currently transposable in clinical routine Significance restricted to SqCC subgroup | 128 | [148] |
| CA-IX, CCL20, CORO1C, CTSC, LDHA, NDRG, PTP4A3, TUBA1B |
Gene expression signature | mRNA expression | Predictive of hypoxic tumours associated with ↓ OS Co-expression with TILs infiltration |
Prognosis | Non-invasive procedure Reproducible Real-time monitoring |
Not currently transposable in clinical routine | 515 | [97] |
3.1. Hypoxic Characterisation by Imaging Techniques
3.1.1. Using Radiomics on Computed Tomography Images to Identify Hypoxic Tumours
3.1.2. Positive Emission Tomography Radiotracers Identify Hypoxia
3.1.3. Conventional [18F]-FDG PET to Explore Hypoxia
3.1.4. Current Limitations for Hypoxia Characterisation by PET
3.2. Circulating Markers to Help Clinicians Classify Hypoxic Tumours
3.2.1. Soluble Blood Proteins Are Promising for the Identification of Hypoxic Tumours
3.2.2. Tumour Circulating DNA/RNA Provide Hypoxia-Related Signatures
3.2.3. Circulating Tumour Cells Associated with Hypoxic Tumours
3.3. Emerging Approaches in Hypoxic-Tumour Identification
4. Prognostic Implications of Hypoxia in Lung Cancer
4.1. Early and Locally Advanced Stages
4.1.1. Hypoxia Is Associated with Higher Tumour Stages
4.1.2. Hypoxia-Associated SNPs Revealed Higher Lung Cancer Risks
4.1.3. Hypoxia as a Prognostic Marker for Resected Tumours
HIF-1α Expression Is Associated with Worse Clinical Outcomes
Hypoxia Related Prognosis and HIFs
4.1.4. Characterisation of Hypoxia to Improve the Clinical Course in Locally Advanced Stages
Current Strategy in Non-Resectable and Local NSCLC
Hypoxic Features to Predict and Monitor Tumour Response
4.2. Metastatic Stages: Potential Hypoxia-Related Treatment Strategies, from Response to Resistance
4.2.1. Hypoxic Tumours Are Associated with a Higher Risk of Distant Metastasis
4.2.2. Chemotherapy
4.2.3. Immunotherapy
Current Use of Immune-Checkpoint Inhibitors
Hypoxia as a Predictor of Response under ICI Regimens
4.2.4. Radiotherapy
Hypoxic Imaging to Guide Radiotherapy Strategies
Biological Hypoxia-Related Markers Improving Radiotherapy Success
4.2.5. Targeted Therapies
KRAS
EGFR
- •
-
Current Clinical Application of anti-EGFR in NSCLC
- •
-
Hypoxia Could Predict Non-Response to EGFR TKIs in Wild-Type EGFR NSCLC
- •
-
Hypoxia Led to EGFR TKIs Resistance in Mutant EGFR NSCLC
- •
-
Promising Strategies to Overcome Hypoxia-Mediated Resistance with EGFR-TKIs
ALK
5. Hypoxia-Related Treatments and Research Development
5.1. Pyruvate Dehydrogenase Kinase (PDK) Inhibitors
5.2. Metformin
5.3. Vorinostat
5.4. Nitroglycerin
5.5. Tirapazamine
5.6. Efaproxiral
5.7. Anti-Angiogenic Therapies
6. Conclusions
In this study, we conducted an extensive review of the potential impact of hypoxia in each stage of NSCLC and highlighted clinical and pathological features related to hypoxic tumours relevant to clinicians. It appears that some tumours are more frequently associated with hypoxic regions than others, such as poorly differentiated SqCC presenting a tumour microenvironment including high tumoural microvessel density and stroma-enriched immune cells harbouring epithelial-to-mesenchymal polarization. The current challenge to identifying hypoxia remains the definition of relevant thresholds for markers discriminating hypoxic from non-hypoxic tumours. Pathological examinations and immunostainings are needed to validate further markers in paired and matched comparison studies. The identification of biological signatures based on nucleic acid expressions may contribute to the development of hypoxic-scores after validation in larger and design-dedicated cohorts. Systematic genetic association studies taking hypoxia as a relevant parameter will ideally complement the translational approach. We also reviewed current promising approaches allowing to evaluate hypoxia in the NSCLC context with a special interest in the most suitable and transposable approaches in clinical routine. Tumour hypoxia biology is complex and in constant evolution over time, given that sequential drugs and radiation use lead to resistance and treatment escape. We finally investigated how hypoxic characterisation could influence the major steps of lung cancer clinical management. For patients with early cured NSCLC, transversal hypoxic tumour determination might also be of interest to isolate and predict those who would benefit from adjuvant therapies to reduce the risk of relapse. Nonetheless, challenges and clinical goals are specific in advanced and metastatic stages when aiming to predict tumour response across various regimens of treatments. In these later stages, longitudinal hypoxic characterisation might be the most relevant approach. Repeated PET/CT scans and more strikingly, circulating hypoxia-related markers may enable monitoring of tumour variations and adaptation of clinical strategies in a personalised approach. Despite limitations to hypoxia implementation in lung cancer clinical management, evidence is accumulating for its consideration, including dedicated hypoxia-related treatments. Hypoxia characterisation could improve the outcome of patients with NSCLC and might represent the next step to a personalised medical protocol in the field of cancer.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640.
- Reck, M.; Rabe, K.F. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 849–861.
- Korpanty, G.J.; Graham, D.M.; Vincent, M.D.; Leighl, N.B. Biomarkers That Currently Affect Clinical Practice in Lung Cancer: EGFR, ALK, MET, ROS-1, and KRAS. Front. Oncol. 2014, 4, 204.
- Prelaj, A.; Tay, R.; Ferrara, R.; Chaput, N.; Besse, B.; Califano, R. Predictive Biomarkers of Response for Immune Checkpoint Inhibitors in Non-Small-Cell Lung Cancer. Eur. J. Cancer 2019, 106, 144–159.
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000-14 (CONCORD-3): Analysis of Individual Records for 37 513 025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075.
- Chen, P.-S.; Chiu, W.-T.; Hsu, P.-L.; Lin, S.-C.; Peng, I.-C.; Wang, C.-Y.; Tsai, S.-J. Pathophysiological Implications of Hypoxia in Human Diseases. J. Biomed. Sci. 2020, 27, 63.
- Mouronte-Roibás, C.; Leiro-Fernández, V.; Fernández-Villar, A.; Botana-Rial, M.; Ramos-Hernández, C.; Ruano-Ravina, A. COPD, Emphysema and the Onset of Lung Cancer. A Systematic Review. Cancer Lett. 2016, 382, 240–244.
- Semenza, G.L. Hypoxia-Inducible Factors in Physiology and Medicine. Cell 2012, 148, 399–408.
- Fajersztajn, L.; Veras, M.M. Hypoxia: From Placental Development to Fetal Programming. Birth Defects Res. 2017, 109, 1377–1385.
- Kurihara, T. Roles of Hypoxia Response in Retinal Development and Pathophysiology. Keio J. Med. 2018, 67, 1–9.
- Vadivel, A.; Alphonse, R.S.; Etches, N.; van Haaften, T.; Collins, J.J.P.; O’Reilly, M.; Eaton, F.; Thébaud, B. Hypoxia-Inducible Factors Promote Alveolar Development and Regeneration. Am. J. Respir. Cell Mol. Biol. 2014, 50, 96–105.
- Woik, N.; Kroll, J. Regulation of Lung Development and Regeneration by the Vascular System. Cell. Mol. Life Sci. 2015, 72, 2709–2718.
- Ullmann, P.; Nurmik, M.; Begaj, R.; Haan, S.; Letellier, E. Hypoxia- and MicroRNA-Induced Metabolic Reprogramming of Tumor-Initiating Cells. Cells 2019, 8, 528.
- Huertas-Castaño, C.; Gómez-Muñoz, M.A.; Pardal, R.; Vega, F.M. Hypoxia in the Initiation and Progression of Neuroblastoma Tumours. Int. J. Mol. Sci. 2019, 21, 39.
- Pouysségur, J.; Dayan, F.; Mazure, N.M. Hypoxia Signalling in Cancer and Approaches to Enforce Tumour Regression. Nature 2006, 441, 437–443.
- Peng, G.; Liu, Y. Hypoxia-Inducible Factors in Cancer Stem Cells and Inflammation. Trends Pharmacol. Sci. 2015, 36, 374–383.
- Schito, L. Hypoxia-Dependent Angiogenesis and Lymphangiogenesis in Cancer. Adv. Exp. Med. Biol. 2019, 1136, 71–85.
- Vito, A.; El-Sayes, N.; Mossman, K. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992.
- Luo, W.; Wang, Y. Hypoxia Mediates Tumor Malignancy and Therapy Resistance. Adv. Exp. Med. Biol. 2019, 1136, 1–18.
- Jackson, A.L.; Zhou, B.; Kim, W.Y. HIF, Hypoxia and the Role of Angiogenesis in Non-Small Cell Lung Cancer. Expert Opin. Ther. Targets 2010, 14, 1047–1057.
- Pugh, C.W.; Ratcliffe, P.J. New Horizons in Hypoxia Signaling Pathways. Exp. Cell Res. 2017, 356, 116–121.
- Keith, B.; Simon, M.C. Hypoxia-Inducible Factors, Stem Cells, and Cancer. Cell 2007, 129, 465–472.
- Zhao, Z.; Mu, H.; Li, Y.; Liu, Y.; Zou, J.; Zhu, Y. Clinicopathological and Prognostic Value of Hypoxia-Inducible Factor-1α in Breast Cancer: A Meta-Analysis Including 5177 Patients. Clin. Transl. Oncol. 2020.
- Dahia, P.L.M.; Toledo, R.A. Recognizing Hypoxia in Phaeochromocytomas and Paragangliomas. Nat. Rev. Endocrinol. 2020, 16, 191–192.
- Fuchs, Q.; Pierrevelcin, M.; Messe, M.; Lhermitte, B.; Blandin, A.-F.; Papin, C.; Coca, A.; Dontenwill, M.; Entz-Werlé, N. Hypoxia Inducible Factors’ Signaling in Pediatric High-Grade Gliomas: Role, Modelization and Innovative Targeted Approaches. Cancers 2020, 12, 979.
- Guo, Y.; Xiao, Z.; Yang, L.; Gao, Y.; Zhu, Q.; Hu, L.; Huang, D.; Xu, Q. Hypoxia-inducible Factors in Hepatocellular Carcinoma (Review). Oncol. Rep. 2020, 43, 3–15.
- Wielockx, B.; Grinenko, T.; Mirtschink, P.; Chavakis, T. Hypoxia Pathway Proteins in Normal and Malignant Hematopoiesis. Cells 2019, 8, 155.
- Bryant, J.L.; Meredith, S.L.; Williams, K.J.; White, A. Targeting Hypoxia in the Treatment of Small Cell Lung Cancer. Lung Cancer 2014, 86, 126–132.
- Nabavi, N.; Bennewith, K.L.; Churg, A.; Wang, Y.; Collins, C.C.; Mutti, L. Switching off Malignant Mesothelioma: Exploiting the Hypoxic Microenvironment. Genes Cancer 2016, 7, 340–354.
- Papkovsky, D.B.; Dmitriev, R.I. Imaging of Oxygen and Hypoxia in Cell and Tissue Samples. Cell. Mol. Life Sci. 2018, 75, 2963–2980.
- Semenza, G.L. HIF-1: Mediator of Physiological and Pathophysiological Responses to Hypoxia. J. Appl. Physiol. 2000, 88, 1474–1480.
- Shimoda, L.A.; Semenza, G.L. HIF and the Lung: Role of Hypoxia-Inducible Factors in Pulmonary Development and Disease. Am. J. Respir. Crit. Care Med. 2011, 183, 152–156.
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia Signaling in Human Diseases and Therapeutic Targets. Exp. Mol. Med. 2019, 51, 1–13.
- Balamurugan, K. HIF-1 at the Crossroads of Hypoxia, Inflammation, and Cancer. Int. J. Cancer 2016, 138, 1058–1066.
- Tirpe, A.A.; Gulei, D.; Ciortea, S.M.; Crivii, C.; Berindan-Neagoe, I. Hypoxia: Overview on Hypoxia-Mediated Mechanisms with a Focus on the Role of HIF Genes. Int. J. Mol. Sci. 2019, 20, 6140.
- Goudar, R.K.; Vlahovic, G. Hypoxia, Angiogenesis, and Lung Cancer. Curr. Oncol. Rep. 2008, 10, 277–282.
- Yang, S.-L.; Ren, Q.-G.; Wen, L.; Hu, J.-L. Clinicopathological and Prognostic Significance of Hypoxia-Inducible Factor-1 Alpha in Lung Cancer: A Systematic Review with Meta-Analysis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 321–327.
- Li, J.; Zhang, G.; Wang, X.; Li, X.-F. Is Carbonic Anhydrase IX a Validated Target for Molecular Imaging of Cancer and Hypoxia? Future Oncol. 2015, 11, 1531–1541.
- Albadari, N.; Deng, S.; Li, W. The Transcriptional Factors HIF-1 and HIF-2 and Their Novel Inhibitors in Cancer Therapy. Expert Opin. Drug Discov. 2019, 14, 667–682.
- Xiong, Q.; Liu, B.; Ding, M.; Zhou, J.; Yang, C.; Chen, Y. Hypoxia and Cancer Related Pathology. Cancer Lett. 2020, 486, 1–7.
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising Targets for the Treatment of Respiratory Disorders. Respir. Med. 2019, 156, 33–46.
- Xu, C.; Wang, W.; Wang, Y.; Zhang, X.; Yan, J.; Yu, L. Serum Angiopoietin-2 as a Clinical Marker for Lung Cancer in Patients with Solitary Pulmonary Nodules. Ann. Clin. Lab. Sci. 2016, 46, 60–64.
- Xu, Y.; Zhang, Y.; Wang, Z.; Chen, N.; Zhou, J.; Liu, L. The Role of Serum Angiopoietin-2 Levels in Progression and Prognosis of Lung Cancer: A Meta-Analysis. Medicine 2017, 96, e8063.
- Kizaka-Kondoh, S.; Konse-Nagasawa, H. Significance of Nitroimidazole Compounds and Hypoxia-Inducible Factor-1 for Imaging Tumor Hypoxia. Cancer Sci. 2009, 100, 1366–1373.
- Qin, S.; Yi, M.; Jiao, D.; Li, A.; Wu, K. Distinct Roles of VEGFA and ANGPT2 in Lung Adenocarcinoma and Squamous Cell Carcinoma. J. Cancer 2020, 11, 153–167.
- Ren, W.; Mi, D.; Yang, K.; Cao, N.; Tian, J.; Li, Z.; Ma, B. The Expression of Hypoxia-Inducible Factor-1α and Its Clinical Significance in Lung Cancer: A Systematic Review and Meta-Analysis. Swiss Med. Wkly. 2013, 143, w13855.
- Liu, J.; Liu, Y.; Gong, W.; Kong, X.; Wang, C.; Wang, S.; Liu, A. Prognostic Value of Insulin-like Growth Factor 2 MRNA-Binding Protein 3 and Vascular Endothelial Growth Factor-A in Patients with Primary Non-Small-Cell Lung Cancer. Oncol. Lett. 2019, 18, 4744–4752.
- Yuan, A.; Yu, C.J.; Chen, W.J.; Lin, F.Y.; Kuo, S.H.; Luh, K.T.; Yang, P.C. Correlation of Total VEGF MRNA and Protein Expression with Histologic Type, Tumor Angiogenesis, Patient Survival and Timing of Relapse in Non-Small-Cell Lung Cancer. Int. J. Cancer 2000, 89, 475–483.
