Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giovanni Ventura | + 2495 word(s) | 2495 | 2021-06-03 05:22:34 | | | |
| 2 | Nora Tang | + 22 word(s) | 2517 | 2021-06-28 04:23:47 | | | | |
| 3 | Nora Tang | + 22 word(s) | 2517 | 2021-06-28 04:24:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ventura, G. Olive Oil Production Wastes. Encyclopedia. Available online: https://encyclopedia.pub/entry/11337 (accessed on 08 February 2026).
Ventura G. Olive Oil Production Wastes. Encyclopedia. Available at: https://encyclopedia.pub/entry/11337. Accessed February 08, 2026.
Ventura, Giovanni. "Olive Oil Production Wastes" Encyclopedia, https://encyclopedia.pub/entry/11337 (accessed February 08, 2026).
Ventura, G. (2021, June 26). Olive Oil Production Wastes. In Encyclopedia. https://encyclopedia.pub/entry/11337
Ventura, Giovanni. "Olive Oil Production Wastes." Encyclopedia. Web. 26 June, 2021.
Copy Citation
The production of high-quality olive oils implies the generation of vast quantities of solid residues and/or wastewaters that may have a great impact on terrestrial and aquatic environments because of their high phytotoxicity. Depending on the techniques used for olive oil production, namely, on the type of horizontal centrifugation (two-phase or three-phase), the process most adopted to separate olive oil from olive paste obtained after malaxation, different by-products are obtained.
LC-ESI-MS
MALDI-MS
bioactive phenolics
olive oil
olive leaves
olive pomace
olive oil mill wastewater
1. Olive Leaves
Olive leaves represent an agricultural waste by-product obtained during the harvesting of olive trees for table olives and olive oil production chains [1][2]. It has been estimated that pruning alone produces annually 25 kg of waste represented by branches and leaves per olive tree [3]. A considerable amount of olive leaves is also discarded during the olive drupes washing process at the beginning of the olive oil production chain (see Figure 1), reaching a value ranging from 8% [4] to 10% [5] of the total weight of olives subjected to milling. In general, olive leaves are not included in the definition of olive mill solid waste [6] but, together with olive stones, they are described as solid residue [7]. The use of solid residue is of great economic and social importance for the Mediterranean area, as it is accumulated in large amounts [7]. Although limited by their pungency, olive leaves are traditionally used in many countries as feed for livestock or simply disposed by burning. Olive leaves represent a potential source of bioactive compounds, as indirectly proved by the use of their extracts in the context of folk medicine for centuries, with the first medicinal application attested in history dating back to the ancient Egyptian civilization [1]. During centuries, the use of olive leaves preparations in traditional medicine has spread in many different countries [8] (see Table 1). Recently, preparations based on olive leaves, in the form of liquid extracts or tablets, have been commercialised as natural supplements against diabetes, high blood pressure, cardiovascular diseases, urinary tract infections, chronic fatigue symptoms and to improve immune system [9]. Olive leaves preparations also find their application in the cosmetic industry for their anti-ageing activity, and due to their antibiotic and antiparasitic properties as supplements for animal health [10]. Moreover, olive leaves extracts may be used as additives to increase food shelf-life, safety and functionality for their antioxidant and antimicrobial features [11][12]. Interestingly, their probiotic properties have been recently found to promote the Lactobacillus casei survival during cold storage of cheese [13]. Their addition to beer [14] and to a Southern Italy traditional cereal-based baked product known as taralli [5] has been found to increase the inherent polyphenols content of these products. It is also worth noting that phenolic compounds and tocopherols, normally found in olive oils, play a protective role against oxidative stress [15] and are able to extend the extra virgin olive oil shelf-life due to their antioxidant properties [16]. Since the use of synthetic antioxidants may lead to health risks, recent papers have featured that olive leaves, having a high antioxidant activity [17][18][19] due to their phenolics, can exhibit strong preventive effects against olive oil oxidation. The addition of olive leaves during oil extraction process, specifically during olive milling, has thus been recently evaluated in detail and found to lead to the enrichment in chlorophyll [20][21], carotenoids, flavonoids, and in the derivatives of oleuropein, which is the main secoiridoid occurring in olive leaves (see Figure 2) [22].
