
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Miguel Burgos | + 1980 word(s) | 1980 | 2021-07-06 11:30:49 | | | |
| 2 | Vicky Zhou | Meta information modification | 1980 | 2021-07-27 03:31:24 | | |
Video Upload Options
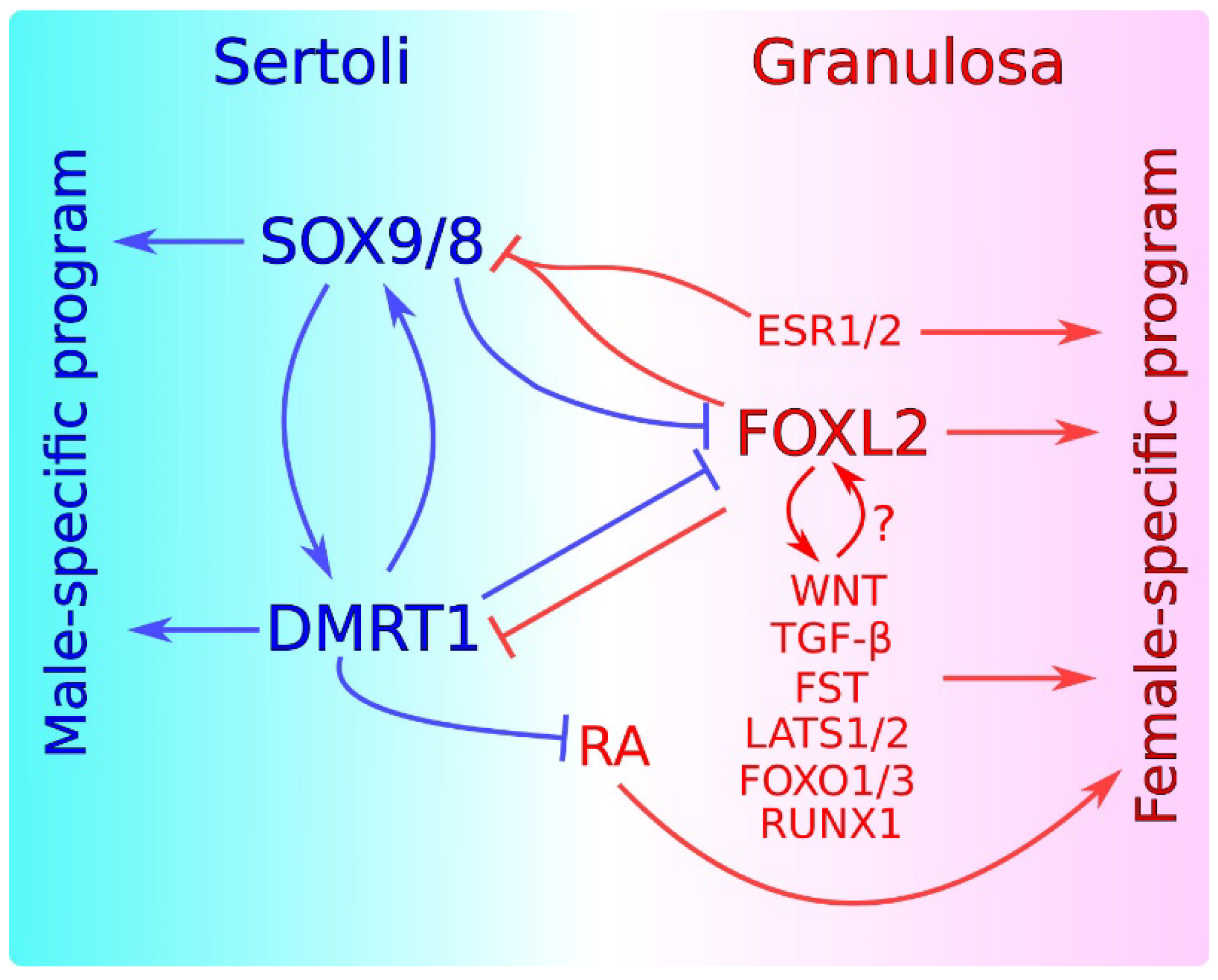
Sex maintenance in mammals is important in sexual differentiation. The crucial event in mammalian sexual differentiation occurs at the embryonic stage of sex determination, when the bipotential gonads differentiate as either testes or ovaries, according to the sex chromosome constitution of the embryo, XY or XX, respectively. Once differentiated, testes produce sexual hormones that induce the subsequent differentiation of the male reproductive tract. On the other hand, the lack of masculinizing hormones in XX embryos permits the formation of the female reproductive tract. It was long assumed that once the gonad is differentiated, this developmental decision is irreversible. However, several findings in the last decade have shown that this is not the case and that a continuous sex maintenance is needed. Deletion of Foxl2 in the adult ovary lead to ovary-to-testis transdifferentiation and deletion of either Dmrt1 or Sox9/Sox8 in the adult testis induces the opposite process. In both cases, mutant gonads were genetically reprogrammed, showing that both the male program in ovaries and the female program in testes must be actively repressed throughout the individual’s life. In addition to these transcription factors, other genes and molecular pathways have also been shown to be involved in this antagonism.
