2. Analysis on Results
A total of 2032 consecutive corneas of 1019 donors harvested between 2014 and 2016 at the Eye Bank of Rhineland-Palatinate in Mainz, Germany, were included in this study. In 1013 cases, both corneas were donated, while only the right cornea was explanted in five donors and only the left cornea in one donor.
2.1. Donors
Of the donors, 585 were male (mean age 73.8 ± 11.6 years, range 23–100 years) and 431 were female (mean age 75.9 ± 13.3 years, range 17–103 years). The most common cause of death was cardiovascular disease (39.2%), followed by cancer (35.2%) and sepsis (15.9%); 3.6% donors received CPR (Table 1).
Table 1. Causes of death, comparison between donor sexes. Multiple causes of death per donor are possible.
| Cause of Death/CPR |
Male |
Female |
Total |
| Cardiovascular/cerebrovascular disease n (%) (n = 837) |
186 (38.2%) |
142 (40.6%) |
328 (39.2%) |
| Cancer n (%) (n = 836) |
181 (37.2%) |
113 (32.3%) |
294 (35.2%) |
| Sepsis n (%) (n = 848) |
71 (14.4%) |
64 (18.1%) |
135 (15.9%) |
| CPR n (%) (n = 928) |
24 (4.4%) |
9 (2.3%) |
33 (3.6%) |
Of all retrieved corneas, 52.4% had an ECD ≥ 2000 cells/mm2, 20.4% of corneas had an ECD < 1500 mm2, and 18.6% 1500 > ECD < 2000 mm2 (8.6% missing values). Other characteristics of the included eyes are described in Table 2.
Table 2. Comparison of endothelial cell density (ECD) and lens status with respect to donor sex.
| |
Male |
Female |
| Mean ECD ± SD (cells/mm2) |
1944 ± 622 |
2018 ± 581 |
| Minimum (cells/mm2) |
32 |
118 |
| Maximum (cells/mm2) |
3272 |
3142 |
| Phakic eyes |
799 (68.9%) |
473 (55.6%) |
| Pseudophakic eyes |
352 (30.3%) |
369 (43.4%) |
| Aphakic eyes |
9 (0.8%) |
8 (0.9%) |
2.2. Causes of Disqualification
Nine hundred five harvested corneas (44.5%) were not suitable for transplantation, and 46.7% of the male and 41.6% of the female donor corneas were discarded. Of the pseudophakic donor corneas, 58.1% and of the phakic corneas, 36.4% were not suitable for transplantation (Table 3).
Table 3. Suitability for transplantation regarding gender, side, and lens status.
| |
Suitable |
Discarded |
| No (%) (n = 2032) |
1127 (55.5%) |
905 (44.5%) |
| Gender (n = 2028) |
|
|
| Corneas from male donors (%) |
622 (53.3%) |
544 (46.7%) |
| Corneas from female donors (%) |
503 (58.4%) |
359 (41.6%) |
| Side (n = 2032) |
|
|
| Right corneas (%) |
573 (56.3%) |
445 (43.7%) |
| Left corneas (%) |
554 (54.6%) |
460 (45.4%) |
| Lens status (n = 2014) |
|
|
| Phakic (%) |
812 (63.6%) |
464 (36.4%) |
| Pseudophakic (%) |
302 (41.9%) |
419 (58.1%) |
| Aphakic (%) |
7 (41.2%) |
10 (58.8%) |
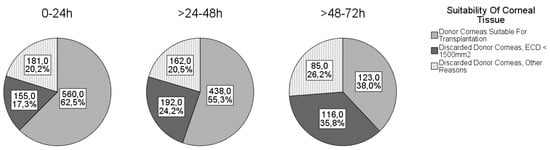
The causes for discarding the cornea are shown in Figure 1. The most common reason for discarding the cornea was ECD < 1500 cells/mm2, followed by positive serology and ECD < 2000 cells/mm2 (and/or overdue corneal culture).
Figure 1. Corneal suitability in different death-to-explantation intervals.