- Gao, Z.-J.; Wang, Y.; Yuan, W.-D.; Yuan, J.-Q.; Yuan, K. HIF-2α Not HIF-1α Overexpression Confers Poor Prognosis in Non-Small Cell Lung Cancer. Tumour Biol. 2017, 39, 1010428317709637.
- Yeh, S.-J.; Chang, C.-A.; Li, C.-W.; Wang, L.H.-C.; Chen, B.-S. Comparing Progression Molecular Mechanisms between Lung Adenocarcinoma and Lung Squamous Cell Carcinoma Based on Genetic and Epigenetic Networks: Big Data Mining and Genome-Wide Systems Identification. Oncotarget 2019, 10, 3760–3806.
- Lee, S.; Kang, H.G.; Choi, J.E.; Lee, J.H.; Kang, H.J.; Baek, S.A.; Lee, E.; Seok, Y.; Lee, W.K.; Lee, S.Y.; et al. The Different Effect of VEGF Polymorphisms on the Prognosis of Non-Small Cell Lung Cancer According to Tumor Histology. J. Korean Med. Sci. 2016, 31, 1735–1741.
- Eilertsen, M.; Pettersen, I.; Andersen, S.; Martinez, I.; Donnem, T.; Busund, L.-T.; Bremnes, R.M. In NSCLC, VEGF-A Response to Hypoxia May Differ between Squamous Cell and Adenocarcinoma Histology. Anticancer Res. 2012, 32, 4729–4736.
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer Stem Cell (CSC) Resistance Drivers. Life Sci. 2019, 234, 116781.
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134.
- Tong, W.-W.; Tong, G.-H.; Liu, Y. Cancer Stem Cells and Hypoxia-Inducible Factors (Review). Int. J. Oncol. 2018, 53, 469–476.
- Bajaj, J.; Diaz, E.; Reya, T. Stem Cells in Cancer Initiation and Progression. J. Cell Biol. 2020, 219.
- Saforo, D.; Omer, L.; Smolenkov, A.; Barve, A.; Casson, L.; Boyd, N.; Clark, G.; Siskind, L.; Beverly, L. Primary Lung Cancer Samples Cultured under Microenvironment-Mimetic Conditions Enrich for Mesenchymal Stem-like Cells That Promote Metastasis. Sci. Rep. 2019, 9, 4177.
- Kang, N.; Choi, S.Y.; Kim, B.N.; Yeo, C.D.; Park, C.K.; Kim, Y.K.; Kim, T.-J.; Lee, S.-B.; Lee, S.H.; Park, J.Y.; et al. Hypoxia-Induced Cancer Stemness Acquisition Is Associated with CXCR4 Activation by Its Aberrant Promoter Demethylation. BMC Cancer 2019, 19, 148.
- Chen, J.; Xu, R.; Xia, J.; Huang, J.; Su, B.; Wang, S. Aspirin Inhibits Hypoxia-Mediated Lung Cancer Cell Stemness and Exosome Function. Pathol. Res. Pract. 2019, 215, 152379.
- Zhao, M.; Zhang, Y.; Zhang, H.; Wang, S.; Zhang, M.; Chen, X.; Wang, H.; Zeng, G.; Chen, X.; Liu, G.; et al. Hypoxia-Induced Cell Stemness Leads to Drug Resistance and Poor Prognosis in Lung Adenocarcinoma. Lung Cancer 2015, 87, 98–106.
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-Induced Epithelial-Mesenchymal Transition in Cancers: HIF-1α and Beyond. Front. Oncol. 2020, 10, 486.
- Yeo, C.D.; Kang, N.; Choi, S.Y.; Kim, B.N.; Park, C.K.; Kim, J.W.; Kim, Y.K.; Kim, S.J. The Role of Hypoxia on the Acquisition of Epithelial-Mesenchymal Transition and Cancer Stemness: A Possible Link to Epigenetic Regulation. Korean J. Intern. Med. 2017, 32, 589–599.
- Emprou, C.; Le Van Quyen, P.; Jégu, J.; Prim, N.; Weingertner, N.; Guérin, E.; Pencreach, E.; Legrain, M.; Voegeli, A.-C.; Leduc, C.; et al. SNAI2 and TWIST1 in Lymph Node Progression in Early Stages of NSCLC Patients. Cancer Med. 2018.
- Wei, L.; Sun, J.-J.; Cui, Y.-C.; Jiang, S.-L.; Wang, X.-W.; Lv, L.-Y.; Xie, L.; Song, X.-R. Twist May Be Associated with Invasion and Metastasis of Hypoxic NSCLC Cells. Tumour Biol. 2016, 37, 9979–9987.
- Hung, J.-J.; Yang, M.-H.; Hsu, H.-S.; Hsu, W.-H.; Liu, J.-S.; Wu, K.-J. Prognostic Significance of Hypoxia-Inducible Factor-1alpha, TWIST1 and Snail Expression in Resectable Non-Small Cell Lung Cancer. Thorax 2009, 64, 1082–1089.
- Zhang, J.; Wang, J.; Xing, H.; Li, Q.; Zhao, Q.; Li, J. Down-Regulation of FBP1 by ZEB1-Mediated Repression Confers to Growth and Invasion in Lung Cancer Cells. Mol. Cell. Biochem. 2016, 411, 331–340.
- Ruan, J.; Zhang, L.; Yan, L.; Liu, Y.; Yue, Z.; Chen, L.; Wang, A.-Y.; Chen, W.; Zheng, S.; Wang, S.; et al. Inhibition of Hypoxia-Induced Epithelial Mesenchymal Transition by Luteolin in Non-Small Cell Lung Cancer Cells. Mol. Med. Rep. 2012, 6, 232–238.
- Kong, X.; Zhao, Y.; Li, X.; Tao, Z.; Hou, M.; Ma, H. Overexpression of HIF-2α-Dependent NEAT1 Promotes the Progression of Non-Small Cell Lung Cancer through MiR-101-3p/SOX9/Wnt/β-Catenin Signal Pathway. Cell. Physiol. Biochem. 2019, 52, 368–381.
- Liu, X.; Chen, H.; Xu, X.; Ye, M.; Cao, H.; Xu, L.; Hou, Y.; Tang, J.; Zhou, D.; Bai, Y.; et al. Insulin-like Growth Factor-1 Receptor Knockdown Enhances Radiosensitivity via the HIF-1α Pathway and Attenuates ATM/H2AX/53BP1 DNA Repair Activation in Human Lung Squamous Carcinoma Cells. Oncol. Lett. 2018, 16, 1332–1340.
- Kim, I.-G.; Lee, J.-H.; Kim, S.-Y.; Hwang, H.-M.; Kim, T.-R.; Cho, E.-W. Hypoxia-Inducible Transgelin 2 Selects Epithelial-to-Mesenchymal Transition and γ-Radiation-Resistant Subtypes by Focal Adhesion Kinase-Associated Insulin-like Growth Factor 1 Receptor Activation in Non-Small-Cell Lung Cancer Cells. Cancer Sci. 2018, 109, 3519–3531.
- Nurwidya, F.; Takahashi, F.; Kobayashi, I.; Murakami, A.; Kato, M.; Minakata, K.; Nara, T.; Hashimoto, M.; Yagishita, S.; Baskoro, H.; et al. Treatment with Insulin-like Growth Factor 1 Receptor Inhibitor Reverses Hypoxia-Induced Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2014, 455, 332–338.
- Wang, J.; Tian, L.; Khan, M.N.; Zhang, L.; Chen, Q.; Zhao, Y.; Yan, Q.; Fu, L.; Liu, J. Ginsenoside Rg3 Sensitizes Hypoxic Lung Cancer Cells to Cisplatin via Blocking of NF-ΚB Mediated Epithelial-Mesenchymal Transition and Stemness. Cancer Lett. 2018, 415, 73–85.
- Wu, C.-E.; Zhuang, Y.-W.; Zhou, J.-Y.; Liu, S.-L.; Zou, X.; Wu, J.; Wang, R.-P.; Shu, P. Nm23-H1 Inhibits Hypoxia Induced Epithelial-Mesenchymal Transition and Stemness in Non-Small Cell Lung Cancer Cells. Biol. Chem. 2019, 400, 765–776.
- Tan, S.; Xia, L.; Yi, P.; Han, Y.; Tang, L.; Pan, Q.; Tian, Y.; Rao, S.; Oyang, L.; Liang, J.; et al. Exosomal MiRNAs in Tumor Microenvironment. J. Exp. Clin. Cancer Res. 2020, 39, 67.
- Laitala, A.; Erler, J.T. Hypoxic Signalling in Tumour Stroma. Front. Oncol. 2018, 8, 189.
- Farina, A.R.; Cappabianca, L.; Sebastiano, M.; Zelli, V.; Guadagni, S.; Mackay, A.R. Hypoxia-Induced Alternative Splicing: The 11th Hallmark of Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 110.
- Zhang, X.; Sai, B.; Wang, F.; Wang, L.; Wang, Y.; Zheng, L.; Li, G.; Tang, J.; Xiang, J. Hypoxic BMSC-Derived Exosomal MiRNAs Promote Metastasis of Lung Cancer Cells via STAT3-Induced EMT. Mol. Cancer 2019, 18, 40.
- Ren, W.; Hou, J.; Yang, C.; Wang, H.; Wu, S.; Wu, Y.; Zhao, X.; Lu, C. Extracellular Vesicles Secreted by Hypoxia Pre-Challenged Mesenchymal Stem Cells Promote Non-Small Cell Lung Cancer Cell Growth and Mobility as Well as Macrophage M2 Polarization via MiR-21-5p Delivery. J. Exp. Clin. Cancer Res. 2019, 38, 62.
- Kao, S.-H.; Cheng, W.-C.; Wang, Y.-T.; Wu, H.-T.; Yeh, H.-Y.; Chen, Y.-J.; Tsai, M.-H.; Wu, K.-J. Regulation of MiRNA Biogenesis and Histone Modification by K63-Polyubiquitinated DDX17 Controls Cancer Stem-like Features. Cancer Res. 2019, 79, 2549–2563.
- Chen, X.; Wu, L.; Li, D.; Xu, Y.; Zhang, L.; Niu, K.; Kong, R.; Gu, J.; Xu, Z.; Chen, Z.; et al. Radiosensitizing Effects of MiR-18a-5p on Lung Cancer Stem-like Cells via Downregulating Both ATM and HIF-1α. Cancer Med. 2018, 7, 3834–3847.
- Kumar, A.; Deep, G. Exosomes in Hypoxia-Induced Remodeling of the Tumor Microenvironment. Cancer Lett. 2020, 488, 1–8.
- Jafari, R.; Rahbarghazi, R.; Ahmadi, M.; Hassanpour, M.; Rezaie, J. Hypoxic Exosomes Orchestrate Tumorigenesis: Molecular Mechanisms and Therapeutic Implications. J. Transl. Med. 2020, 18, 474.
- Noman, M.Z.; Messai, Y.; Muret, J.; Hasmim, M.; Chouaib, S. Crosstalk between CTC, Immune System and Hypoxic Tumor Microenvironment. Cancer Microenviron. 2014, 7, 153–160.
- Wang, B.; Zhao, Q.; Zhang, Y.; Liu, Z.; Zheng, Z.; Liu, S.; Meng, L.; Xin, Y.; Jiang, X. Targeting Hypoxia in the Tumor Microenvironment: A Potential Strategy to Improve Cancer Immunotherapy. J. Exp. Clin. Cancer Res. 2021, 40, 24.
- Hajizadeh, F.; Okoye, I.; Esmaily, M.; Ghasemi Chaleshtari, M.; Masjedi, A.; Azizi, G.; Irandoust, M.; Ghalamfarsa, G.; Jadidi-Niaragh, F. Hypoxia Inducible Factors in the Tumor Microenvironment as Therapeutic Targets of Cancer Stem Cells. Life Sci. 2019, 237, 116952.
- Giannone, G.; Ghisoni, E.; Genta, S.; Scotto, G.; Tuninetti, V.; Turinetto, M.; Valabrega, G. Immuno-Metabolism and Microenvironment in Cancer: Key Players for Immunotherapy. Int. J. Mol. Sci. 2020, 21, 4414.
- Mittal, V.; El Rayes, T.; Narula, N.; McGraw, T.E.; Altorki, N.K.; Barcellos-Hoff, M.H. The Microenvironment of Lung Cancer and Therapeutic Implications. Adv. Exp. Med. Biol. 2016, 890, 75–110.
- D’Ignazio, L.; Batie, M.; Rocha, S. Hypoxia and Inflammation in Cancer, Focus on HIF and NF-ΚB. Biomedicines 2017, 5, 21.
- Cassetta, L.; Kitamura, T. Macrophage Targeting: Opening New Possibilities for Cancer Immunotherapy. Immunology 2018, 155, 285–293.
- Bremnes, R.M.; Busund, L.-T.; Kilvær, T.L.; Andersen, S.; Richardsen, E.; Paulsen, E.E.; Hald, S.; Khanehkenari, M.R.; Cooper, W.A.; Kao, S.C.; et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 789–800.
- Henze, A.-T.; Mazzone, M. The Impact of Hypoxia on Tumor-Associated Macrophages. J. Clin. Investig. 2016, 126, 3672–3679.
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49.
- Jeong, H.; Kim, S.; Hong, B.-J.; Lee, C.-J.; Kim, Y.-E.; Bok, S.; Oh, J.-M.; Gwak, S.-H.; Yoo, M.Y.; Lee, M.S.; et al. Tumor-Associated Macrophages Enhance Tumor Hypoxia and Aerobic Glycolysis. Cancer Res. 2019, 79, 795–806.
- Silva, V.L.; Al-Jamal, W.T. Exploiting the Cancer Niche: Tumor-Associated Macrophages and Hypoxia as Promising Synergistic Targets for Nano-Based Therapy. J. Control. Release 2017, 253, 82–96.
- Noël, G.; Langouo Fontsa, M.; Willard-Gallo, K. The Impact of Tumor Cell Metabolism on T Cell-Mediated Immune Responses and Immuno-Metabolic Biomarkers in Cancer. Semin. Cancer Biol. 2018, 52, 66–74.
- Chang, W.H.; Forde, D.; Lai, A.G. A Novel Signature Derived from Immunoregulatory and Hypoxia Genes Predicts Prognosis in Liver and Five Other Cancers. J. Transl. Med. 2019, 17, 14.
- Giatromanolaki, A.; Harris, A.L.; Banham, A.H.; Contrafouris, C.A.; Koukourakis, M.I. Carbonic Anhydrase 9 (CA9) Expression in Non-Small-Cell Lung Cancer: Correlation with Regulatory FOXP3+T-Cell Tumour Stroma Infiltration. Br. J. Cancer 2020, 122, 1205–1210.
- Akbarpour, M.; Khalyfa, A.; Qiao, Z.; Gileles-Hillel, A.; Almendros, I.; Farré, R.; Gozal, D. Altered CD8+ T-Cell Lymphocyte Function and TC1 Cell Stemness Contribute to Enhanced Malignant Tumor Properties in Murine Models of Sleep Apnea. Sleep 2017, 40.
- Noman, M.Z.; Buart, S.; Romero, P.; Ketari, S.; Janji, B.; Mari, B.; Mami-Chouaib, F.; Chouaib, S. Hypoxia-Inducible MiR-210 Regulates the Susceptibility of Tumor Cells to Lysis by Cytotoxic T Cells. Cancer Res. 2012, 72, 4629–4641.