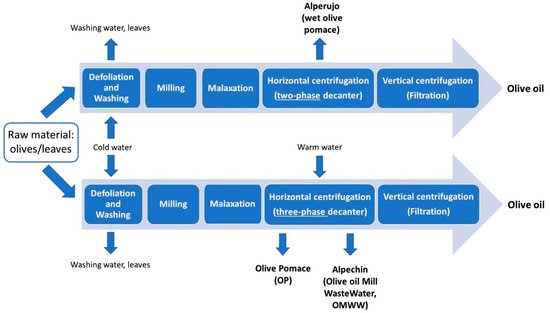
Figure 1. Layout of the main steps of olive oil production involving two- or three-phase horizontal centrifugation and the main by-products obtained.
Table 1. Main uses of olive leaves in traditional medicine occurring worldwide.
| Country | Assumption Form of Olive Leaf-Based Products | Route | Treatments | References |
|---|---|---|---|---|
| Arabic countries | Dried plant fumigation | Nasal | Abortifacient, and treatment of cystitis and sore throat | [23] |
| Brazil | Herbal tea of the fresh leaves | Oral | To induce diuresis, and treatment of hypertension | [24] |
| Canary Islands | An infusion prepared from the fresh or dried leaves | Oral/rectal | Treatment of diabetes; hypertension and haemorrhoids | [25] |
| France | Powdered dried leaves as hard capsules | Oral | To promote urinary and digestive elimination functions | [26] |
| Germany | Extract with ethanol 96% v/v as liquid or coated tablet | Oral | Treatment of atherosclerosis and hypertension | [26] |
| Italy | Infusion of the dried leaf Infusion of the fresh leaf |
Oral | Treatment of hypertension and anti-inflammatory; for wound healing, emollient for ingrown nails and to restore epithelium | [27][28] |
| Morocco | Leaves and essential oil from the leaves | Oral Topical |
Treatment of stomach and intestinal disease and as a mouth cleanser; treatment of constipation and liver pain; tonic for hairs | [29] |
| Trinidad | Hot water extract of the leaf | Oral | To increase milk supply of nursing mother | [30] |
| Ukraine | Hot water extract of dried plant | Oral | Treatment of bronchial asthma | [31] |
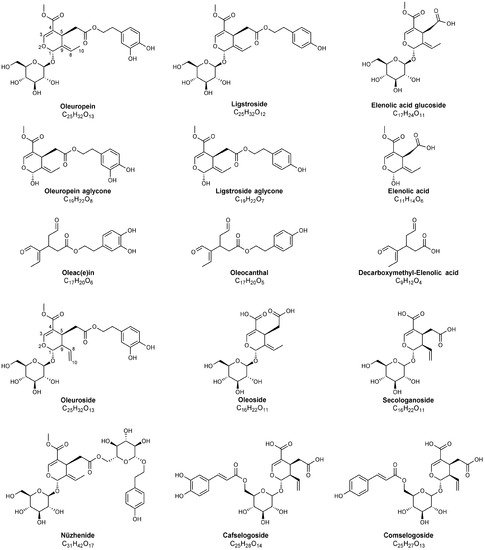
Figure 2. Chemical structures of major secoiridoids identified in olive leaves, pomace and in olive oil mill wastewater using MS techniques. Note that hemiacetalic forms of elenolic acid and of oleuropein and ligstroside aglycones are reported; dialdehydic open-structure isoforms are indicated for oleac(e)in, oleocanthal and decarboxymethyl-elenolic acid. The carbon atoms numbering typically adopted for secoiridoids has been reported for oleuropein and oleuroside to emphasize the positional isomerism occurring for these compounds, related to the position of the exocyclic C=C bond.