The most common cause of discarding the cornea was low ECD, which decreased proportionally with donor age. The suitability of corneal tissue according to donor age is shown in Table 4. Of note, 45% of corneas from donors older than 80 years and 49% of corneas from donors older than 90 years were suitable for transplantation.
Table 4. ECD in relation to life decade.
| Decade of Life |
ECD ± SD (cells/mm2) |
n |
| <50 years |
2316 ± 47 |
63 |
| 50–59 years |
2233 ± 49 |
166 |
| 60–69 years |
2137 ± 59 |
325 |
| 70–79 years |
1949 ± 58 |
541 |
| 80–89 years |
1846 ± 63 |
613 |
| ≥90 years |
1839 ± 59 |
147 |
The mean DEI was 30.7 ± 16.4 h and ranged from 1 to 72 h. The proportions of corneas suitable for transplantation according to the different DEIs are shown in Figure 1. As expected, the number of discarded tissues increased with increasing DEI.
2.3. Associations
The chi-square test revealed significant differences between lens status (p < 0.001), sex (p = 0.006), and received CPR (p = 0.002) corneal usability (suitable, discarded due to low ECD < 1500 cells/mm2, discarded for other reasons). Phakic lens status, female sex, and CPR positively influenced corneal tissue usability. There were no significant differences in corneal usability with respect to causes of death (cardiovascular no/yes, cancer no/yes or sepsis no/yes).
Pearson correlation analyses showed a significant negative correlation between age and ECD (r = −0.26, p < 0.001) and between DEI and ECD (r = −0.19, p < 0.001).
In multivariable regression analysis, higher ECD was associated with female sex, younger age, phakic lens status, and shorter DEI (Table 5).
Table 5. Association analysis of ECD with ocular and donor characteristics. Linear regression analysis using a mixed model.
| Parameter |
Estimate ECD ± SD (cells/mm2) |
95% CI
(cells/mm2) |
p-Value |
| Intercept |
2919 ± 149 |
(2626; 3212) |
|
| Sex (ref: female) |
|
|
|
| Male |
−189 ± 44 |
(−275; −102) |
<0.001 |
| Age (year) |
−6 ± 2 |
(−10; −2) |
0.001 |
| DEI (hour) |
−7 ± 2 |
(−10; −5) |
<0.001 |
| Lens status (ref: phakic) |
|
|
|
| Aphakic |
−87 ± 156 |
(−393; 218) |
0.574 |
| Pseudophakic |
−378 ± 42 |
(−461; −295) |
<0.001 |
Fixed effects estimation shows that corneas from pseudophakic eyes had −375 cells/mm2, CI = (−460.4; −294.5), p < 0.001) less compared with phakic eyes. For aphakic eyes, the results were not significant (p = 0574) and had a wide confidence interval with positive and negative values (−329.6; 217.8).
Corneas from male donors had a reduced ECD of −188.5 cells/mm2 compared with corneas from female eyes.
Age had a significant effect on ECD. The fixed effects estimate of −6.3 cells/mm2 indicates that corneas lost 6.2 cells/mm2 per year.
DEI had a significant effect on ECD. The fixed effects estimate of −7.3 cells/mm2 indicated that corneas lost 7.3 cells/mm2 per hour that elapses between death and explantation.
The variable donor, as a random effect, was also significant (p < 0.001). The large standard deviation (454 ± 124 cells/mm2) implies that there are serious differences between individuals and that other donor characteristics must also be considered as influencing factors.
The following formula summarizes the final linear mixed model:
Based on this formula, ECD can be anticipated by inserting the parameters and predictors.
In terms of corneal usability, logistic regression showed that older donor, longer DEI, pseudophakia, male sex, and not receiving CPR were associated with impaired corneal usability (Table 6). The accuracy of corneal usability separation using this logistic regression model was assessed with a classification table. The overall percentage correct was 64%, and the logistic regression more accurately predicted the suitability of corneas for transplantation compared with discarded corneas (percentage correct 74% and 51%, respectively).