- Noman, M.Z.; Janji, B.; Kaminska, B.; Van Moer, K.; Pierson, S.; Przanowski, P.; Buart, S.; Berchem, G.; Romero, P.; Mami-Chouaib, F.; et al. Blocking Hypoxia-Induced Autophagy in Tumors Restores Cytotoxic T-Cell Activity and Promotes Regression. Cancer Res. 2011, 71, 5976–5986.
- Dong, Z.-Y.; Wu, S.-P.; Liao, R.-Q.; Huang, S.-M.; Wu, Y.-L. Potential Biomarker for Checkpoint Blockade Immunotherapy and Treatment Strategy. Tumour Biol. 2016, 37, 4251–4261.
- Giatromanolaki, A.; Koukourakis, I.M.; Balaska, K.; Mitrakas, A.G.; Harris, A.L.; Koukourakis, M.I. Programmed Death-1 Receptor (PD-1) and PD-Ligand-1 (PD-L1) Expression in Non-Small Cell Lung Cancer and the Immune-Suppressive Effect of Anaerobic Glycolysis. Med. Oncol. 2019, 36, 76.
- Koh, Y.W.; Lee, S.J.; Han, J.-H.; Haam, S.; Jung, J.; Lee, H.W. PD-L1 Protein Expression in Non-Small-Cell Lung Cancer and Its Relationship with the Hypoxia-Related Signaling Pathways: A Study Based on Immunohistochemistry and RNA Sequencing Data. Lung Cancer 2019, 129, 41–47.
- Corrales, L.; Scilla, K.; Caglevic, C.; Miller, K.; Oliveira, J.; Rolfo, C. Immunotherapy in Lung Cancer: A New Age in Cancer Treatment. Adv. Exp. Med. Biol. 2018, 995, 65–95.
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167.
- Gatenby, R.A.; Gillies, R.J. Why Do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899.
- Koukourakis, M.I.; Giatromanolaki, A.; Bougioukas, G.; Sivridis, E. Lung Cancer: A Comparative Study of Metabolism Related Protein Expression in Cancer Cells and Tumor Associated Stroma. Cancer Biol. Ther. 2007, 6, 1476–1479.
- Haider, S.; McIntyre, A.; van Stiphout, R.G.P.M.; Winchester, L.M.; Wigfield, S.; Harris, A.L.; Buffa, F.M. Genomic Alterations Underlie a Pan-Cancer Metabolic Shift Associated with Tumour Hypoxia. Genome Biol. 2016, 17, 140.
- Chang, W.H.; Lai, A.G. Transcriptional Landscape of DNA Repair Genes Underpins a Pan-Cancer Prognostic Signature Associated with Cell Cycle Dysregulation and Tumor Hypoxia. DNA Repair 2019, 78, 142–153.
- Wang, Z.; Wei, Y.; Zhang, R.; Su, L.; Gogarten, S.M.; Liu, G.; Brennan, P.; Field, J.K.; McKay, J.D.; Lissowska, J.; et al. Multi-Omics Analysis Reveals a HIF Network and Hub Gene EPAS1 Associated with Lung Adenocarcinoma. EBioMedicine 2018, 32, 93–101.
- Harris, B.H.L.; Barberis, A.; West, C.M.L.; Buffa, F.M. Gene Expression Signatures as Biomarkers of Tumour Hypoxia. Clin. Oncol. 2015, 27, 547–560.
- Buffa, F.M.; Harris, A.L.; West, C.M.; Miller, C.J. Large Meta-Analysis of Multiple Cancers Reveals a Common, Compact and Highly Prognostic Hypoxia Metagene. Br. J. Cancer 2010, 102, 428–435.
- Chen, Y.-L.; Zhang, Y.; Wang, J.; Chen, N.; Fang, W.; Zhong, J.; Liu, Y.; Qin, R.; Yu, X.; Sun, Z.; et al. A 17 Gene Panel for Non-Small-Cell Lung Cancer Prognosis Identified through Integrative Epigenomic-Transcriptomic Analyses of Hypoxia-Induced Epithelial-Mesenchymal Transition. Mol. Oncol. 2019, 13, 1490–1502.
- Ferrara, M.G.; Di Noia, V.; D’Argento, E.; Vita, E.; Damiano, P.; Cannella, A.; Ribelli, M.; Pilotto, S.; Milella, M.; Tortora, G.; et al. Oncogene-Addicted Non-Small-Cell Lung Cancer: Treatment Opportunities and Future Perspectives. Cancers 2020, 12, 1196.
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.-J.; Vujcic, T.; Huang, X.; et al. Molecular Landmarks of Tumor Hypoxia across Cancer Types. Nat. Genet. 2019, 51, 308–318.
- Bhandari, V.; Li, C.H.; Bristow, R.G.; Boutros, P.C. PCAWG Consortium Divergent Mutational Processes Distinguish Hypoxic and Normoxic Tumours. Nat. Commun. 2020, 11, 737.
- Hu, X.; Fang, Y.; Zheng, J.; He, Y.; Zan, X.; Lin, S.; Li, X.; Li, H.; You, C. The Association between HIF-1α Polymorphism and Cancer Risk: A Systematic Review and Meta-Analysis. Tumour Biol. 2014, 35, 903–916.
- Li, Y.; Li, C.; Shi, H.; Lou, L.; Liu, P. The Association between the Rs11549465 Polymorphism in the Hif-1α Gene and Cancer Risk: A Meta-Analysis. Int. J. Clin. Exp. Med. 2015, 8, 1561–1574.
- Jain, L.; Vargo, C.A.; Danesi, R.; Sissung, T.M.; Price, D.K.; Venzon, D.; Venitz, J.; Figg, W.D. The Role of Vascular Endothelial Growth Factor SNPs as Predictive and Prognostic Markers for Major Solid Tumors. Mol. Cancer Ther. 2009, 8, 2496–2508.
- Heist, R.S.; Zhai, R.; Liu, G.; Zhou, W.; Lin, X.; Su, L.; Asomaning, K.; Lynch, T.J.; Wain, J.C.; Christiani, D.C. VEGF Polymorphisms and Survival in Early-Stage Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 856–862.
- Wouters, A.; Boeckx, C.; Vermorken, J.B.; Van den Weyngaert, D.; Peeters, M.; Lardon, F. The Intriguing Interplay between Therapies Targeting the Epidermal Growth Factor Receptor, the Hypoxic Microenvironment and Hypoxia-Inducible Factors. Curr. Pharm. Des. 2013, 19, 907–917.
- Qi, H.; Wang, S.; Wu, J.; Yang, S.; Gray, S.; Ng, C.S.H.; Du, J.; Underwood, M.J.; Li, M.-Y.; Chen, G.G. EGFR-AS1/HIF2A Regulates the Expression of FOXP3 to Impact the Cancer Stemness of Smoking-Related Non-Small Cell Lung Cancer. Ther. Adv. Med. Oncol. 2019, 11.
- Wang, T.; Niki, T.; Goto, A.; Ota, S.; Morikawa, T.; Nakamura, Y.; Ohara, E.; Ishikawa, S.; Aburatani, H.; Nakajima, J.; et al. Hypoxia Increases the Motility of Lung Adenocarcinoma Cell Line A549 via Activation of the Epidermal Growth Factor Receptor Pathway. Cancer Sci. 2007, 98, 506–511.
- Swinson, D.E.B.; O’Byrne, K.J. Interactions between Hypoxia and Epidermal Growth Factor Receptor in Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2006, 7, 250–256.
- Swinson, D.E.B.; Jones, J.L.; Cox, G.; Richardson, D.; Harris, A.L.; O’Byrne, K.J. Hypoxia-Inducible Factor-1 Alpha in Non Small Cell Lung Cancer: Relation to Growth Factor, Protease and Apoptosis Pathways. Int. J. Cancer 2004, 111, 43–50.
- Yuan, X.-H.; Yang, J.; Wang, X.-Y.; Zhang, X.-L.; Qin, T.-T.; Li, K. Association between EGFR/KRAS Mutation and Expression of VEGFA, VEGFR and VEGFR2 in Lung Adenocarcinoma. Oncol. Lett. 2018, 16, 2105–2112.
- Meng, S.; Wang, G.; Lu, Y.; Fan, Z. Functional Cooperation between HIF-1α and c-Jun in Mediating Primary and Acquired Resistance to Gefitinib in NSCLC Cells with Activating Mutation of EGFR. Lung Cancer 2018, 121, 82–90.
- Lu, Y.; Liu, Y.; Oeck, S.; Zhang, G.J.; Schramm, A.; Glazer, P.M. Hypoxia Induces Resistance to EGFR Inhibitors in Lung Cancer Cells via Upregulation of FGFR1 and the MAPK Pathway. Cancer Res. 2020.
- Reiterer, M.; Colaço, R.; Emrouznejad, P.; Jensen, A.; Rundqvist, H.; Johnson, R.S.; Branco, C. Acute and Chronic Hypoxia Differentially Predispose Lungs for Metastases. Sci. Rep. 2019, 9, 10246.
- Bader, S.B.; Dewhirst, M.W.; Hammond, E.M. Cyclic Hypoxia: An Update on Its Characteristics, Methods to Measure It and Biological Implications in Cancer. Cancers 2020, 13, 23.
- Moldogazieva, N.T.; Mokhosoev, I.M.; Terentiev, A.A. Metabolic Heterogeneity of Cancer Cells: An Interplay between HIF-1, GLUTs, and AMPK. Cancers 2020, 12, 862.
- Qian, J.; Rankin, E.B. Hypoxia-Induced Phenotypes That Mediate Tumor Heterogeneity. Adv. Exp. Med. Biol. 2019, 1136, 43–55.
- Castello, A.; Grizzi, F.; Toschi, L.; Rossi, S.; Rahal, D.; Marchesi, F.; Russo, C.; Finocchiaro, G.; Lopci, E. Tumor Heterogeneity, Hypoxia, and Immune Markers in Surgically Resected Non-Small-Cell Lung Cancer. Nucl. Med. Commun. 2018, 39, 636–644.
- Thunnissen, E.; Kerr, K.M.; Herth, F.J.F.; Lantuejoul, S.; Papotti, M.; Rintoul, R.C.; Rossi, G.; Skov, B.G.; Weynand, B.; Bubendorf, L.; et al. The Challenge of NSCLC Diagnosis and Predictive Analysis on Small Samples. Practical Approach of a Working Group. Lung Cancer 2012, 76, 1–18.
- O’Connor, J.P.B.; Robinson, S.P.; Waterton, J.C. Imaging Tumour Hypoxia with Oxygen-Enhanced MRI and BOLD MRI. Br. J. Radiol. 2019, 92, 20180642.
- Salem, A.; Little, R.A.; Latif, A.; Featherstone, A.K.; Babur, M.; Peset, I.; Cheung, S.; Watson, Y.; Tessyman, V.; Mistry, H.; et al. Oxygen-Enhanced MRI Is Feasible, Repeatable, and Detects Radiotherapy-Induced Change in Hypoxia in Xenograft Models and in Patients with Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 3818–3829.
- Challapalli, A.; Carroll, L.; Aboagye, E.O. Molecular Mechanisms of Hypoxia in Cancer. Clin. Transl. Imaging 2017, 5, 225–253.
- Thawani, R.; McLane, M.; Beig, N.; Ghose, S.; Prasanna, P.; Velcheti, V.; Madabhushi, A. Radiomics and Radiogenomics in Lung Cancer: A Review for the Clinician. Lung Cancer 2018, 115, 34–41.
- Seijo, L.M.; Peled, N.; Ajona, D.; Boeri, M.; Field, J.K.; Sozzi, G.; Pio, R.; Zulueta, J.J.; Spira, A.; Massion, P.P.; et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J. Thorac. Oncol. 2019, 14, 343–357.
- Ganeshan, B.; Goh, V.; Mandeville, H.C.; Ng, Q.S.; Hoskin, P.J.; Miles, K.A. Non-Small Cell Lung Cancer: Histopathologic Correlates for Texture Parameters at CT. Radiology 2013, 266, 326–336.
- Mandeville, H.C.; Ng, Q.S.; Daley, F.M.; Barber, P.R.; Pierce, G.; Finch, J.; Burke, M.; Bell, A.; Townsend, E.R.; Kozarski, R.; et al. Operable Non-Small Cell Lung Cancer: Correlation of Volumetric Helical Dynamic Contrast-Enhanced CT Parameters with Immunohistochemical Markers of Tumor Hypoxia. Radiology 2012, 264, 581–589.
- Even, A.J.G.; Reymen, B.; La Fontaine, M.D.; Das, M.; Jochems, A.; Mottaghy, F.M.; Belderbos, J.S.A.; De Ruysscher, D.; Lambin, P.; van Elmpt, W. Predicting Tumor Hypoxia in Non-Small Cell Lung Cancer by Combining CT, FDG PET and Dynamic Contrast-Enhanced CT. Acta Oncol. 2017, 56, 1591–1596.
- Marcu, L.G.; Forster, J.C.; Bezak, E. The Potential Role of Radiomics and Radiogenomics in Patient Stratification by Tumor Hypoxia Status. J. Am. Coll. Radiol. 2019, 16, 1329–1337.
- Lubner, M.G.; Smith, A.D.; Sandrasegaran, K.; Sahani, D.V.; Pickhardt, P.J. CT Texture Analysis: Definitions, Applications, Biologic Correlates, and Challenges. Radiographics 2017, 37, 1483–1503.
- Yip, C.; Blower, P.J.; Goh, V.; Landau, D.B.; Cook, G.J.R. Molecular Imaging of Hypoxia in Non-Small-Cell Lung Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 956–976.
- Kelada, O.J.; Rockwell, S.; Zheng, M.-Q.; Huang, Y.; Liu, Y.; Booth, C.J.; Decker, R.H.; Oelfke, U.; Carson, R.E.; Carlson, D.J. Quantification of Tumor Hypoxic Fractions Using Positron Emission Tomography with [18F]Fluoromisonidazole ([18F]FMISO) Kinetic Analysis and Invasive Oxygen Measurements. Mol. Imaging Biol. 2017, 19, 893–902.
- Xu, Z.; Li, X.-F.; Zou, H.; Sun, X.; Shen, B. 18F-Fluoromisonidazole in Tumor Hypoxia Imaging. Oncotarget 2017, 8, 94969–94979.
- Nehmeh, S.A.; Schwartz, J.; Grkovski, M.; Yeung, I.; Laymon, C.M.; Muzi, M.; Humm, J.L. Inter-Operator Variability in Compartmental Kinetic Analysis of 18F-Fluoromisonidazole Dynamic PET. Clin. Imaging 2018, 49, 121–127.
- Schwartz, J.; Grkovski, M.; Rimner, A.; Schöder, H.; Zanzonico, P.B.; Carlin, S.D.; Staton, K.D.; Humm, J.L.; Nehmeh, S.A. Pharmacokinetic Analysis of Dynamic 18F-Fluoromisonidazole PET Data in Non-Small Cell Lung Cancer. J. Nucl. Med. 2017, 58, 911–919.
- Thureau, S.; Chaumet-Riffaud, P.; Modzelewski, R.; Fernandez, P.; Tessonnier, L.; Vervueren, L.; Cachin, F.; Berriolo-Riedinger, A.; Olivier, P.; Kolesnikov-Gauthier, H.; et al. Interobserver Agreement of Qualitative Analysis and Tumor Delineation of 18F-Fluoromisonidazole and 3’-Deoxy-3’-18F-Fluorothymidine PET Images in Lung Cancer. J. Nucl. Med. 2013, 54, 1543–1550.
- Texte, E.; Gouel, P.; Thureau, S.; Lequesne, J.; Barres, B.; Edet-Sanson, A.; Decazes, P.; Vera, P.; Hapdey, S. Impact of the Bayesian Penalized Likelihood Algorithm (Q.Clear®) in Comparison with the OSEM Reconstruction on Low Contrast PET Hypoxic Images. EJNMMI Phys. 2020, 7, 28.