2. Olive Pomace and Olive Oil Mill Wastewater
According to Decision 2000/532/EC of the European Union Commission and to the Eurostat database classification [34], waste from agricultural activities (i.e., corn, wheat, fruit, vegetables, rice, pomace, olive wastes) are included in the agricultural livestock waste (ALW) category. Just to give an idea of the amount of ALW in 2016 resulting from olive oil production, 22 and 14 thousands of tons of olive pomace were generated, respectively, in Apulia and Sicily, two of the major olive oil producers in Italy [35]. Such amounts are not surprising, since approximately 800 kg of pomace and 200 kg of wastewater are on average obtained for 200 kg of extra virgin olive oils. To avoid endangering human health and to protect the environment, a rational waste management including prevention, reuse, recycling, recovery, and disposal is crucial also for such by-products [36].
As previously mentioned, the type of horizontal centrifugation adopted during olive oil production can determine the main features of OP, which is humid and semisolid, with a moisture content between 50 and 70%, when two-phase decanters are used, whereas moisture is decreased if three-phase decanters (35–40%) or traditional press mills (20–25%) are employed [37]. The average rough composition of OP has been reported as follows: water (60–70%), lignin (13–15%), cellulose and hemicellulose (18–20%), olive oil retained in the pulp (2.5–3%) and mineral solids (2.5%) [38]. Major organic compounds are sugars (3%), volatile fatty acids (C2–C7) (1%), poly-alcohols (0.2%), proteins (1.5%), polyphenols (0.2%) and pigments (0.5%) [38]. The profile vitamin E occurring in OP includes α-tocopherol, β-tocopherol, α-tocotrienol and γ-tocopherol, being α-tocopherol the major form (>2.6 mg/100 g). The lipid fraction is particularly rich in oleic acid (ca. 75%), followed by palmitic, linoleic, and stearic acids; hydroxytyrosol and comselogoside represent around the 80% of the total phenolics in OP [39].
Since OP consists essentially of olive pulp, olive stone and vegetation water, it includes many of the valuable components of the olive fruit so it can be subjected to biorefining, i.e., to the extraction of valuable compounds and energy. OP is mostly used for the recovery of pomace oil by solid-liquid extraction with hexane, followed by distillation and solvent recycling. The crude oil is then refined and typically blended with a small amount of virgin olive oil. Furthermore, after OP is evaporated and thermally concentrated, it can be applied to cultivated soils as herbicide, insecticide, and compost. Indeed, due to the polysaccharide occurrence, dried OP represents a potential source of gelling pectic material. As an example, Lama-Munoz et al. isolated sugars from alperujo by ethanol/water precipitation, obtaining various oligosaccharide fractions as pectin, neutral and acidic xylo-oligosaccharides, and xyloglucan oligosaccharides with diverse applications as gelling agents, stabilizers and emulsifiers for the food industry [40]. Alperujo compost at different doses was evaluated by Tortosa et al. [41] as an organic manure, mixed with a minimal amount of inorganic fertilizers, for pepper growth in greenhouse cultivations. The authors speculated that the organic matter from alperujo compost positively affects pepper oxidative metabolism by increasing the antioxidative enzymatic activities. Hence, the yield and physiological growth of plants would be improved or rather comparable to standard nutrient solutions. OP is also considered a source of bioethanol, biogas, and methane [42]; residuals of olive stones in OP can be the substrate to obtain activated carbon that can be employed as fuel for the generation of heat and electricity [7]. Moreover, alperujo oil has been used as a non-edible substrate with a high-free fatty acid content for the synthesis of biodiesel through an enzymatic path based on recombinant 1,3-positional specific Rhizopus oryzae lipase, avoiding the generation of glycerol as a co-product [43]. Another fascinating alternative of OP valorization is its use as a substrate for growing microorganisms of biotechnological interest [44]. This strategy offers the opportunity of producing high value-added products, such as enzymes, biopolymers, and pigments with a concomitant reduction of organic wastes. Ghilardi et al. explored the possibility to produce carotenoids by a strain of Rhodotorula mucilaginosa using different media derived from alperujo as a cheap substrate [44]. According to the medium used, it was possible to obtain a mixture of carotenoids enriched in torulene, torularhodin and/or neurosporene, thus showing that alperujo can be used to produce carotenoids exploitable at industrial scale, as additives in pharmaceutical, food and feed products [44].