1. Introduction
2. Antagonism between Male and Female Factors in Sexual Cell Fate Maintenance
References
- Engle, E.T. Tubular Adenomas and Testis-Like Tubules of the Ovaries of Aged Rats. Cancer Res. 1946, 6, 578–582.
- Taketo-Hosotani, T.; Merchant-Larios, H.; Thau, R.B.; Koide, S.S. Testicular Cell Differentiation in Fetal Mouse Ovaries Following Transplantation into Adult Male Mice. J. Exp. Zool. 1985, 236, 229–237.
- Prépin, J.; Hida, N. Influence of Age and Medium on Formation of Epithelial Cords in the Rat Fetal Ovary In Vitro. Reproduction 1989, 87, 375–382.
- Whitworth, D.J.; Shaw, G.; Renfree, M.B. Gonadal Sex Reversal of the Developing Marsupial Ovary In Vivo and In Vitro. Development 1996, 122, 4057–4063.
- Guigon, C.J.; Coudouel, N.; Mazaud-Guittot, S.; Forest, M.G.; Magre, S. Follicular Cells Acquire Sertoli Cell Characteristics after Oocyte Loss. Endocrinology 2005, 146, 2992–3004.
- Barrionuevo, F.J.; Zurita, F.; Burgos, M.; Jiménez, R. Testis-like Development of Gonads in Female Moles. New Insights on Mammalian Gonad Organogenesis. Dev. Biol. 2004, 268, 39–52.
- Jost, A.; Perchellet, J.P.; Prepin, J.; Vigier, B. The Prenatal Development of Bovine Freemartins. In Intersexuality in the Animal Kingdom; Reinboth, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; pp. 392–406. ISBN 978-3-642-66069-6.
- Dominguez, M.M.; Liptrap, R.M.; Croy, B.A.; Basrur, P.K. Hormonal Correlates of Ovarian Alterations in Bovine Freemartin Fetuses. Anim. Reprod. Sci. 1990, 22, 181–201.
- Behringer, R.R.; Finegold, M.J.; Cate, R.L. Müllerian-Inhibiting Substance Function during Mammalian Sexual Development. Cell 1994, 79, 415–425.
- Vigier, B.; Watrin, F.; Magre, S.; Tran, D.; Josso, N. Purified Bovine AMH Induces a Characteristic Freemartin Effect in Fetal Rat Prospective Ovaries Exposed to It In Vitro. Development 1987, 100, 43–55.
- Whitworth, D.J. XX Germ Cells: The Difference between an Ovary and a Testis. Trends Endocrinol. Metab. 1998, 9, 2–6.
- Rios-Rojas, C.; Bowles, J.; Koopman, P. On the Role of Germ Cells in Mammalian Gonad Development: Quiet Passengers or Back-Seat Drivers? Reproduction 2015, 149, R181–R191.
- Maatouk, D.M.; Mork, L.; Hinson, A.; Kobayashi, A.; McMahon, A.P.; Capel, B. Germ Cells Are Not Required to Establish the Female Pathway in Mouse Fetal Gonads. PLoS ONE 2012, 7, e47238.
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Eisenberger, T.; Sekido, R.; Kress, J.; Treier, A.-C.; Klugmann, C.; Klasen, C.; Holter, N.I.; et al. Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell 2009, 139, 1130–1142.
- Durmuş, Y.; Kılıç, Ç.; Çakır, C.; Yüksel, D.; Boran, N.; Karalök, A.; Boyraz, G.; Turan, A.T. Sertoli–Leydig Cell Tumor of the Ovary: Analysis of a Single Institution Database and Review of the Literature. J. Obstet. Gynaecol. Res. 2019, 45, 1311–1318.