- Zschaeck, S.; Steinbach, J.; Troost, E.G.C. FMISO as a Biomarker for Clinical Radiation Oncology. Recent Results Cancer Res. 2016, 198, 189–201.
- Zegers, C.M.L.; van Elmpt, W.; Szardenings, K.; Kolb, H.; Waxman, A.; Subramaniam, R.M.; Moon, D.H.; Brunetti, J.C.; Srinivas, S.M.; Lambin, P.; et al. Repeatability of Hypoxia PET Imaging Using [18F]HX4 in Lung and Head and Neck Cancer Patients: A Prospective Multicenter Trial. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1840–1849.
- Zegers, C.M.L.; van Elmpt, W.; Reymen, B.; Even, A.J.G.; Troost, E.G.C.; Ollers, M.C.; Hoebers, F.J.P.; Houben, R.M.A.; Eriksson, J.; Windhorst, A.D.; et al. In Vivo Quantification of Hypoxic and Metabolic Status of NSCLC Tumors Using [18F]HX4 and [18F]FDG-PET/CT Imaging. Clin. Cancer Res. 2014, 20, 6389–6397.
- Zegers, C.M.L.; van Elmpt, W.; Wierts, R.; Reymen, B.; Sharifi, H.; Öllers, M.C.; Hoebers, F.; Troost, E.G.C.; Wanders, R.; van Baardwijk, A.; et al. Hypoxia Imaging with [18F]HX4 PET in NSCLC Patients: Defining Optimal Imaging Parameters. Radiother. Oncol. 2013, 109, 58–64.
- Wack, L.J.; Mönnich, D.; van Elmpt, W.; Zegers, C.M.L.; Troost, E.G.C.; Zips, D.; Thorwarth, D. Comparison of [18F]-FMISO, [18F]-FAZA and [18F]-HX4 for PET Imaging of Hypoxia--a Simulation Study. Acta Oncol. 2015, 54, 1370–1377.
- Peeters, S.G.J.A.; Zegers, C.M.L.; Lieuwes, N.G.; van Elmpt, W.; Eriksson, J.; van Dongen, G.A.M.S.; Dubois, L.; Lambin, P. A Comparative Study of the Hypoxia PET Tracers [18F]HX4, [18F]FAZA, and [18F]FMISO in a Preclinical Tumor Model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 351–359.
- Huang, T.; Civelek, A.C.; Li, J.; Jiang, H.; Ng, C.K.; Postel, G.C.; Shen, B.; Li, X.-F. Tumor Microenvironment-Dependent 18F-FDG, 18F-Fluorothymidine, and 18F-Misonidazole Uptake: A Pilot Study in Mouse Models of Human Non-Small Cell Lung Cancer. J. Nucl. Med. 2012, 53, 1262–1268.
- Yu, B.; Zhu, X.; Liang, Z.; Sun, Y.; Zhao, W.; Chen, K. Clinical Usefulness of 18F-FDG PET/CT for the Detection of Distant Metastases in Patients with Non-Small Cell Lung Cancer at Initial Staging: A Meta-Analysis. Cancer Manag. Res. 2018, 10, 1859–1864.
- Surov, A.; Meyer, H.J.; Wienke, A. Standardized Uptake Values Derived from 18F-FDG PET May Predict Lung Cancer Microvessel Density and Expression of KI 67, VEGF, and HIF-1α but Not Expression of Cyclin D1, PCNA, EGFR, PD L1, and P53. Contrast Media Mol. Imaging 2018, 2018, 9257929.
- Heiden, B.T.; Chen, G.; Hermann, M.; Brown, R.K.J.; Orringer, M.B.; Lin, J.; Chang, A.C.; Carrott, P.W.; Lynch, W.R.; Zhao, L.; et al. 18F-FDG PET Intensity Correlates with a Hypoxic Gene Signature and Other Oncogenic Abnormalities in Operable Non-Small Cell Lung Cancer. PLoS ONE 2018, 13, e0199970.
- Thureau, S.; Modzelewski, R.; Bohn, P.; Hapdey, S.; Gouel, P.; Dubray, B.; Vera, P. Comparison of Hypermetabolic and Hypoxic Volumes Delineated on [18F]FDG and [18F]Fluoromisonidazole PET/CT in Non-Small-Cell Lung Cancer Patients. Mol. Imaging Biol. 2019.
- Sanduleanu, S.; van der Wiel, A.M.A.; Lieverse, R.I.Y.; Marcus, D.; Ibrahim, A.; Primakov, S.; Wu, G.; Theys, J.; Yaromina, A.; Dubois, L.J.; et al. Hypoxia PET Imaging with [18F]-HX4-A Promising Next-Generation Tracer. Cancers 2020, 12, 1322.
- Vera, P.; Bohn, P.; Edet-Sanson, A.; Salles, A.; Hapdey, S.; Gardin, I.; Ménard, J.-F.; Modzelewski, R.; Thiberville, L.; Dubray, B. Simultaneous Positron Emission Tomography (PET) Assessment of Metabolism with 18F-Fluoro-2-Deoxy-d-Glucose (FDG), Proliferation with 18F-Fluoro-Thymidine (FLT), and Hypoxia with 18fluoro-Misonidazole (F-Miso) before and during Radiotherapy in Patients with Non-Small-Cell Lung Cancer (NSCLC): A Pilot Study. Radiother. Oncol. 2011, 98, 109–116.
- Manafi-Farid, R.; Karamzade-Ziarati, N.; Vali, R.; Mottaghy, F.M.; Beheshti, M. 2-[18F]FDG PET/CT Radiomics in Lung Cancer: An Overview of the Technical Aspect and Its Emerging Role in Management of the Disease. Methods 2020.
- van Elmpt, W.; Zegers, C.M.L.; Reymen, B.; Even, A.J.G.; Dingemans, A.-M.C.; Oellers, M.; Wildberger, J.E.; Mottaghy, F.M.; Das, M.; Troost, E.G.C.; et al. Multiparametric Imaging of Patient and Tumour Heterogeneity in Non-Small-Cell Lung Cancer: Quantification of Tumour Hypoxia, Metabolism and Perfusion. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 240–248.
- Carvalho, S.; Troost, E.G.C.; Bons, J.; Menheere, P.; Lambin, P.; Oberije, C. Prognostic Value of Blood-Biomarkers Related to Hypoxia, Inflammation, Immune Response and Tumour Load in Non-Small Cell Lung Cancer-A Survival Model with External Validation. Radiother. Oncol. 2016, 119, 487–494.
- Ostheimer, C.; Bache, M.; Güttler, A.; Kotzsch, M.; Vordermark, D. A Pilot Study on Potential Plasma Hypoxia Markers in the Radiotherapy of Non-Small Cell Lung Cancer. Osteopontin, Carbonic Anhydrase IX and Vascular Endothelial Growth Factor. Strahlenther. Onkol. 2014, 190, 276–282.
- Ostheimer, C.; Gunther, S.; Bache, M.; Vordermark, D.; Multhoff, G. Dynamics of Heat Shock Protein 70 Serum Levels as a Predictor of Clinical Response in Non-Small-Cell Lung Cancer and Correlation with the Hypoxia-Related Marker Osteopontin. Front. Immunol. 2017, 8, 1305.
- Ostheimer, C.; Evers, C.; Bache, M.; Reese, T.; Vordermark, D. Prognostic Implications of the Co-Detection of the Urokinase Plasminogen Activator System and Osteopontin in Patients with Non-Small-Cell Lung Cancer Undergoing Radiotherapy and Correlation with Gross Tumor Volume. Strahlenther. Onkol. 2018, 194, 539–551.
- Ostheimer, C.; Schweyer, F.; Reese, T.; Bache, M.; Vordermark, D. The Relationship between Tumor Volume Changes and Serial Plasma Osteopontin Detection during Radical Radiotherapy of Non-Small-Cell Lung Cancer. Oncol. Lett. 2016, 12, 3449–3456.
- Afsar, C.U.; Uysal, P. HIF-1α Levels in Patients Receiving Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Carcinoma. Rev. Assoc. Med. Bras. 2019, 65, 1295–1299.
- Bremnes, R.M.; Camps, C.; Sirera, R. Angiogenesis in Non-Small Cell Lung Cancer: The Prognostic Impact of Neoangiogenesis and the Cytokines VEGF and BFGF in Tumours and Blood. Lung Cancer 2006, 51, 143–158.
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268.
- Cortese, R.; Almendros, I.; Wang, Y.; Gozal, D. Tumor Circulating DNA Profiling in Xenografted Mice Exposed to Intermittent Hypoxia. Oncotarget 2015, 6, 556–569.
- Kulshreshtha, R.; Ferracin, M.; Wojcik, S.E.; Garzon, R.; Alder, H.; Agosto-Perez, F.J.; Davuluri, R.; Liu, C.-G.; Croce, C.M.; Negrini, M.; et al. A MicroRNA Signature of Hypoxia. Mol. Cell. Biol. 2007, 27, 1859–1867.
- Goodall, G.J.; Wickramasinghe, V.O. RNA in Cancer. Nat. Rev. Cancer 2021, 21, 22–36.
- Papadaki, C.; Monastirioti, A.; Rounis, K.; Makrakis, D.; Kalbakis, K.; Nikolaou, C.; Mavroudis, D.; Agelaki, S. Circulating MicroRNAs Regulating DNA Damage Response and Responsiveness to Cisplatin in the Prognosis of Patients with Non-Small Cell Lung Cancer Treated with First-Line Platinum Chemotherapy. Cancers 2020, 12, 1282.
- Tartarone, A.; Rossi, E.; Lerose, R.; Mambella, G.; Calderone, G.; Zamarchi, R.; Aieta, M. Possible Applications of Circulating Tumor Cells in Patients with Non Small Cell Lung Cancer. Lung Cancer 2017, 107, 59–64.
- Kallergi, G.; Markomanolaki, H.; Giannoukaraki, V.; Papadaki, M.A.; Strati, A.; Lianidou, E.S.; Georgoulias, V.; Mavroudis, D.; Agelaki, S. Hypoxia-Inducible Factor-1alpha and Vascular Endothelial Growth Factor Expression in Circulating Tumor Cells of Breast Cancer Patients. Breast Cancer Res. 2009, 11, R84.
- Manjunath, Y.; Upparahalli, S.V.; Avella, D.M.; Deroche, C.B.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Smith, C.J.; Li, G.; Kaifi, J.T. PD-L1 Expression with Epithelial Mesenchymal Transition of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Non-Small Cell Lung Cancer. Cancers 2019, 11, 806.
- Francart, M.-E.; Lambert, J.; Vanwynsberghe, A.M.; Thompson, E.W.; Bourcy, M.; Polette, M.; Gilles, C. Epithelial-Mesenchymal Plasticity and Circulating Tumor Cells: Travel Companions to Metastases. Dev. Dyn. 2018, 247, 432–450.
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-Invasive Early Detection of Cancer Four Years before Conventional Diagnosis Using a Blood Test. Nat. Commun. 2020, 11, 3475.
- Chang, S.; Yim, S.; Park, H. The Cancer Driver Genes IDH1/2, JARID1C/KDM5C, and UTX/KDM6A: Crosstalk between Histone Demethylation and Hypoxic Reprogramming in Cancer Metabolism. Exp. Mol. Med. 2019, 51, 1–17.
- Zhang, K.; Wang, J.; Yang, L.; Yuan, Y.-C.; Tong, T.R.; Wu, J.; Yun, X.; Bonner, M.; Pangeni, R.; Liu, Z.; et al. Targeting Histone Methyltransferase G9a Inhibits Growth and Wnt Signaling Pathway by Epigenetically Regulating HP1α and APC2 Gene Expression in Non-Small Cell Lung Cancer. Mol. Cancer 2018, 17, 153.
- Xu, X.-H.; Bao, Y.; Wang, X.; Yan, F.; Guo, S.; Ma, Y.; Xu, D.; Jin, L.; Xu, J.; Wang, J. Hypoxic-Stabilized EPAS1 Proteins Transactivate DNMT1 and Cause Promoter Hypermethylation and Transcription Inhibition of EPAS1 in Non-Small Cell Lung Cancer. FASEB J. 2018, fj201700715.
- Li, T.; Mao, C.; Wang, X.; Shi, Y.; Tao, Y. Epigenetic Crosstalk between Hypoxia and Tumor Driven by HIF Regulation. J. Exp. Clin. Cancer Res. 2020, 39, 224.
- Cheng, X.; Yang, Y.; Fan, Z.; Yu, L.; Bai, H.; Zhou, B.; Wu, X.; Xu, H.; Fang, M.; Shen, A.; et al. MKL1 Potentiates Lung Cancer Cell Migration and Invasion by Epigenetically Activating MMP9 Transcription. Oncogene 2015, 34, 5570–5581.
- Oleksiewicz, U.; Liloglou, T.; Tasopoulou, K.-M.; Daskoulidou, N.; Gosney, J.R.; Field, J.K.; Xinarianos, G. COL1A1, PRPF40A, and UCP2 Correlate with Hypoxia Markers in Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1133–1141.
- Xu, X.-L.; Gong, Y.; Zhao, D.-P. Elevated PHD2 Expression Might Serve as a Valuable Biomarker of Poor Prognosis in Lung Adenocarcinoma, but No Lung Squamous Cell Carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8731–8739.
- Li, H.; Tong, L.; Tao, H.; Liu, Z. Genome-Wide Analysis of the Hypoxia-Related DNA Methylation-Driven Genes in Lung Adenocarcinoma Progression. Biosci. Rep. 2020, 40.
- Zhang, R.; Lai, L.; He, J.; Chen, C.; You, D.; Duan, W.; Dong, X.; Zhu, Y.; Lin, L.; Shen, S.; et al. EGLN2 DNA Methylation and Expression Interact with HIF1A to Affect Survival of Early-Stage NSCLC. Epigenetics 2019, 14, 118–129.
- Brahimi-Horn, M.C.; Giuliano, S.; Saland, E.; Lacas-Gervais, S.; Sheiko, T.; Pelletier, J.; Bourget, I.; Bost, F.; Féral, C.; Boulter, E.; et al. Knockout of Vdac1 Activates Hypoxia-Inducible Factor through Reactive Oxygen Species Generation and Induces Tumor Growth by Promoting Metabolic Reprogramming and Inflammation. Cancer Metab. 2015, 3, 8.
- Putra, A.C.; Tanimoto, K.; Arifin, M.; Hiyama, K. Hypoxia-Inducible Factor-1α Polymorphisms Are Associated with Genetic Aberrations in Lung Cancer. Respirology 2011, 16, 796–802.
- Xiao, P.; Chen, J.; Zhou, F.; Lu, C.; Yang, Q.; Tao, G.; Tao, Y.; Chen, J. Methylation of P16 in Exhaled Breath Condensate for Diagnosis of Non-Small Cell Lung Cancer. Lung Cancer 2014, 83, 56–60.
- Campanella, A.; De Summa, S.; Tommasi, S. Exhaled Breath Condensate Biomarkers for Lung Cancer. J. Breath Res. 2019, 13, 044002.
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499.
- Wu, J.-Y.; Huang, T.-W.; Hsieh, Y.-T.; Wang, Y.-F.; Yen, C.-C.; Lee, G.-L.; Yeh, C.-C.; Peng, Y.-J.; Kuo, Y.-Y.; Wen, H.-T.; et al. Cancer-Derived Succinate Promotes Macrophage Polarization and Cancer Metastasis via Succinate Receptor. Mol. Cell 2020, 77, 213–227.e5.