OP has been applied even in the construction field. De la Casa et al. [45] reported the addition of alperujo to the ceramic paste of bricks, lowering density and thermal conductivity. The OP residue was also used as a substitute for clay in 1.25, 2.5 and 5% (wt) of artificial lightweight aggregate manufactures [46]. The results indicated that the addition of OP in the mixture is beneficial in terms of environmental impact compared to that related to aggregates made with clay.
Besides current uses, including composting, soil amendment, animal feed, OP can be considered a valuable source of bioactive substances with well-recognized benefits for human health and well-being. As aforementioned, it is rich in phenolic compounds and triterpenic acids, for which numerous biological activities, including anti-inflammatory, antitumor, antimicrobial, antioxidant, antidiabetic, and cardio-protective activities, have been reported [47]. Among bioactive phenols, hydroxytyrosol (HT) stands out among compounds with highest added value that can be recovered from the solid by-product, due to its high oxidative stability and antioxidant activity and it is currently used as a therapeutic agent, dietary supplement or natural ingredient in food and feed industries. For instance, various contents (65–195 μg) of HT were added to 100 g of fresh prepared mayonnaise and several quality parameters as free acidity, peroxide value and concentration of conjugated dienes, as well as the content of polyphenols and squalene in the lipid fraction [48], were evaluated [49]. The authors demonstrated that HT improves the mayonnaise hydrolytic stability reducing the formation of oxidation by-products during storage at room temperature and in the dark up to four weeks. Antioxidant-rich extracts from olive mill pomace related to tree different cultivars of Southern Italy (Nocellara, Roggianella and Carolea) were dissolved in a commercial pear juice using inulin as a carrier system of bioactive compounds [50]. In vitro evaluation highlighted outstanding antioxidant features of fortified juice in terms of both antioxidant and scavenging performances, representing an attractive source of functional foods. Very recently, Di Nunzio et al. [51] prepared bakery products (biscuits and breads) using a variety of flours and fermentation protocols also enriched with defatted OP. To assess the effects in a biological system, the digested fractions were supplemented to intestinal cultured enterocytes cells, used as model system, and the secretion of cytokines was measured. OP extracts were also marketed as feed additives, especially fish feed [52], while an olive oil bioactive extract was tested in fish diet, favouring growth of rainbow trout and gilthead sea bream [53]. Other important examples of the use of by-products for functional and food applications have been summarized by Nunes et al. [54] in a more focused review.
As shown in Figure 1, OMWW (also called alpechin) is a secondary product of the olive oil extraction process generated from three-phase decanters. It has a distinctive odour, pH = 4.0–5.5, and high electrical conductivity (6000–16000 mS/cm); it also contains large amounts of suspended solids and high concentrations of polyaromatic compounds [55]. Despite the fact that three-phase centrifugation causes the reduction of natural antioxidants in olive oil and a considerable volume of OMWW, it is currently largely used for oil production, having high working capacity and enabling the automation of industrial plants, leading to a lessening of manual labour and olive-processing costs [56]. The physicochemical features of OMWW largely depends on the oil extraction and processing methods, climate, as well as olives maturity, cultivar and origin [57][58][59]. From the chemical composition point of view, OMWW consists mainly of water (80–92%), and contains 3–15% organic matter (olive oils, carbohydrates, lipids, pectin, organic acids, polysaccharides, phenols, tannins, and lignin) and minerals [60]. Phenolic compounds, sugars, and organic acids are the main components of the OMWW organic fraction, while, among minerals, the potassium ion content is relatively high (see Table 1 in Ref. [61]). Long-chain fatty acids contained in OMWW represent a pollution concern, being toxic to soil micro-organisms and plants [57] it is considered the most polluting waste generated by the agri-food industries [60]. Although it has been spread for many years into soil or nearby streams and rivers, OMWW can be very harmful to soil microflora, plants and freshwater species [62] since it is characterized by high values for key pollution parameters, such as biological oxygen demand (BOD5, 40–95 g/L) and chemical oxygen demand (COD, 50–180 g/L) and high concentrations of phenols and flavonoids (from 0.5 to 24 g/L), having strong antimicrobial and phytotoxic properties [63]. At present, more than 50 different phenolic compounds, in particular hydrophilic species such as phenolic alcohols and acids and, at in a minor amount, secoiridoids, have been identified in OMWW [64][65][66][67]. Due to its inherent features and with the aim of giving value to a waste, different employments of OMWW have been evaluated in the last 30 years.