Table 6. Logistic regression.
| |
Odds Ratio |
95% CI for Odds Ratio |
Regression Coefficient b |
p-Value |
| Age |
0.99 |
(0.98; 0.99) |
−0.01 (−0.02; −0.01) |
p = 0.001 |
| DEI |
0.98 |
(0.97; 0.98) |
−0.02 (−0.03; −0.02) |
p < 0.001 |
| Lens status (pseudophakic) |
0.48 |
(0.39; 0.60) |
−0.73 (−0.96; −0.52) |
p < 0.001 |
| Sex (male) |
0.72 |
(0.59; 0.89) |
−0.32 (−0.52; −0.12) |
p = 0.002 |
| CPR (no) |
0.45 |
(0.25; 0.81) |
−0.80 (−1.56; −0.25) |
p = 0.008 |
3. Current Insights
In this study, we investigated the influence of certain donor characteristics on corneal suitability for transplantation in a large donor cohort from Germany. The results show that older donor age, male sex, longer DEI, and pseudophakic lens status are associated with lower ECD and higher discard rates. Furthermore, the cause of death seems to be irrelevant to the quality of the harvested corneas.
DEI is limited to a maximum of 72 h in Germany
[25]. However, some German eye banks allow DEI up to a maximum of 48 h according to their own regulations. This could be one of the reasons for the discrepancy between the average German discard rate of 33% and 44% at the Eye Bank in Rhineland-Palatinate. Nevertheless, German regulations are very liberal compared with other countries. Although no restrictions regarding DEI are specified in the guidelines and regulations, the actual postmortem times in many European countries and in the USA are significantly lower than the German time frame
[26]. For example, the Cornea Donor Study (USA) included only corneas with a time from death to preservation of less than 12 h (refrigeration/cooling of body/eyes) or less than 8 h (no refrigeration)
[27]. In the UK, guidelines for blood transfusion services recommend limiting DEI to 24 h
[28]. Our study confirms that a longer DEI leads to a lower ECD. Relative to a mean DEI of 30.7 h in our cohort, this reduction is 224 cells/mm
2 after this time, making it a matter of debate whether such ECD loss should be considered clinically significant. With a DEI between 24 and 48 h, 55.3% of the harvested corneas had sufficient quality, and with a DEI between 48 and 72 h, as many as 38.0% were still good enough to be transplanted. Similar to our study, Boehringer et al. and to a lesser extent Linke at al. found a negative linear effect of DEI on ECD after penetrating keratoplasty
[23][29]. Several other studies could not find a relevant correlation between DEI and ECD changes
[16][21][24]. One reason for these results could be the much stricter regulations regarding the allowed DEI. Since the allowed collection time in the Eye Bank of Rhineland-Palatinate is up to 72 h, we were able to detect effects that were not detectable with shorter DEI. To our knowledge, this is the first study to investigate the influence of such long DEI on the quality of donor corneas. We think that the high demand for donor corneas allows corneas to be excised for up to 72 h, even at the risk of the ECD perhaps being lower and the discard rate higher. At least half of the corneas excised after 24 h were still suitable for transplantation. On the other hand, DEI is a factor influenced by conditions such as distance to donor or availability of trained personnel at the time of donor death.
In our study, 35.8% of all corneas were from pseudophakic eyes. In the donor age groups of 80–89 years and 90–99 years, 53% and 69% of the eyes were pseudophakic. The mean ECD of pseudophakic eyes was significantly lower than that of phakic eyes (1687 ± 619 cells/mm
2 vs. 2152 ± 523 cells/mm2). The mean ECD values in this study are lower than those obtained in other studies such as Schaub et al. (phakic 2936 ± 262 cells/mm
2, pseudophakic 2645 ± 200 cells/mm
2), but the donor population differs in terms of the lower proportion of pseudophakic eyes and the higher proportion of female donors compared with our study. Compared with other studies showing a negative influence of pseudophakia on corneal ECD
[13][30], the negative influence determined in our study seems rather strong (378 cells/mm
2 less in pseudophakic eyes), although a direct comparison with other studies is not possible because of different study designs.