- Navarro, P.; Bueno, M.J.; Zagorac, I.; Mondejar, T.; Sanchez, J.; Mourón, S.; Muñoz, J.; Gómez-López, G.; Jimenez-Renard, V.; Mulero, F.; et al. Targeting Tumor Mitochondrial Metabolism Overcomes Resistance to Antiangiogenics. Cell Rep. 2016, 15, 2705–2718.
- Dang, N.-H.T.; Singla, A.K.; Mackay, E.M.; Jirik, F.R.; Weljie, A.M. Targeted Cancer Therapeutics: Biosynthetic and Energetic Pathways Characterized by Metabolomics and the Interplay with Key Cancer Regulatory Factors. Curr. Pharm. Des. 2014, 20, 2637–2647.
- Pernemalm, M.; De Petris, L.; Branca, R.M.; Forshed, J.; Kanter, L.; Soria, J.-C.; Girard, P.; Validire, P.; Pawitan, Y.; van den Oord, J.; et al. Quantitative Proteomics Profiling of Primary Lung Adenocarcinoma Tumors Reveals Functional Perturbations in Tumor Metabolism. J. Proteome Res. 2013, 12, 3934–3943.
- Ping, W.; Jiang, W.-Y.; Chen, W.-S.; Sun, W.; Fu, X.-N. Expression and Significance of Hypoxia Inducible Factor-1α and Lysyl Oxidase in Non-Small Cell Lung Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 3613–3618.
- Carrillo de Santa Pau, E.; Arias, F.C.; Caso Peláez, E.; Muñoz Molina, G.M.; Sánchez Hernández, I.; Muguruza Trueba, I.; Moreno Balsalobre, R.; Sacristán López, S.; Gómez Pinillos, A.; del Val Toledo Lobo, M. Prognostic Significance of the Expression of Vascular Endothelial Growth Factors A, B, C, and D and Their Receptors R1, R2, and R3 in Patients with Nonsmall Cell Lung Cancer. Cancer 2009, 115, 1701–1712.
- Feng, Y.; Wang, W.; Hu, J.; Ma, J.; Zhang, Y.; Zhang, J. Expression of VEGF-C and VEGF-D as Significant Markers for Assessment of Lymphangiogenesis and Lymph Node Metastasis in Non-Small Cell Lung Cancer. Anat. Rec. 2010, 293, 802–812.
- Saintigny, P.; Kambouchner, M.; Ly, M.; Gomes, N.; Sainte-Catherine, O.; Vassy, R.; Czernichow, S.; Letoumelin, P.; Breau, J.-L.; Bernaudin, J.-F.; et al. Vascular Endothelial Growth Factor-C and Its Receptor VEGFR-3 in Non-Small-Cell Lung Cancer: Concurrent Expression in Cancer Cells from Primary Tumour and Metastatic Lymph Node. Lung Cancer 2007, 58, 205–213.
- Maekawa, S.; Iwasaki, A.; Shirakusa, T.; Enatsu, S.; Kawakami, T.; Kuroki, M.; Kuroki, M. Correlation between Lymph Node Metastasis and the Expression of VEGF-C, VEGF-D and VEGFR-3 in T1 Lung Adenocarcinoma. Anticancer Res. 2007, 27, 3735–3741.
- Takizawa, H.; Kondo, K.; Fujino, H.; Kenzaki, K.; Miyoshi, T.; Sakiyama, S.; Tangoku, A. The Balance of VEGF-C and VEGFR-3 MRNA Is a Predictor of Lymph Node Metastasis in Non-Small Cell Lung Cancer. Br. J. Cancer 2006, 95, 75–79.
- Lee, S.J.; Lee, S.Y.; Jeon, H.-S.; Park, S.H.; Jang, J.S.; Lee, G.Y.; Son, J.W.; Kim, C.H.; Lee, W.K.; Kam, S.; et al. Vascular Endothelial Growth Factor Gene Polymorphisms and Risk of Primary Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 571–575.
- Liu, C.; Zhou, X.; Gao, F.; Qi, Z.; Zhang, Z.; Guo, Y. Correlation of Genetic Polymorphism of Vascular Endothelial Growth Factor Gene with Susceptibility to Lung Cancer. Cancer Gene Ther. 2015, 22, 312–316.
- Zhai, R.; Liu, G.; Zhou, W.; Su, L.; Heist, R.S.; Lynch, T.J.; Wain, J.C.; Asomaning, K.; Lin, X.; Christiani, D.C. Vascular Endothelial Growth Factor Genotypes, Haplotypes, Gender, and the Risk of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2008, 14, 612–617.
- Yang, F.; Qin, Z.; Shao, C.; Liu, W.; Ma, L.; Shu, Y.; Shen, H. Association between VEGF Gene Polymorphisms and the Susceptibility to Lung Cancer: An Updated Meta-Analysis. Biomed. Res. Int. 2018, 2018, 9271215.
- Zhao, H.-L.; Yu, J.-H.; Huang, L.-S.; Li, P.-Z.; Lao, M.; Zhu, B.; Ou, C. Relationship between Vascular Endothelial Growth Factor -2578C > a Gene Polymorphism and Lung Cancer Risk: A Meta-Analysis. BMC Med. Genet. 2020, 21, 17.
- Li, H.-N.; He, T.; Zha, Y.-J.; Du, F.; Liu, J.; Lin, H.-R.; Yang, W.-Z. HIF-1α Rs11549465 C>T Polymorphism Contributes to Increased Cancer Susceptibility: Evidence from 49 Studies. J. Cancer 2019, 10, 5955–5963.
- Yan, Q.; Chen, P.; Wang, S.; Liu, N.; Zhao, P.; Gu, A. Association between HIF-1α C1772T/G1790A Polymorphisms and Cancer Susceptibility: An Updated Systematic Review and Meta-Analysis Based on 40 Case-Control Studies. BMC Cancer 2014, 14, 950.
- Xu, S.; Ying, K. Association between HIF-1α Gene Polymorphisms and Lung Cancer: A Meta-Analysis. Medicine 2020, 99, e20610.
- Burdett, S.; Pignon, J.P.; Tierney, J.; Tribodet, H.; Stewart, L.; Le Pechoux, C.; Aupérin, A.; Le Chevalier, T.; Stephens, R.J.; Arriagada, R.; et al. Adjuvant Chemotherapy for Resected Early-Stage Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2015, CD011430.
- Hui, E.P.; Chan, A.T.C.; Pezzella, F.; Turley, H.; To, K.-F.; Poon, T.C.W.; Zee, B.; Mo, F.; Teo, P.M.L.; Huang, D.P.; et al. Coexpression of Hypoxia-Inducible Factors 1alpha and 2alpha, Carbonic Anhydrase IX, and Vascular Endothelial Growth Factor in Nasopharyngeal Carcinoma and Relationship to Survival. Clin. Cancer Res. 2002, 8, 2595–2604.
- Yohena, T.; Yoshino, I.; Takenaka, T.; Kameyama, T.; Ohba, T.; Kuniyoshi, Y.; Maehara, Y. Upregulation of Hypoxia-Inducible Factor-1alpha MRNA and Its Clinical Significance in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 284–290.
- Honguero Martínez, A.F.; Arnau Obrer, A.; Figueroa Almazán, S.; Martínez Hernández, N.; Guijarro Jorge, R. Prognostic value of the expression of vascular endothelial growth factor A and hypoxia-inducible factor 1alpha in patients undergoing surgery for non-small cell lung cancer. Med. Clin. 2014, 142, 432–437.
- Kim, S.J.; Rabbani, Z.N.; Dewhirst, M.W.; Vujaskovic, Z.; Vollmer, R.T.; Schreiber, E.-G.; Oosterwijk, E.; Kelley, M.J. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in Surgically Resected Non-Small Cell Lung Cancer. Lung Cancer 2005, 49, 325–335.
- Zheng, C.-L.; Qiu, C.; Shen, M.-X.; Qu, X.; Zhang, T.-H.; Zhang, J.-H.; Du, J.-J. Prognostic Impact of Elevation of Vascular Endothelial Growth Factor Family Expression in Patients with Non-Small Cell Lung Cancer: An Updated Meta-Analysis. Asian Pac. J. Cancer Prev. 2015, 16, 1881–1895.
- Liu, C.; Zhou, X.; Zhang, Z.; Guo, Y. Correlation of Gene Polymorphisms of Vascular Endothelial Growth Factor with Grade and Prognosis of Lung Cancer. BMC Med. Genet. 2020, 21, 86.
- Moreno Roig, E.; Yaromina, A.; Houben, R.; Groot, A.J.; Dubois, L.; Vooijs, M. Prognostic Role of Hypoxia-Inducible Factor-2α Tumor Cell Expression in Cancer Patients: A Meta-Analysis. Front. Oncol. 2018, 8, 224.
- Kinoshita, T.; Fujii, H.; Hayashi, Y.; Kamiyama, I.; Ohtsuka, T.; Asamura, H. Prognostic Significance of Hypoxic PET Using (18)F-FAZA and (62)Cu-ATSM in Non-Small-Cell Lung Cancer. Lung Cancer 2016, 91, 56–66.
- Even, A.J.G.; Reymen, B.; La Fontaine, M.D.; Das, M.; Mottaghy, F.M.; Belderbos, J.S.A.; De Ruysscher, D.; Lambin, P.; van Elmpt, W. Clustering of Multi-Parametric Functional Imaging to Identify High-Risk Subvolumes in Non-Small Cell Lung Cancer. Radiother. Oncol. 2017, 125, 379–384.
- Nardone, V.; Tini, P.; Pastina, P.; Botta, C.; Reginelli, A.; Carbone, S.F.; Giannicola, R.; Calabrese, G.; Tebala, C.; Guida, C.; et al. Radiomics Predicts Survival of Patients with Advanced Non-Small Cell Lung Cancer Undergoing PD-1 Blockade Using Nivolumab. Oncol. Lett. 2020, 19, 1559–1566.
- Baldini, E.; Tibaldi, C.; Delli Paoli, C. Chemo-Radiotherapy Integration in Unresectable Locally Advanced Non-Small-Cell Lung Cancer: A Review. Clin. Transl. Oncol. 2020.
- Grass, G.D.; Naghavi, A.O.; Abuodeh, Y.A.; Perez, B.A.; Dilling, T.J. Analysis of Relapse Events After Definitive Chemoradiotherapy in Locally Advanced Non-Small-Cell Lung Cancer Patients. Clin. Lung Cancer 2019, 20, e1–e7.
- Li, L.; Wei, Y.; Huang, Y.; Yu, Q.; Liu, W.; Zhao, S.; Zheng, J.; Lu, H.; Yu, J.; Yuan, S. To Explore a Representative Hypoxic Parameter to Predict the Treatment Response and Prognosis Obtained by [18F]FMISO-PET in Patients with Non-Small Cell Lung Cancer. Mol. Imaging Biol. 2018, 20, 1061–1067.
- Trinkaus, M.E.; Blum, R.; Rischin, D.; Callahan, J.; Bressel, M.; Segard, T.; Roselt, P.; Eu, P.; Binns, D.; MacManus, M.P.; et al. Imaging of Hypoxia with 18F-FAZA PET in Patients with Locally Advanced Non-Small Cell Lung Cancer Treated with Definitive Chemoradiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 475–481.
- Guan, X.; Yin, M.; Wei, Q.; Zhao, H.; Liu, Z.; Wang, L.-E.; Yuan, X.; O’Reilly, M.S.; Komaki, R.; Liao, Z. Genotypes and Haplotypes of the VEGF Gene and Survival in Locally Advanced Non-Small Cell Lung Cancer Patients Treated with Chemoradiotherapy. BMC Cancer 2010, 10, 431.
- Bollineni, V.R.; Koole, M.J.B.; Pruim, J.; Brouwer, C.L.; Wiegman, E.M.; Groen, H.J.M.; Vlasman, R.; Halmos, G.B.; Oosting, S.F.; Langendijk, J.A.; et al. Dynamics of Tumor Hypoxia Assessed by 18F-FAZA PET/CT in Head and Neck and Lung Cancer Patients during Chemoradiation: Possible Implications for Radiotherapy Treatment Planning Strategies. Radiother. Oncol. 2014, 113, 198–203.
- Lavigne, J.; Suissa, A.; Verger, N.; Dos Santos, M.; Benadjaoud, M.; Mille-Hamard, L.; Momken, I.; Soysouvanh, F.; Buard, V.; Guipaud, O.; et al. Lung Stereotactic Arc Therapy in Mice: Development of Radiation Pneumopathy and Influence of HIF-1α Endothelial Deletion. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 279–290.
- Minami-Shimmyo, Y.; Ohe, Y.; Yamamoto, S.; Sumi, M.; Nokihara, H.; Horinouchi, H.; Yamamoto, N.; Sekine, I.; Kubota, K.; Tamura, T. Risk Factors for Treatment-Related Death Associated with Chemotherapy and Thoracic Radiotherapy for Lung Cancer. J. Thorac. Oncol. 2012, 7, 177–182.
- Nieder, C.; Bremnes, R.M. Effects of Smoking Cessation on Hypoxia and Its Potential Impact on Radiation Treatment Effects in Lung Cancer Patients. Strahlenther. Onkol. 2008, 184, 605–609.
- Yin, M.; Liao, Z.; Yuan, X.; Guan, X.; O’Reilly, M.S.; Welsh, J.; Wang, L.-E.; Wei, Q. Polymorphisms of the Vascular Endothelial Growth Factor Gene and Severe Radiation Pneumonitis in Non-Small Cell Lung Cancer Patients Treated with Definitive Radiotherapy. Cancer Sci. 2012, 103, 945–950.
- Rankin, E.B.; Giaccia, A.J. Hypoxic Control of Metastasis. Science 2016, 352, 175–180.
- Najafi, M.; Farhood, B.; Mortezaee, K.; Kharazinejad, E.; Majidpoor, J.; Ahadi, R. Hypoxia in Solid Tumors: A Key Promoter of Cancer Stem Cell (CSC) Resistance. J. Cancer Res. Clin. Oncol. 2020, 146, 19–31.
- Schöning, J.P.; Monteiro, M.; Gu, W. Drug Resistance and Cancer Stem Cells: The Shared but Distinct Roles of Hypoxia-Inducible Factors HIF1α and HIF2α. Clin. Exp. Pharmacol. Physiol. 2017, 44, 153–161.
- Rohwer, N.; Cramer, T. Hypoxia-Mediated Drug Resistance: Novel Insights on the Functional Interaction of HIFs and Cell Death Pathways. Drug Resist. Updat. 2011, 14, 191–201.
- Xia, Y.; Jiang, L.; Zhong, T. The Role of HIF-1α in Chemo-/Radioresistant Tumors. Onco Targets Ther. 2018, 11, 3003–3011.
- Zhao, J.; Du, F.; Luo, Y.; Shen, G.; Zheng, F.; Xu, B. The Emerging Role of Hypoxia-Inducible Factor-2 Involved in Chemo/Radioresistance in Solid Tumors. Cancer Treat. Rev. 2015, 41, 623–633.
- Rankin, E.B.; Nam, J.-M.; Giaccia, A.J. Hypoxia: Signaling the Metastatic Cascade. Trends Cancer 2016, 2, 295–304.
- Johnson, R.W.; Sowder, M.E.; Giaccia, A.J. Hypoxia and Bone Metastatic Disease. Curr. Osteoporos. Rep. 2017, 15, 231–238.
- Pezzuto, A.; Perrone, G.; Orlando, N.; Citarella, F.; Ciccozzi, M.; Scarlata, S.; Tonini, G. A Close Relationship between HIF-1α Expression and Bone Metastases in Advanced NSCLC, a Retrospective Analysis. Oncotarget 2019, 10, 7071–7079.