The effects of spreading OMWW on soil properties and crops was reviewed by Barbera et al. [68]; within the European Union each State has established different limits (for example, in Italy the legal limit is 80 m3/ha) and the authors, by using an holistic approach, concluded that direct application of OMWW exerts a temporary positive effect on soil physical properties, if some outlined restrictions based on soil characteristics are applied. Moreover, they emphasized that polyphenols are the most limiting factor for spreading OMWW on soils because of their antimicrobial and phytotoxic effects [68].
Due to the well-known beneficial effects of phenols contained in OMWW, its potential application for the preparation of functional beverages has been also explored [56]. However, some issues were evidenced, such as the stability of bioactive constituents during preservation procedures, processing, and storage that require appropriate thermal and light-exposure conditions. Moreover, as already discussed, the OMWW composition largely depends on specific factors, which makes a “standardization” of its phenolic extract not straightforward. Olive oils enriched with phenolic compounds extracted from OMWW have been also proposed [69][70][71]; despite further studies are needed, this is an intriguing example of the possibility to create added value by using a waste of olive oil production. As reviewed by Caporaso et al. [71], a similar approach was used for milk and derivatives and also for meat-based products, such as lard and hamburgers.
References
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279.
- Flamminii, F.; Di Mattia, C.D.; Nardella, M.; Chiarini, M.; Valbonetti, L.; Neri, L.; Difonzo, G.; Pittia, P. Structuring alginate beads with different biopolymers for the development of functional ingredients loaded with olive leaves phenolic extract. Food Hydrocoll. 2020, 108, 105849.
- Alcaide, E.M.; Nefzaoui, A. Recycling of Olive Oil By-Products: Possibilities of Utilization in Animal Nutrition. Int. Biodeterior. Biodegrad. 1996, 38, 227–235.
- Hidalgo-Carrillo, J.; Martín-Gómez, J.; Herrera-Beurnio, M.C.; Estévez, R.C.; Urbano, F.J.; Marinas, A. Olive leaves as biotemplates for enhanced solar-light harvesting by a titania-based solid. Nanomaterials 2020, 10, 1057.
- Cedola, A.; Palermo, C.; Centonze, D.; Del Nobile, M.A.; Conte, A. Characterization and bio-accessibility evaluation of olive leaf extract-enriched “Taralli”. Foods 2020, 9, 1268.
- Inglezakis, V.J.; Moreno, J.L.; Doula, M.K. Olive oil waste management EU legislation: Current situation and policy recommendations. Int. J. Chem. Environ. Eng. Syst. 2012, 3, 65–77.
- Guinda, Á. Use of solid residue from the olive industry. Grasas y Aceites 2006, 57, 107–115.
- Kamran, M. Olive (Olea Europaea L.) Leaf Biophenols as Nutraceuticals; Charles Sturt University: Bathurst, Australia, 2016.
- Ben Salem, M.; Affes, H.; Ksouda, K.; Sahnoun, Z.; Zeghal, K.M.; Hammami, S. Pharmacological Activities of Olea europaea Leaves. J. Food Process. Preserv. 2015, 39, 3128–3136.
- Ranalli, A.; Contento, S.; Lucera, L.; Di Febo, M.; Marchegiani, D.; Di Fonzo, V. Factors affecting the contents of iridoid oleuropein in olive leaves (Olea europaea L.). J. Agric. Food Chem. 2006, 54, 434–440.
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70.
- Liu, Y.; McKeever, L.C.; Suo, Y.; Jin, T.Z.; Malik, N.S.A. Antimicrobial Activities of Olive Leaf Extract and Its Potential Use in Food Industry. ACS Symp. Ser. 2018, 1287, 119–132.
- Noori, N.; Rajabian, M.; Nasrabadi, H.G.; Soofiani, M.R.A. Effect of Olea europaea Leaf Extract as A Prebiotic on Survival of Lactobacillus casei in UF Cheese During Cold Storage. J. Veterenary Res. 2020, 75, FA38–FA46.