Differences in endothelial cell loss may be due to different surgical techniques. The effect of ocular surgical trauma on the cornea is well known
[31], but it is not an exclusion criterion for corneal donation. In our retrospective study design, the ophthalmologic history of the donors was mostly unknown. Our collective consists of a high proportion of eyes that underwent cataract surgery and thus have a lower ECD. The result of our study confirms that lens status is an important factor for corneal quality. It must be considered whether the additional effort to determine the donor’s lens status before corneal harvesting by taking a medical history from the relatives or the treating ophthalmologist is reasonable.
Age is certainly the best studied factor influencing corneal quality. In Germany, there is no age limit for corneal donation. We found that age has a significant negative impact on ECD results. Interestingly, our results show a lower ECD and a wider standard deviation compared with other studies. Gain et al. reported a mean ECD of 2135 cells/mm
2 by donors older than 85 years
[8]. Gavrilov et al. found a mean ECD of 2059 ± 313 cells/mm
2 in donors older than 80 years
[7]. Our study revealed a mean ECD of 1846 ± 627 cells/mm
2 in donors aged between 80 and 89 years (
n = 613) and a mean ECD of 1839 ± 586 cells/mm
2 in donors aged 90 years and older (
n = 147). The donor age-related ECD loss in our study (6.2 cells/mm
2/year) is comparable with the results reported by McGlumphy et al. (5.2 cells/mm
2/year). It needs to be discussed whether an age restriction should be applied to corneal donors because of the negative influence of aging on ECD. In our department, as in many others, corneas from old donors are considered necessary to meet the high demand for grafts. Remarkably, as many as 45% of corneas harvested from donors older than 80 years and 49% of corneas harvested from donors older than 90 years were suitable for transplantation. In addition, there are other positive aspects of older donors that should considered, such as the easier preparation and intraoperative handling of the Descemet endothelial complex for DMEK.
In our study, donor sex was found to have a significant effect on ECD, with corneas from male donors having an average of 189 cells/mm
2 less than those from female donors, as also found in other studies
[15][16]. However, a number of studies found no association between female sex and higher ECD under in vivo conditions
[10][17][18], so the reasons for the difference in ECD between males and females in donor corneas are not yet understood. Other authors found only minor differences in ECD between male and female corneas in living subjects
[15][32].
To the best of our knowledge, the possible influence of CPR on corneal ECD and quality has not been investigated in previous studies. Interestingly, the ECD of corneas from donors who received CPR was significantly higher than that of donors who did not receive CPR. However, this difference could be explained by age because the mean age of all donors who received CPR was 70.5 years, whereas the mean age of all donors who did not receive CPR was 74.7 years. It is conceivable that CPR is more likely to be performed in younger donors with higher ECD than in older, multimorbid patients, some of whom may decline CPR (for example by living will).
In summary, the aim of our analysis was to identify factors that significantly influence the suitability of donor corneas for transplantation. Age, male sex, longer DEI, and pseudophakic lens status negatively influenced ECD and thus graft usability. Cause of death did not appear to be relevant to donor quality. To meet the growing demand for donor tissue, we do not recommend limiting the donor age or collection time to 24 h or excluding oncologic or septic donors, as is done in many countries. We propose a model that can be used to predict the “usability” of the donor based on all known parameters to reduce the number of unnecessary declarations and to economize this process.
This model takes into account the factors that most influence the quality of the corneas harvested, such as donor age, sex, DEI, and lens status.
This model provides an estimate of ECD based on factors that can be determined prior to corneal harvest. However, the donor itself and the general random effect also influence the ECD. Nevertheless, our model predicts a trend for expected ECD. In practice, this may be useful when a qualitative consideration becomes necessary. Occasionally, the situation arises where corneas could be collected from two donors simultaneously, but human or organizational resources only allow corneas to be collected from one donor in the allowed period. In this case, the model could be used to predict corneas with a higher probability of high ECD based on donor-dependent factors.