- Pacheco, J.M.; Camidge, D.R.; Doebele, R.C.; Schenk, E. A Changing of the Guard: Immune Checkpoint Inhibitors With and Without Chemotherapy as First Line Treatment for Metastatic Non-Small Cell Lung Cancer. Front. Oncol. 2019, 9, 195.
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Şenler, F.Ç.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051.
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092.
- Manoochehri Khoshinani, H.; Afshar, S.; Najafi, R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Investig. 2016, 34, 536–545.
- Li, R.; Gu, J.; Heymach, J.V.; Shu, X.; Zhao, L.; Han, B.; Ye, Y.; Roth, J.; Wu, X. Hypoxia Pathway Genetic Variants Predict Survival of Non-Small-Cell Lung Cancer Patients Receiving Platinum-Based Chemotherapy. Carcinogenesis 2017, 38, 419–424.
- Wu, F.; Zhang, J.; Liu, Y.; Zheng, Y.; Hu, N. HIF1α Genetic Variants and Protein Expressions Determine the Response to Platinum Based Chemotherapy and Clinical Outcome in Patients with Advanced NSCLC. Cell. Physiol. Biochem. 2013, 32, 1566–1576.
- Shingyoji, M.; Ando, S.; Nishimura, H.; Nakajima, T.; Ishikawa, A.; Itakura, M.; Iizasa, T.; Kimura, H. VEGF in Patients with Non-Small Cell Lung Cancer during Combination Chemotherapy of Carboplatin and Paclitaxel. Anticancer Res. 2009, 29, 2635–2639.
- Naumnik, W.; Izycki, T.; Swidzińska, E.; Ossolińiska, M.; Chyczewska, E. Serum Levels of VEGF-C, VEGF-D, and SVEGF-R2 in Patients with Lung Cancer during Chemotherapy. Oncol. Res. 2007, 16, 445–451.
- Zeng, L.; Kizaka-Kondoh, S.; Itasaka, S.; Xie, X.; Inoue, M.; Tanimoto, K.; Shibuya, K.; Hiraoka, M. Hypoxia Inducible Factor-1 Influences Sensitivity to Paclitaxel of Human Lung Cancer Cell Lines under Normoxic Conditions. Cancer Sci. 2007, 98, 1394–1401.
- Sowa, T.; Menju, T.; Chen-Yoshikawa, T.F.; Takahashi, K.; Nishikawa, S.; Nakanishi, T.; Shikuma, K.; Motoyama, H.; Hijiya, K.; Aoyama, A.; et al. Hypoxia-Inducible Factor 1 Promotes Chemoresistance of Lung Cancer by Inducing Carbonic Anhydrase IX Expression. Cancer Med. 2017, 6, 288–297.
- Guo, Q.; Lan, F.; Yan, X.; Xiao, Z.; Wu, Y.; Zhang, Q. Hypoxia Exposure Induced Cisplatin Resistance Partially via Activating P53 and Hypoxia Inducible Factor-1α in Non-Small Cell Lung Cancer A549 Cells. Oncol. Lett. 2018, 16, 801–808.
- Deben, C.; Deschoolmeester, V.; De Waele, J.; Jacobs, J.; Van den Bossche, J.; Wouters, A.; Peeters, M.; Rolfo, C.; Smits, E.; Lardon, F.; et al. Hypoxia-Induced Cisplatin Resistance in Non-Small Cell Lung Cancer Cells Is Mediated by HIF-1α and Mutant P53 and Can Be Overcome by Induction of Oxidative Stress. Cancers 2018, 10, 126.
- Zhang, F.; Duan, S.; Tsai, Y.; Keng, P.C.; Chen, Y.; Lee, S.O.; Chen, Y. Cisplatin Treatment Increases Stemness through Upregulation of Hypoxia-Inducible Factors by Interleukin-6 in Non-Small Cell Lung Cancer. Cancer Sci. 2016, 107, 746–754.
- Shi, Y.; Fan, S.; Wu, M.; Zuo, Z.; Li, X.; Jiang, L.; Shen, Q.; Xu, P.; Zeng, L.; Zhou, Y.; et al. YTHDF1 Links Hypoxia Adaptation and Non-Small Cell Lung Cancer Progression. Nat. Commun. 2019, 10, 4892.
- Lu, Y.; Yu, L.-Q.; Zhu, L.; Zhao, N.; Zhou, X.-J.; Lu, X. Expression of HIF-1α and P-Gp in Non-Small Cell Lung Cancer and the Relationship with HPV Infection. Oncol. Lett. 2016, 12, 1455–1459.
- Shen, X.; Zhi, Q.; Wang, Y.; Li, Z.; Zhou, J.; Huang, J. Hypoxia Induces Multidrug Resistance via Enhancement of Epidermal Growth Factor-Like Domain 7 Expression in Non-Small Lung Cancer Cells. Chemotherapy 2017, 62, 172–180.
- Liu, S.; Qin, T.; Jia, Y.; Li, K. PD-L1 Expression Is Associated with VEGFA and LADC Patients’ Survival. Front. Oncol. 2019, 9, 189.
- Cubillos-Zapata, C.; Almendros, I.; Díaz-García, E.; Toledano, V.; Casitas, R.; Galera, R.; López-Collazo, E.; Farre, R.; Gozal, D.; García-Rio, F. Differential Effect of Intermittent Hypoxia and Sleep Fragmentation on PD-1/PD-L1 Upregulation. Sleep 2020, 43.
- Huang, M.-H.; Zhang, X.-B.; Wang, H.-L.; Li, L.-X.; Zeng, Y.-M.; Wang, M.; Zeng, H.-Q. Intermittent Hypoxia Enhances the Tumor Programmed Death Ligand 1 Expression in a Mouse Model of Sleep Apnea. Ann. Transl. Med. 2019, 7, 97.
- Cubillos-Zapata, C.; Balbás-García, C.; Avendaño-Ortiz, J.; Toledano, V.; Torres, M.; Almendros, I.; Casitas, R.; Zamarrón, E.; García-Sánchez, A.; Feliu, J.; et al. Age-Dependent Hypoxia-Induced PD-L1 Upregulation in Patients with Obstructive Sleep Apnoea. Respirology 2019, 24, 684–692.
- Chang, W.H.; Lai, A.G. Aberrations in Notch-Hedgehog Signalling Reveal Cancer Stem Cells Harbouring Conserved Oncogenic Properties Associated with Hypoxia and Immunoevasion. Br. J. Cancer 2019, 121, 666–678.
- Hatfield, S.; Veszeleiova, K.; Steingold, J.; Sethuraman, J.; Sitkovsky, M. Mechanistic Justifications of Systemic Therapeutic Oxygenation of Tumors to Weaken the Hypoxia Inducible Factor 1α-Mediated Immunosuppression. Adv. Exp. Med. Biol. 2019, 1136, 113–121.
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological Mechanisms of the Antitumor Effects of Supplemental Oxygenation. Sci. Transl. Med. 2015, 7, 277ra30.
- Eckert, F.; Zwirner, K.; Boeke, S.; Thorwarth, D.; Zips, D.; Huber, S.M. Rationale for Combining Radiotherapy and Immune Checkpoint Inhibition for Patients with Hypoxic Tumors. Front. Immunol. 2019, 10, 407.
- Dewhirst, M.W.; Mowery, Y.M.; Mitchell, J.B.; Cherukuri, M.K.; Secomb, T.W. Rationale for Hypoxia Assessment and Amelioration for Precision Therapy and Immunotherapy Studies. J. Clin. Investig. 2019, 129, 489–491.
- Carbone, F.; Grossi, F.; Bonaventura, A.; Vecchié, A.; Minetti, S.; Bardi, N.; Elia, E.; Ansaldo, A.M.; Ferrara, D.; Rijavec, E.; et al. Baseline Serum Levels of Osteopontin Predict Clinical Response to Treatment with Nivolumab in Patients with Non-Small Cell Lung Cancer. Clin. Exp. Metastasis 2019, 36, 449–456.
- Stieb, S.; Eleftheriou, A.; Warnock, G.; Guckenberger, M.; Riesterer, O. Longitudinal PET Imaging of Tumor Hypoxia during the Course of Radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2201–2217.
- Eschmann, S.-M.; Paulsen, F.; Reimold, M.; Dittmann, H.; Welz, S.; Reischl, G.; Machulla, H.-J.; Bares, R. Prognostic Impact of Hypoxia Imaging with 18F-Misonidazole PET in Non-Small Cell Lung Cancer and Head and Neck Cancer before Radiotherapy. J. Nucl. Med. 2005, 46, 253–260.
- Yang, Y.; Han, G.; Xu, W. The Diagnostic Value of 99TcM-2-(2-Methyl-5-Nitro-1H-Imidazol-1-Yl) Ethyl Dihydrogen Phosphate Hypoxia Imaging and Its Evaluation Performance for Radiotherapy Efficacy in Non-Small-Cell Lung Cancer. Onco Targets Ther. 2016, 9, 6499–6509.
- Watanabe, S.; Inoue, T.; Okamoto, S.; Magota, K.; Takayanagi, A.; Sakakibara-Konishi, J.; Katoh, N.; Hirata, K.; Manabe, O.; Toyonaga, T.; et al. Combination of FDG-PET and FMISO-PET as a Treatment Strategy for Patients Undergoing Early-Stage NSCLC Stereotactic Radiotherapy. EJNMMI Res. 2019, 9, 104.
- Li, L.; Yu, J.; Xing, L.; Ma, K.; Zhu, H.; Guo, H.; Sun, X.; Li, J.; Yang, G.; Li, W.; et al. Serial Hypoxia Imaging with 99mTc-HL91 SPECT to Predict Radiotherapy Response in Nonsmall Cell Lung Cancer. Am. J. Clin. Oncol. 2006, 29, 628–633.
- Grootjans, W.; de Geus-Oei, L.-F.; Bussink, J. Image-Guided Adaptive Radiotherapy in Patients with Locally Advanced Non-Small Cell Lung Cancer: The Art of PET. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 369–384.
- Bollineni, V.R.; Wiegman, E.M.; Pruim, J.; Groen, H.J.M.; Langendijk, J.A. Hypoxia Imaging Using Positron Emission Tomography in Non-Small Cell Lung Cancer: Implications for Radiotherapy. Cancer Treat. Rev. 2012, 38, 1027–1032.
- Askoxylakis, V.; Dinkel, J.; Eichinger, M.; Stieltjes, B.; Sommer, G.; Strauss, L.G.; Dimitrakopoulou-Strauss, A.; Kopp-Schneider, A.; Haberkorn, U.; Huber, P.E.; et al. Multimodal Hypoxia Imaging and Intensity Modulated Radiation Therapy for Unresectable Non-Small-Cell Lung Cancer: The HIL Trial. Radiat. Oncol. 2012, 7, 157.
- Li, H.; Xu, D.; Han, X.; Ruan, Q.; Zhang, X.; Mi, Y.; Dong, M.; Guo, S.; Lin, Y.; Wang, B.; et al. Dosimetry Study of 18F-FMISO + PET/CT Hypoxia Imaging Guidance on Intensity-Modulated Radiation Therapy for Non-Small Cell Lung Cancer. Clin. Transl. Oncol. 2018, 20, 1329–1336.
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging Hypoxia to Improve Radiotherapy Outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687.
- Vera, P.; Mihailescu, S.-D.; Lequesne, J.; Modzelewski, R.; Bohn, P.; Hapdey, S.; Pépin, L.-F.; Dubray, B.; Chaumet-Riffaud, P.; Decazes, P.; et al. Radiotherapy Boost in Patients with Hypoxic Lesions Identified by 18F-FMISO PET/CT in Non-Small-Cell Lung Carcinoma: Can We Expect a Better Survival Outcome without Toxicity? [RTEP5 Long-Term Follow-Up]. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1448–1456.
- Yang, L.; West, C.M. Hypoxia Gene Expression Signatures as Predictive Biomarkers for Personalising Radiotherapy. Br. J. Radiol. 2019, 92, 20180036.
- Peitzsch, C.; Perrin, R.; Hill, R.P.; Dubrovska, A.; Kurth, I. Hypoxia as a Biomarker for Radioresistant Cancer Stem Cells. Int. J. Radiat. Biol. 2014, 90, 636–652.
- Sørensen, B.S.; Horsman, M.R. Tumor Hypoxia: Impact on Radiation Therapy and Molecular Pathways. Front. Oncol. 2020, 10, 562.
- Moreno Roig, E.; Groot, A.J.; Yaromina, A.; Hendrickx, T.C.; Barbeau, L.M.O.; Giuranno, L.; Dams, G.; Ient, J.; Olivo Pimentel, V.; van Gisbergen, M.W.; et al. HIF-1α and HIF-2α Differently Regulate the Radiation Sensitivity of NSCLC Cells. Cells 2019, 8, 45.
- Harada, H. Hypoxia-Inducible Factor 1-Mediated Characteristic Features of Cancer Cells for Tumor Radioresistance. J. Radiat. Res. 2016, 57 (Suppl. 1), i99–i105.
- Gong, C.; Gu, R.; Jin, H.; Sun, Y.; Li, Z.; Chen, J.; Wu, G. Lysyl Oxidase Mediates Hypoxia-Induced Radioresistance in Non-Small Cell Lung Cancer A549 Cells. Exp. Biol. Med. 2016, 241, 387–395.
- Gu, Q.; He, Y.; Ji, J.; Yao, Y.; Shen, W.; Luo, J.; Zhu, W.; Cao, H.; Geng, Y.; Xu, J.; et al. Hypoxia-Inducible Factor 1α (HIF-1α) and Reactive Oxygen Species (ROS) Mediates Radiation-Induced Invasiveness through the SDF-1α/CXCR4 Pathway in Non-Small Cell Lung Carcinoma Cells. Oncotarget 2015, 6, 10893–10907.
- Schilling, D.; Bayer, C.; Li, W.; Molls, M.; Vaupel, P.; Multhoff, G. Radiosensitization of Normoxic and Hypoxic H1339 Lung Tumor Cells by Heat Shock Protein 90 Inhibition Is Independent of Hypoxia Inducible Factor-1α. PLoS ONE 2012, 7, e31110.
- Kim, W.-Y.; Oh, S.H.; Woo, J.-K.; Hong, W.K.; Lee, H.-Y. Targeting Heat Shock Protein 90 Overrides the Resistance of Lung Cancer Cells by Blocking Radiation-Induced Stabilization of Hypoxia-Inducible Factor-1alpha. Cancer Res. 2009, 69, 1624–1632.
- Devlin, C.; Greco, S.; Martelli, F.; Ivan, M. MiR-210: More than a Silent Player in Hypoxia. IUBMB Life 2011, 63, 94–100.
- Grosso, S.; Doyen, J.; Parks, S.K.; Bertero, T.; Paye, A.; Cardinaud, B.; Gounon, P.; Lacas-Gervais, S.; Noël, A.; Pouysségur, J.; et al. MiR-210 Promotes a Hypoxic Phenotype and Increases Radioresistance in Human Lung Cancer Cell Lines. Cell Death Dis. 2013, 4, e544.
- Saki, M.; Makino, H.; Javvadi, P.; Tomimatsu, N.; Ding, L.-H.; Clark, J.E.; Gavin, E.; Takeda, K.; Andrews, J.; Saha, D.; et al. EGFR Mutations Compromise Hypoxia-Associated Radiation Resistance through Impaired Replication Fork-Associated DNA Damage Repair. Mol. Cancer Res. 2017, 15, 1503–1516.
- Yang, H.; Liang, S.-Q.; Schmid, R.A.; Peng, R.-W. New Horizons in KRAS-Mutant Lung Cancer: Dawn After Darkness. Front. Oncol. 2019, 9, 953.