- Guglielmotti, M.; Passaghe, P.; Buiatti, S. Use of olive (Olea europaea L.) leaves as beer ingredient, and their influence on beer chemical composition and antioxidant activity. J. Food Sci. 2020, 85, 2278–2285.
- Gambacorta, G.; Faccia, M.; Previtali, M.A.; Pati, S.; Notte, E.L.; Baiano, A. Effects of olive maturation and stoning on quality indices and antioxidant content of extra virgin oils (cv. coratina) during storage. J. Food Sci. 2010, 75, C229–C235.
- Cinquanta, L.; Esti, M.; La Notte, E. Evolution of phenolic compounds in virgin olive oil during storage. J. Am. Oil Chem. Soc. 1997, 74, 1259–1264.
- Malheiro, R.; Casal, S.; Teixeira, H.; Bento, A.; Pereira, J.A. Effect of Olive Leaves Addition during the Extraction Process of Overmature Fruits on Olive Oil Quality. Food Bioprocess. Technol. 2013, 6, 509–521.
- Kiritsakis, K.; Kontominas, M.G.; Kontogiorgis, C.; Hadjipavlou-Litina, D.; Moustakas, A.; Kiritsakis, A. Composition and antioxidant activity of olive leaf extracts from Greek olive cultivars. J. Am. Oil Chem. Soc. 2010, 87, 369–376.
- Paiva-Martins, F.; Correia, R.; Félix, S.; Ferreira, P.; Gordon, M.H. Effects of enrichment of refined olive oil with phenolic compounds from olive leaves. J. Agric. Food Chem. 2007, 55, 4139–4143.
- Calvano, C.D.; Ventura, G.; Cataldi, T.R.I.; Palmisano, F. Improvement of chlorophyll identification in foodstuffs by MALDI ToF/ToF mass spectrometry using 1,5-diaminonaphthalene electron transfer secondary reaction matrix. Anal. Bioanal. Chem. 2015, 407, 6369–6379.
- Calvano, C.D.; Ventura, G.; Trotta, M.; Bianco, G.; Cataldi, T.R.I.; Palmisano, F. Electron-Transfer Secondary Reaction Matrices for MALDI MS Analysis of Bacteriochlorophyll a in Rhodobacter sphaeroides and Its Zinc and Copper Analogue Pigments. J. Am. Soc. Mass Spectrom. 2017, 28, 125–135.
- Tarchoune, I.; Sgherri, C.; Eddouzi, J.; Zinnai, A.; Quartacci, M.F.; Zarrouk, M. Olive leaf addition increases olive oil nutraceutical properties. Molecules 2019, 24, 545.
- Ross, I.A. Olea europaea. In Medicinal Plants of the World; Humana Press: Totowa, NJ, USA, 2007; Volume 3, pp. 373–400.
- Ribeiro, R.D.A.; De Melo, M.R.; De Barros, F.; Gomes, C.; Trolin, G. Acute antihypertensive effect in conscious rats produced by some medicinal plants used in the state of São Paulo. J. Ethnopharmacol. 1986, 15, 261–269.
- Darias, V.; Bravo, L.; Barquin, E.; Herrera, D.M.; Fraile, C. Contribution to the ethnopharmacological study of the Canary Islands. J. Ethnopharmacol. 1986, 15, 169–193.
- Reynolds, J.E.F. Martindale: The Extra Pharmacopoeia, 31st ed.; Royal Pharmaceutical Society of Great Britain, Ed.; Royal Pharmaceutical Society: London, UK, 1996.
- De Feo, V.; Senatore, F. Medicinal plants and phytotherapy in the Amalfitan Coast, Salerno Province, Campania, Southern Italy. J. Ethnopharmacol. 1993, 39, 39–51.
- Pieroni, A.; Heimler, D.; Pieters, L.; Van Poel, B.; Vlietinck, A.J. In vitro anti-complementary activity of flavonoids from olive (Olea europaea L.) leaves. Pharmazie 1996, 51, 765–768.
- Bellakhdar, J.; Claisse, R.; Fleurentin, J.; Younos, C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. J. Ethnopharmacol. 1991, 35, 123–143.