- Aran, V.; Omerovic, J. Current Approaches in NSCLC Targeting K-RAS and EGFR. Int. J. Mol. Sci. 2019, 20, 5701.
- De la Garza, M.M.; Cumpian, A.M.; Daliri, S.; Castro-Pando, S.; Umer, M.; Gong, L.; Khosravi, N.; Caetano, M.S.; Ramos-Castañeda, M.; Flores, A.G.; et al. COPD-Type Lung Inflammation Promotes K-Ras Mutant Lung Cancer through Epithelial HIF-1α Mediated Tumor Angiogenesis and Proliferation. Oncotarget 2018, 9, 32972–32983.
- Gong, L.; da Silva Caetano, M.; Cumpian, A.M.; Daliri, S.; Garza Flores, A.; Chang, S.H.; Ochoa, C.E.; Evans, C.M.; Yu, Z.; Moghaddam, S.J. Tumor Necrosis Factor Links Chronic Obstructive Pulmonary Disease and K-Ras Mutant Lung Cancer through Induction of an Immunosuppressive pro-Tumor Microenvironment. Oncoimmunology 2016, 5, e1229724.
- Deng, S.; Clowers, M.J.; Velasco, W.V.; Ramos-Castaneda, M.; Moghaddam, S.J. Understanding the Complexity of the Tumor Microenvironment in K-Ras Mutant Lung Cancer: Finding an Alternative Path to Prevention and Treatment. Front. Oncol. 2019, 9, 1556.
- Yoshikawa, Y.; Takano, O.; Kato, I.; Takahashi, Y.; Shima, F.; Kataoka, T. Ras Inhibitors Display an Anti-Metastatic Effect by Downregulation of Lysyl Oxidase through Inhibition of the Ras-PI3K-Akt-HIF-1α Pathway. Cancer Lett. 2017, 410, 82–91.
- Orue, A.; Chavez, V.; Strasberg-Rieber, M.; Rieber, M. Hypoxic Resistance of KRAS Mutant Tumor Cells to 3-Bromopyruvate Is Counteracted by Prima-1 and Reversed by N-Acetylcysteine. BMC Cancer 2016, 16, 902.
- Wu, S.-G.; Shih, J.-Y. Management of Acquired Resistance to EGFR TKI-Targeted Therapy in Advanced Non-Small Cell Lung Cancer. Mol. Cancer 2018, 17, 38.
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2005, 353, 123–132.
- Pore, N.; Jiang, Z.; Gupta, A.; Cerniglia, G.; Kao, G.D.; Maity, A. EGFR Tyrosine Kinase Inhibitors Decrease VEGF Expression by Both Hypoxia-Inducible Factor (HIF)-1-Independent and HIF-1-Dependent Mechanisms. Cancer Res. 2006, 66, 3197–3204.
- An, S.-M.; Lei, H.-M.; Ding, X.-P.; Sun, F.; Zhang, C.; Tang, Y.-B.; Chen, H.-Z.; Shen, Y.; Zhu, L. Interleukin-6 Identified as an Important Factor in Hypoxia- and Aldehyde Dehydrogenase-Based Gefitinib Adaptive Resistance in Non-Small Cell Lung Cancer Cells. Oncol. Lett. 2017, 14, 3445–3454.
- Park, S.; Ha, S.Y.; Cho, H.Y.; Chung, D.H.; Kim, N.R.; Hong, J.; Cho, E.K. Prognostic Implications of Hypoxia-Inducible Factor-1α in Epidermal Growth Factor Receptor-Negative Non-Small Cell Lung Cancer. Lung Cancer 2011, 72, 100–107.
- Franzini, A.; Baty, F.; Macovei, I.I.; Dürr, O.; Droege, C.; Betticher, D.; Grigoriu, B.D.; Klingbiel, D.; Zappa, F.; Brutsche, M.H. Gene Expression Signatures Predictive of Bevacizumab/Erlotinib Therapeutic Benefit in Advanced Nonsquamous Non-Small Cell Lung Cancer Patients (SAKK 19/05 Trial). Clin. Cancer Res. 2015, 21, 5253–5263.
- Minakata, K.; Takahashi, F.; Nara, T.; Hashimoto, M.; Tajima, K.; Murakami, A.; Nurwidya, F.; Yae, S.; Koizumi, F.; Moriyama, H.; et al. Hypoxia Induces Gefitinib Resistance in Non-Small-Cell Lung Cancer with Both Mutant and Wild-Type Epidermal Growth Factor Receptors. Cancer Sci. 2012, 103, 1946–1954.
- Lu, Y.; Liang, K.; Li, X.; Fan, Z. Responses of Cancer Cells with Wild-Type or Tyrosine Kinase Domain-Mutated Epidermal Growth Factor Receptor (EGFR) to EGFR-Targeted Therapy Are Linked to Downregulation of Hypoxia-Inducible Factor-1alpha. Mol. Cancer 2007, 6, 63.
- Jin, Q.; Zhou, J.; Xu, X.; Huang, F.; Xu, W. Hypoxia-Inducible Factor-1 Signaling Pathway Influences the Sensitivity of HCC827 Cells to Gefitinib. Oncol. Lett. 2019, 17, 4034–4043.
- Xu, L.; Nilsson, M.B.; Saintigny, P.; Cascone, T.; Herynk, M.H.; Du, Z.; Nikolinakos, P.G.; Yang, Y.; Prudkin, L.; Liu, D.; et al. Epidermal Growth Factor Receptor Regulates MET Levels and Invasiveness through Hypoxia-Inducible Factor-1a in Non-Small Cell Lung Cancer Cells. Oncogene 2010, 29, 2616–2627.
- Murakami, A.; Takahashi, F.; Nurwidya, F.; Kobayashi, I.; Minakata, K.; Hashimoto, M.; Nara, T.; Kato, M.; Tajima, K.; Shimada, N.; et al. Hypoxia Increases Gefitinib-Resistant Lung Cancer Stem Cells through the Activation of Insulin-like Growth Factor 1 Receptor. PLoS ONE 2014, 9, e86459.
- Lu, Y.; Liu, Y.; Oeck, S.; Glazer, P.M. Hypoxia Promotes Resistance to EGFR Inhibition in NSCLC Cells via the Histone Demethylases, LSD1 and PLU-1. Mol. Cancer Res. 2018, 16, 1458–1469.
- Kani, K.; Garri, C.; Tiemann, K.; Malihi, P.D.; Punj, V.; Nguyen, A.L.; Lee, J.; Hughes, L.D.; Alvarez, R.M.; Wood, D.M.; et al. JUN-Mediated Downregulation of EGFR Signaling Is Associated with Resistance to Gefitinib in EGFR-Mutant NSCLC Cell Lines. Mol. Cancer Ther. 2017, 16, 1645–1657.
- Chen, Z.; Tian, D.; Liao, X.; Zhang, Y.; Xiao, J.; Chen, W.; Liu, Q.; Chen, Y.; Li, D.; Zhu, L.; et al. Apigenin Combined With Gefitinib Blocks Autophagy Flux and Induces Apoptotic Cell Death Through Inhibition of HIF-1α, c-Myc, p-EGFR, and Glucose Metabolism in EGFR L858R+T790M-Mutated H1975 Cells. Front. Pharmacol. 2019, 10, 260.
- Kobayashi, M.; Saito, R.; Miki, Y.; Nanamiya, R.; Inoue, C.; Abe, J.; Sato, I.; Okada, Y.; Sasano, H. The Correlation of P22phox and Chemosensitivity in EGFR-TKI Resistant Lung Adenocarcinoma. Oncotarget 2019, 10, 1119–1131.
- Lee, J.G.; Wu, R. Erlotinib-Cisplatin Combination Inhibits Growth and Angiogenesis through c-MYC and HIF-1α in EGFR-Mutated Lung Cancer in Vitro and in Vivo. Neoplasia 2015, 17, 190–200.
- Saito, H.; Fukuhara, T.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; Yoshimori, K.; et al. Erlotinib plus Bevacizumab versus Erlotinib Alone in Patients with EGFR-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (NEJ026). Lancet Oncol. 2019, 20, 625–635.
- Ninomiya, T.; Ishikawa, N.; Inoue, K.; Kubo, T.; Yasugi, M.; Shibayama, T.; Maeda, T.; Fujitaka, K.; Kodani, M.; Yokoyama, T.; et al. Phase 2 Study of Afatinib Alone or Combined with Bevacizumab in Chemonaive Patients With Advanced Non-Small-Cell Lung Cancer Harboring EGFR Mutations: AfaBev-CS Study Protocol. Clin. Lung Cancer 2019, 20, 134–138.
- Hu, H.; Miao, X.-K.; Li, J.-Y.; Zhang, X.-W.; Xu, J.-J.; Zhang, J.-Y.; Zhou, T.-X.; Hu, M.-N.; Yang, W.-L.; Mou, L.-Y. YC-1 Potentiates the Antitumor Activity of Gefitinib by Inhibiting HIF-1α and Promoting the Endocytic Trafficking and Degradation of EGFR in Gefitinib-Resistant Non-Small-Cell Lung Cancer Cells. Eur. J. Pharmacol. 2020, 874, 172961.
- Li, F.; Mei, H.; Gao, Y.; Xie, X.; Nie, H.; Li, T.; Zhang, H.; Jia, L. Co-Delivery of Oxygen and Erlotinib by Aptamer-Modified Liposomal Complexes to Reverse Hypoxia-Induced Drug Resistance in Lung Cancer. Biomaterials 2017, 145, 56–71.
- Du, X.; Shao, Y.; Qin, H.-F.; Tai, Y.-H.; Gao, H.-J. ALK-Rearrangement in Non-Small-Cell Lung Cancer (NSCLC). Thorac. Cancer 2018, 9, 423–430.
- Martinengo, C.; Poggio, T.; Menotti, M.; Scalzo, M.S.; Mastini, C.; Ambrogio, C.; Pellegrino, E.; Riera, L.; Piva, R.; Ribatti, D.; et al. ALK-Dependent Control of Hypoxia-Inducible Factors Mediates Tumor Growth and Metastasis. Cancer Res. 2014, 74, 6094–6106.
- Sharma, G.G.; Mota, I.; Mologni, L.; Patrucco, E.; Gambacorti-Passerini, C.; Chiarle, R. Tumor Resistance against ALK Targeted Therapy-Where It Comes from and Where It Goes. Cancers 2018, 10, 62.
- Ma, Y.; Yu, C.; Mohamed, E.M.; Shao, H.; Wang, L.; Sundaresan, G.; Zweit, J.; Idowu, M.; Fang, X. A Causal Link from ALK to Hexokinase II Overexpression and Hyperactive Glycolysis in EML4-ALK-Positive Lung Cancer. Oncogene 2016, 35, 6132–6142.
- Kogita, A.; Togashi, Y.; Hayashi, H.; Sogabe, S.; Terashima, M.; De Velasco, M.A.; Sakai, K.; Fujita, Y.; Tomida, S.; Takeyama, Y.; et al. Hypoxia Induces Resistance to ALK Inhibitors in the H3122 Non-Small Cell Lung Cancer Cell Line with an ALK Rearrangement via Epithelial-Mesenchymal Transition. Int. J. Oncol. 2014, 45, 1430–1436.
- Koh, J.; Jang, J.-Y.; Keam, B.; Kim, S.; Kim, M.-Y.; Go, H.; Kim, T.M.; Kim, D.-W.; Kim, C.-W.; Jeon, Y.K.; et al. EML4-ALK Enhances Programmed Cell Death-Ligand 1 Expression in Pulmonary Adenocarcinoma via Hypoxia-Inducible Factor (HIF)-1α and STAT3. Oncoimmunology 2016, 5, e1108514.
- Bielec, B.; Schueffl, H.; Terenzi, A.; Berger, W.; Heffeter, P.; Keppler, B.K.; Kowol, C.R. Development and Biological Investigations of Hypoxia-Sensitive Prodrugs of the Tyrosine Kinase Inhibitor Crizotinib. Bioorg. Chem. 2020, 99, 103778.
- Denko, N.C. Hypoxia, HIF1 and Glucose Metabolism in the Solid Tumour. Nat. Rev. Cancer 2008, 8, 705–713.
- Kim, J.; Tchernyshyov, I.; Semenza, G.L.; Dang, C.V. HIF-1-Mediated Expression of Pyruvate Dehydrogenase Kinase: A Metabolic Switch Required for Cellular Adaptation to Hypoxia. Cell Metab. 2006, 3, 177–185.
- Yang, R.; Guo, C. Discovery of Potent Pyruvate Dehydrogenase Kinase Inhibitors and Evaluation of Their Anti-Lung Cancer Activity under Hypoxia. Medchemcomm 2018, 9, 1843–1849.
- Liu, T.; Yin, H. PDK1 Promotes Tumor Cell Proliferation and Migration by Enhancing the Warburg Effect in Non-Small Cell Lung Cancer. Oncol. Rep. 2017, 37, 193–200.
- Yang, Z.; Tam, K.Y. Anti-Cancer Synergy of Dichloroacetate and EGFR Tyrosine Kinase Inhibitors in NSCLC Cell Lines. Eur. J. Pharmacol. 2016, 789, 458–467.
- Yang, Z.; Hu, X.; Zhang, S.; Zhang, W.; Tam, K.Y. Pharmacological Synergism of 2,2-Dichloroacetophenone and EGFR-TKi to Overcome TKi-Induced Resistance in NSCLC Cells. Eur. J. Pharmacol. 2017, 815, 80–87.
- Romero, Y.; Castillejos-López, M.; Romero-García, S.; Aguayo, A.S.; Herrera, I.; Garcia-Martin, M.O.; Torres-Espíndola, L.M.; Negrete-García, M.C.; Olvera, A.C.; Huerta-Cruz, J.C.; et al. Antitumor Therapy under Hypoxic Microenvironment by the Combination of 2-Methoxyestradiol and Sodium Dichloroacetate on Human Non-Small-Cell Lung Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 3176375.
- Garon, E.B.; Christofk, H.R.; Hosmer, W.; Britten, C.D.; Bahng, A.; Crabtree, M.J.; Hong, C.S.; Kamranpour, N.; Pitts, S.; Kabbinavar, F.; et al. Dichloroacetate Should Be Considered with Platinum-Based Chemotherapy in Hypoxic Tumors rather than as a Single Agent in Advanced Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2014, 140, 443–452.
- Yang, Z.; Zhang, S.-L.; Hu, X.; Tam, K.Y. Inhibition of Pyruvate Dehydrogenase Kinase 1 Enhances the Anti-Cancer Effect of EGFR Tyrosine Kinase Inhibitors in Non-Small Cell Lung Cancer. Eur. J. Pharmacol. 2018, 838, 41–52.
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159.
- Guo, C.-Y.; Zhu, Q.; Tou, F.-F.; Wen, X.-M.; Kuang, Y.-K.; Hu, H. The Prognostic Value of PKM2 and Its Correlation with Tumour Cell PD-L1 in Lung Adenocarcinoma. BMC Cancer 2019, 19, 289.
- Su, Q.; Luo, S.; Tan, Q.; Deng, J.; Zhou, S.; Peng, M.; Tao, T.; Yang, X. The Role of Pyruvate Kinase M2 in Anticancer Therapeutic Treatments. Oncol. Lett. 2019, 18, 5663–5672.
- Mallik, R.; Chowdhury, T.A. Metformin in Cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419.
- Chun, S.G.; Liao, Z.; Jeter, M.D.; Chang, J.Y.; Lin, S.H.; Komaki, R.U.; Guerrero, T.M.; Mayo, R.C.; Korah, B.M.; Koshy, S.M.; et al. Metabolic Responses to Metformin in Inoperable Early-Stage Non-Small Cell Lung Cancer Treated with Stereotactic Radiotherapy: Results of a Randomized Phase II Clinical Trial. Am. J. Clin. Oncol. 2020, 43, 231–235.