- Simpson, G.E. Folk Medicine in Trinidad. J. Am. Folk. 1962, 75, 326.
- Vardanian, S.A. Phytotherapy of bronchial asthma in medieval Armenian medicine. Ter. Arkhiv 1978, 50, 133–136.
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857.
- Bastante, C.C.; Cardoso, L.C.; Fernández-Ponce, M.T.; Serrano, C.M.; de la Ossa, E.J.M. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338.
- European Environment Agency. Diverting waste from landfill: Effectiveness of waste management policies in the European Union. EEA Rep. 2009, 1–68.
- Istituto Superiore per la protezione e la Ricerca Ambientale (ISPRA). Rapporto Rifiuti Speciali Edizione 2017. Available online: (accessed on 18 April 2021).
- Demichelis, F.; Piovano, F.; Fiore, S. Biowaste Management in Italy: Challenges and Perspectives. Sustainability 2019, 11, 4213.
- Malapert, A.; Reboul, E.; Loonis, M.; Dangles, O.; Tomao, V. Direct and Rapid Profiling of Biophenols in Olive Pomace by UHPLC-DAD-MS. Food Anal. Methods 2018, 11, 1001–1010.
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200.
- Nunes, M.A.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236.
- Lama-Muñoz, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Fernández-Bolaños, J. Production, characterization and isolation of neutral and pectic oligosaccharides with low molecular weights from olive by-products thermally treated. Food Hydrocoll. 2012, 28, 92–104.
- Tortosa, G.; González-Gordo, S.; Ruiz, C.; Bedmar, E.J.; Palma, J.M. “Alperujo” compost improves the ascorbate (Vitamin C) content in pepper (Capsicum annuum L.) fruits and influences their oxidative metabolism. Agronomy 2018, 8, 82.
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432.
- Bonet-Ragel, K.; Canet, A.; Benaiges, M.D.; Valero, F. Synthesis of biodiesel from high FFA alperujo oil catalysed by immobilised lipase. Fuel 2015, 161, 12–17.
- Ghilardi, C.; Negrete, P.S.; Carelli, A.A.; Borroni, V. Evaluation of olive mill waste as substrate for carotenoid production by Rhodotorula mucilaginosa. Bioresour. Bioprocess. 2020, 7, 1–11.
- de la Casa, J.A.; Lorite, M.; Jiménez, J.; Castro, E. Valorisation of wastewater from two-phase olive oil extraction in fired clay brick production. J. Hazard. Mater. 2009, 169, 271–278.
- Uceda-Rodríguez, M.; López-García, A.B.; Moreno-Maroto, J.M.; Cobo-Ceacero, C.J.; Cotes-Palomino, M.T.; García, C.M. Evaluation of the environmental benefits associated with the addition of olive pomace in the manufacture of lightweight aggregates. Materials 2020, 13, 2351.
- Medina, E.; Romero, C.; Brenes, M. Residual Olive Paste as a Source of Phenolic Compounds and Triterpenic Acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700368.
- Zambonin, C.G.; Calvano, C.D.; D’Accolti, L.; Palmisano, F. Laser desorption/ionization time-of-flight mass spectrometry of squalene in oil samples. Rapid Commun. Mass Spectrom. 2006, 20, 325–327.
- Rigane, G.; Salem, R. Ben Microwave-assisted extraction of hydroxytyrosol from alperujo and its impact on the stability of mayonnaise. J. Indian Chem. Soc. 2020, 97, 67–74.
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2020, 14591.
- Di Nunzio, M.; Picone, G.; Pasini, F.; Chiarello, E.; Caboni, M.F.; Capozzi, F.; Gianotti, A.; Bordoni, A. Olive oil by-product as functional ingredient in bakery products. Influence of processing and evaluation of biological effects. Food Res. Int. 2020, 131, 108940.
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem. 2014, 145, 1097–1105.
- Gisbert, E.; Andree, K.B.; Quintela, J.C.; Calduch-Giner, J.A.; Ipharraguerre, I.R.; Pérez-Sánchez, J. Olive oil bioactive compounds increase body weight, and improve gut health and integrity in gilthead sea bream (Sparus aurata). Br. J. Nutr. 2017, 117, 351–363.