- Kubo, T.; Ninomiya, T.; Hotta, K.; Kozuki, T.; Toyooka, S.; Okada, H.; Fujiwara, T.; Udono, H.; Kiura, K. Study Protocol: Phase-Ib Trial of Nivolumab Combined with Metformin for Refractory/Recurrent Solid Tumors. Clin. Lung Cancer 2018, 19, e861–e864.
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical Outcomes in Non-Small-Cell Lung Cancer Patients Receiving Concurrent Metformin and Immune Checkpoint Inhibitors. Lung Cancer Manag. 2019, 8, LMT11.
- Ahmed, I.; Ferro, A.; Cohler, A.; Langenfeld, J.; Surakanti, S.G.; Aisner, J.; Zou, W.; Haffty, B.G.; Jabbour, S.K. Impact of Metformin Use on Survival in Locally-Advanced, Inoperable Non-Small Cell Lung Cancer Treated with Definitive Chemoradiation. J. Thorac. Dis. 2015, 7, 346–355.
- Stang, K.; Alite, F.; Adams, W.; Altoos, B.; Small, C.; Melian, E.; Emami, B.; Harkenrider, M. Impact of Concurrent Coincident Use of Metformin During Lung Stereotactic Body Radiation Therapy. Cureus 2021, 13, e14157.
- Wink, K.C.J.; Belderbos, J.S.A.; Dieleman, E.M.T.; Rossi, M.; Rasch, C.R.N.; Damhuis, R.A.M.; Houben, R.M.A.; Troost, E.G.C. Improved Progression Free Survival for Patients with Diabetes and Locally Advanced Non-Small Cell Lung Cancer (NSCLC) Using Metformin during Concurrent Chemoradiotherapy. Radiother. Oncol. 2016, 118, 453–459.
- Brancher, S.; Støer, N.C.; Weiderpass, E.; Damhuis, R.A.M.; Johannesen, T.B.; Botteri, E.; Strand, T.-E. Metformin Use and Lung Cancer Survival: A Population-Based Study in Norway. Br. J. Cancer 2021, 124, 1018–1025.
- Li, L.; Jiang, L.; Wang, Y.; Zhao, Y.; Zhang, X.-J.; Wu, G.; Zhou, X.; Sun, J.; Bai, J.; Ren, B.; et al. Combination of Metformin and Gefitinib as First-Line Therapy for Nondiabetic Advanced NSCLC Patients with EGFR Mutations: A Randomized, Double-Blind Phase II Trial. Clin. Cancer Res. 2019, 25, 6967–6975.
- Zhang, B.; Xie, Z.; Li, B. The Clinicopathologic Impacts and Prognostic Significance of GLUT1 Expression in Patients with Lung Cancer: A Meta-Analysis. Gene 2019, 689, 76–83.
- Shriwas, P.; Roberts, D.; Li, Y.; Wang, L.; Qian, Y.; Bergmeier, S.; Hines, J.; Adhicary, S.; Nielsen, C.; Chen, X. A Small-Molecule Pan-Class I Glucose Transporter Inhibitor Reduces Cancer Cell Proliferation in Vitro and Tumor Growth in Vivo by Targeting Glucose-Based Metabolism. Cancer Metab. 2021, 9, 14.
- Hutt, D.M.; Roth, D.M.; Vignaud, H.; Cullin, C.; Bouchecareilh, M. The Histone Deacetylase Inhibitor, Vorinostat, Represses Hypoxia Inducible Factor 1 Alpha Expression through Translational Inhibition. PLoS ONE 2014, 9, e106224.
- Zhang, C.; Yang, C.; Feldman, M.J.; Wang, H.; Pang, Y.; Maggio, D.M.; Zhu, D.; Nesvick, C.L.; Dmitriev, P.; Bullova, P.; et al. Vorinostat Suppresses Hypoxia Signaling by Modulating Nuclear Translocation of Hypoxia Inducible Factor 1 Alpha. Oncotarget 2017, 8, 56110–56125.
- Traynor, A.M.; Dubey, S.; Eickhoff, J.C.; Kolesar, J.M.; Schell, K.; Huie, M.S.; Groteluschen, D.L.; Marcotte, S.M.; Hallahan, C.M.; Weeks, H.R.; et al. Vorinostat (NSC# 701852) in Patients with Relapsed Non-Small Cell Lung Cancer: A Wisconsin Oncology Network Phase II Study. J. Thorac. Oncol. 2009, 4, 522–526.
- Krug, L.M.; Kindler, H.L.; Calvert, H.; Manegold, C.; Tsao, A.S.; Fennell, D.; Öhman, R.; Plummer, R.; Eberhardt, W.E.E.; Fukuoka, K.; et al. Vorinostat in Patients with Advanced Malignant Pleural Mesothelioma Who Have Progressed on Previous Chemotherapy (VANTAGE-014): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Oncol. 2015, 16, 447–456.
- Vansteenkiste, J.; Van Cutsem, E.; Dumez, H.; Chen, C.; Ricker, J.L.; Randolph, S.S.; Schöffski, P. Early Phase II Trial of Oral Vorinostat in Relapsed or Refractory Breast, Colorectal, or Non-Small Cell Lung Cancer. Investig. New Drugs 2008, 26, 483–488.
- Ramalingam, S.S.; Maitland, M.L.; Frankel, P.; Argiris, A.E.; Koczywas, M.; Gitlitz, B.; Thomas, S.; Espinoza-Delgado, I.; Vokes, E.E.; Gandara, D.R.; et al. Carboplatin and Paclitaxel in Combination with either Vorinostat or Placebo for First-Line Therapy of Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2010, 28, 56–62.
- Choi, C.Y.H.; Wakelee, H.A.; Neal, J.W.; Pinder-Schenck, M.C.; Yu, H.-H.M.; Chang, S.D.; Adler, J.R.; Modlin, L.A.; Harsh, G.R.; Soltys, S.G. Vorinostat and Concurrent Stereotactic Radiosurgery for Non-Small Cell Lung Cancer Brain Metastases: A Phase 1 Dose Escalation Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 16–21.
- Reguart, N.; Rosell, R.; Cardenal, F.; Cardona, A.F.; Isla, D.; Palmero, R.; Moran, T.; Rolfo, C.; Pallarès, M.C.; Insa, A.; et al. Phase I/II Trial of Vorinostat (SAHA) and Erlotinib for Non-Small Cell Lung Cancer (NSCLC) Patients with Epidermal Growth Factor Receptor (EGFR) Mutations after Erlotinib Progression. Lung Cancer 2014, 84, 161–167.
- Han, J.-Y.; Lee, S.H.; Lee, G.K.; Yun, T.; Lee, Y.J.; Hwang, K.H.; Kim, J.Y.; Kim, H.T. Phase I/II Study of Gefitinib (Iressa®) and Vorinostat (IVORI) in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer. Cancer Chemother. Pharmacol. 2015, 75, 475–483.
- Takeuchi, S.; Hase, T.; Shimizu, S.; Ando, M.; Hata, A.; Murakami, H.; Kawakami, T.; Nagase, K.; Yoshimura, K.; Fujiwara, T.; et al. Phase I Study of Vorinostat with Gefitinib in BIM Deletion Polymorphism/Epidermal Growth Factor Receptor Mutation Double-Positive Lung Cancer. Cancer Sci. 2020, 111, 561–570.
- Gray, J.E.; Saltos, A.; Tanvetyanon, T.; Haura, E.B.; Creelan, B.; Antonia, S.J.; Shafique, M.; Zheng, H.; Dai, W.; Saller, J.J.; et al. Phase I/Ib Study of Pembrolizumab Plus Vorinostat in Advanced/Metastatic Non-Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 6623–6632.
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized Phase II Trial Comparing Nitroglycerin plus Vinorelbine and Cisplatin with Vinorelbine and Cisplatin Alone in Previously Untreated Stage IIIB/IV Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2006, 24, 688–694.
- Dingemans, A.-M.C.; Groen, H.J.M.; Herder, G.J.M.; Stigt, J.A.; Smit, E.F.; Bahce, I.; Burgers, J.A.; van den Borne, B.E.E.M.; Biesma, B.; Vincent, A.; et al. A Randomized Phase II Study Comparing Paclitaxel-Carboplatin-Bevacizumab with or without Nitroglycerin Patches in Patients with Stage IV Nonsquamous Nonsmall-Cell Lung Cancer: NVALT12 (NCT01171170). Ann. Oncol. 2015, 26, 2286–2293.
- Davidson, A.; Veillard, A.-S.; Tognela, A.; Chan, M.M.K.; Hughes, B.G.M.; Boyer, M.; Briscoe, K.; Begbie, S.; Abdi, E.; Crombie, C.; et al. A Phase III Randomized Trial of Adding Topical Nitroglycerin to First-Line Chemotherapy for Advanced Nonsmall-Cell Lung Cancer: The Australasian Lung Cancer Trials Group NITRO Trial. Ann. Oncol. 2015, 26, 2280–2286.
- Reymen, B.J.T.; van Gisbergen, M.W.; Even, A.J.G.; Zegers, C.M.L.; Das, M.; Vegt, E.; Wildberger, J.E.; Mottaghy, F.M.; Yaromina, A.; Dubois, L.J.; et al. Nitroglycerin as a Radiosensitizer in Non-Small Cell Lung Cancer: Results of a Prospective Imaging-Based Phase II Trial. Clin. Transl. Radiat. Oncol. 2020, 21, 49–55.
- Reddy, S.B.; Williamson, S.K. Tirapazamine: A Novel Agent Targeting Hypoxic Tumor Cells. Expert Opin. Investig. Drugs 2009, 18, 77–87.
- von Pawel, J.; von Roemeling, R.; Gatzemeier, U.; Boyer, M.; Elisson, L.O.; Clark, P.; Talbot, D.; Rey, A.; Butler, T.W.; Hirsh, V.; et al. Tirapazamine plus Cisplatin versus Cisplatin in Advanced Non-Small-Cell Lung Cancer: A Report of the International CATAPULT I Study Group. Cisplatin and Tirapazamine in Subjects with Advanced Previously Untreated Non-Small-Cell Lung Tumors. J. Clin. Oncol. 2000, 18, 1351–1359.
- Huang, W.; Zeng, Y.C. A Candidate for Lung Cancer Treatment: Arsenic Trioxide. Clin. Transl. Oncol. 2019, 21, 1115–1126.
- Choy, H.; Nabid, A.; Stea, B.; Scott, C.; Roa, W.; Kleinberg, L.; Ayoub, J.; Smith, C.; Souhami, L.; Hamburg, S.; et al. Phase II Multicenter Study of Induction Chemotherapy Followed by Concurrent Efaproxiral (RSR13) and Thoracic Radiotherapy for Patients with Locally Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2005, 23, 5918–5928.
- Suh, J.H.; Stea, B.; Nabid, A.; Kresl, J.J.; Fortin, A.; Mercier, J.-P.; Senzer, N.; Chang, E.L.; Boyd, A.P.; Cagnoni, P.J.; et al. Phase III Study of Efaproxiral as an Adjunct to Whole-Brain Radiation Therapy for Brain Metastases. J. Clin. Oncol. 2006, 24, 106–114.
- McGowan, D.R.; Skwarski, M.; Bradley, K.M.; Campo, L.; Fenwick, J.D.; Gleeson, F.V.; Green, M.; Horne, A.; Maughan, T.S.; McCole, M.G.; et al. Buparlisib with Thoracic Radiotherapy and Its Effect on Tumour Hypoxia: A Phase I Study in Patients with Advanced Non-Small Cell Lung Carcinoma. Eur. J. Cancer 2019, 113, 87–95.
- Kabakov, A.E.; Yakimova, A.O. Hypoxia-Induced Cancer Cell Responses Driving Radioresistance of Hypoxic Tumors: Approaches to Targeting and Radiosensitizing. Cancers 2021, 13, 1102.
- Tubin, S.; Khan, M.K.; Salerno, G.; Mourad, W.F.; Yan, W.; Jeremic, B. Mono-Institutional Phase 2 Study of Innovative Stereotactic Body RadioTherapy Targeting PArtial Tumor HYpoxic (SBRT-PATHY) Clonogenic Cells in Unresectable Bulky Non-Small Cell Lung Cancer: Profound Non-Targeted Effects by Sparing Peri-Tumoral Immune Microenvironment. Radiat. Oncol. 2019, 14, 212.
- Tubin, S.; Gupta, S.; Grusch, M.; Popper, H.H.; Brcic, L.; Ashdown, M.L.; Khleif, S.N.; Peter-Vörösmarty, B.; Hyden, M.; Negrini, S.; et al. Shifting the Immune-Suppressive to Predominant Immune-Stimulatory Radiation Effects by SBRT-PArtial Tumor Irradiation Targeting HYpoxic Segment (SBRT-PATHY). Cancers 2020, 13, 50.
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017.
- Alshangiti, A.; Chandhoke, G.; Ellis, P.M. Antiangiogenic Therapies in Non-Small-Cell Lung Cancer. Curr. Oncol. 2018, 25, S45–S58.
- Shukla, N.A.; Yan, M.N.; Hanna, N. The Story of Angiogenesis Inhibitors in Non-Small-Cell Lung Cancer: The Past, Present, and Future. Clin. Lung Cancer 2020.
- Qiang, H.; Chang, Q.; Xu, J.; Qian, J.; Zhang, Y.; Lei, Y.; Han, B.; Chu, T. New Advances in Antiangiogenic Combination Therapeutic Strategies for Advanced Non-Small Cell Lung Cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 631–645.
- Cantelmo, A.R.; Dejos, C.; Kocher, F.; Hilbe, W.; Wolf, D.; Pircher, A. Angiogenesis Inhibition in Non-Small Cell Lung Cancer: A Critical Appraisal, Basic Concepts and Updates from American Society for Clinical Oncology 2019. Curr. Opin. Oncol. 2020, 32, 44–53.
- Manegold, C.; Dingemans, A.-M.C.; Gray, J.E.; Nakagawa, K.; Nicolson, M.; Peters, S.; Reck, M.; Wu, Y.-L.; Brustugun, O.T.; Crinò, L.; et al. The Potential of Combined Immunotherapy and Antiangiogenesis for the Synergistic Treatment of Advanced NSCLC. J. Thorac. Oncol. 2017, 12, 194–207.
- Shiraishi, Y.; Kishimoto, J.; Tanaka, K.; Sugawara, S.; Daga, H.; Hirano, K.; Azuma, K.; Hataji, O.; Hayashi, H.; Tachihara, M.; et al. Treatment Rationale and Design for APPLE (WJOG11218L): A Multicenter, Open-Label, Randomized Phase 3 Study of Atezolizumab and Platinum/Pemetrexed With or Without Bevacizumab for Patients with Advanced Nonsquamous Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2020, 21, 472–476.
- Taylor, M.H.; Lee, C.-H.; Makker, V.; Rasco, D.; Dutcus, C.E.; Wu, J.; Stepan, D.E.; Shumaker, R.C.; Motzer, R.J. Phase IB/II Trial of Lenvatinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma, Endometrial Cancer, and Other Selected Advanced Solid Tumors. J. Clin. Oncol. 2020, 38, 1154–1163.
- Nishio, M.; Horai, T.; Horiike, A.; Nokihara, H.; Yamamoto, N.; Takahashi, T.; Murakami, H.; Yamamoto, N.; Koizumi, F.; Nishio, K.; et al. Phase 1 Study of Lenvatinib Combined with Carboplatin and Paclitaxel in Patients with Non-Small-Cell Lung Cancer. Br. J. Cancer 2013, 109, 538–544.