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148.
- La Scalia, G.; Micale, R.; Cannizzaro, L.; Marra, F.P. A sustainable phenolic compound extraction system from olive oil mill wastewater. J. Clean. Prod. 2017, 142, 3782–3788.
- Zbakh, H.; El Abbassi, A. Potential use of olive mill wastewater in the preparation of functional beverages: A review. J. Funct. Foods 2012, 4, 53–65.
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485.
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2009, 82, 25–39.
- Aviani, I.; Raviv, M.; Hadar, Y.; Saadi, I.; Dag, A.; Ben-Gal, A.; Yermiyahu, U.; Zipori, I.; Laor, Y. Effects of harvest date, irrigation level, cultivar type and fruit water content on olive mill wastewater generated by a laboratory scale “Abencor” milling system. Bioresour. Technol. 2012, 107, 87–96.
- Khdair, A.; Abu-Rumman, G. Sustainable Environmental Management and Valorization Options for Olive Mill Byproducts in the Middle East and North Africa (MENA) Region. Processes 2020, 8, 671.
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process. Biochem. 2013, 48, 1532–1552.
- Justino, C.I.L.; Pereira, R.; Freitas, A.C.; Rocha-Santos, T.A.P.; Panteleitchouk, T.S.L.; Duarte, A.C. Olive oil mill wastewaters before and after treatment: A critical review from the ecotoxicological point of view. Ecotoxicology 2012, 21, 615–629.
- Ouzounidou, G.; Zervakis, G.; Gaitis, F. Raw and microbiologically detoxified olive mill waste and their impact on plant growth. Terr. Aquat. Environ. Toxicol. 2010, 4, 21–38.
- Torrecilla, J.S. Phenolic Compounds in Olive Oil Mill Wastewater. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2010; pp. 357–365. ISBN 9780123744203.
- Bonvino, N.P.; Liang, J.; McCord, E.D.; Zafiris, E.; Benetti, N.; Ray, N.B.; Hung, A.; Boskou, D.; Karagiannis, T.C. OliveNetTM: A comprehensive library of compounds from Olea europaea. Database 2018, 2018, 1–9.
- Ventura, G.; Calvano, C.D.; Abbattista, R.; Bianco, M.; De Ceglie, C.; Losito, I.; Palmisano, F.; Cataldi, T.R.I. Characterization of bioactive and nutraceutical compounds occurring in olive oil processing wastes. Rapid Commun. Mass Spectrom. 2019, 33, 1670–1681.
- Obied, H.K.; Allen, M.S.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Investigation of Australian Olive Mill Waste for Recovery of Biophenols. J. Agric. Food Chem. 2005, 53, 9911–9920.
- Barbera, A.C.; Maucieri, C.; Cavallaro, V.; Ioppolo, A.; Spagna, G. Effects of spreading olive mill wastewater on soil properties and crops, a review. Agric. Water Manag. 2013, 119, 43–53.
- Venturi, F.; Sanmartin, C.; Taglieri, I.; Nari, A.; Andrich, G.; Terzuoli, E.; Donnini, S.; Nicolella, C.; Zinnai, A. Development of phenol-enriched olive oil with phenolic compounds extracted from wastewater produced by physical refining. Nutrients 2017, 9, 916.
- Servili, M.; Esposto, S.; Veneziani, G.; Urbani, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Sordini, B.; Montedoro, G.F. Improvement of bioactive phenol content in virgin olive oil with an olive-vegetation water concentrate produced by membrane treatment. Food Chem. 2011, 124, 1308–1315.
- Caporaso, N.; Formisano, D.; Genovese, A. Use of phenolic compounds from olive mill wastewater as valuable ingredients for functional foods. Crit. Rev. Food Sci. Nutr. 2018, 58, 2829–2841.
More
Information
Subjects:
Nutrition & Dietetics; Green & Sustainable Science & Technology; Agricultural Economics & Policy
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.6K
Entry Collection:
Wastewater Treatment
Revisions:
3 times
(View History)
Update Date:
28 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