- Kao, C.-S.; Cornejo, K.M.; Ulbright, T.M.; Young, R.H. Juvenile Granulosa Cell Tumors of the Testis. Am. J. Surg. Pathol. 2015, 39, 1159–1169.
- Cornejo, K.M.; Young, R.H. Adult Granulosa Cell Tumors of the Testis: A Report of 32 Cases. Am. J. Surg. Pathol. 2014, 38, 1242–1250.
- Hiramatsu, R.; Matoba, S.; Kanai-Azuma, M.; Tsunekawa, N.; Katoh-Fukui, Y.; Kurohmaru, M.; Morohashi, K.; Wilhelm, D.; Koopman, P.; Kanai, Y. A Critical Time Window of Sry Action in Gonadal Sex Determination in Mice. Development 2009, 136, 129–138.
- Harikae, K.; Miura, K.; Shinomura, M.; Matoba, S.; Hiramatsu, R.; Tsunekawa, N.; Kanai-Azuma, M.; Kurohmaru, M.; Morohashi, K.; Kanai, Y. Heterogeneity in Sexual Bipotentiality and Plasticity of Granulosa Cells in Developing Mouse Ovaries. J. Cell Sci. 2013, 126, 2834–2844.
- Maatouk, D.M.; Natarajan, A.; Shibata, Y.; Song, L.; Crawford, G.E.; Ohler, U.; Capel, B. Genome-Wide Identification of Regulatory Elements in Sertoli Cells. Development 2017, 144, 720–730.
- Garcia-Moreno, S.A.; Futtner, C.R.; Salamone, I.M.; Gonen, N.; Lovell-Badge, R.; Maatouk, D.M. Gonadal Supporting Cells Acquire Sex-Specific Chromatin Landscapes during Mammalian Sex Determination. Dev. Biol. 2019, 446, 168–179.
- Garcia-Moreno, S.A.; Lin, Y.-T.; Futtner, C.R.; Salamone, I.M.; Capel, B.; Maatouk, D.M. CBX2 Is Required during Male Sex Determination to Repress Female Fate at Bivalent Loci. bioRxiv 2018.
- Barrionuevo, F.J.; Burgos, M.; Scherer, G.; Jiménez, R. Genes Promoting and Disturbing Testis Development. Histol. Histopatol. 2012, 11, 1361–1383.
- Sekido, R.; Lovell-Badge, R. Genetic Control of Testis Development. Sex. Dev. 2013, 7, 21–32.
- Svingen, T.; Koopman, P. Building the Mammalian Testis: Origins, Differentiation, and Assembly of the Component Cell Populations. Genes Dev. 2013, 27, 2409–2426.
- Ewen, K.A.; Koopman, P. Mouse Germ Cell Development: From Specification to Sex Determination. Mol. Cell. Endocrinol. 2010, 323, 76–93.
- Lin, Y.-T.; Capel, B. Cell Fate Commitment during Mammalian Sex Determination. Curr. Opin. Genet. Dev. 2015, 32, 144–152.
- Nef, S.; Stévant, I.; Greenfield, A. Chapter Six—Characterizing the bipotential mammalian gonad. In Current Topics in Developmental Biology; Capel, B., Ed.; Sex Determination in Vertebrates; Academic Press: Cambridge, MA, USA, 2019; Volume 134, pp. 167–194.
- Vining, B.; Ming, Z.; Bagheri-Fam, S.; Harley, V. Diverse Regulation but Conserved Function: SOX9 in Vertebrate Sex Determination. Genes 2021, 12, 486.
- Georg, I.; Barrionuevo, F.; Wiech, T.; Scherer, G. Sox9 and Sox8 Are Required for Basal Lamina Integrity of Testis Cords and for Suppression of FOXL2 during Embryonic Testis Development in Mice1. Biol. Reprod. 2012, 87, 1–11.
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 Prevents Female Reprogramming in the Postnatal Mammalian Testis. Nature 2011, 476, 101–104.
- Minkina, A.; Matson, C.K.; Lindeman, R.E.; Ghyselinck, N.B.; Bardwell, V.J.; Zarkower, D. DMRT1 Protects Male Gonadal Cells from Retinoid-Dependent Sexual Transdifferentiation. Dev. Cell 2014, 29, 511–520.
- Lindeman, R.E.; Murphy, M.W.; Agrimson, K.S.; Gewiss, R.L.; Bardwell, V.J.; Gearhart, M.D.; Zarkower, D. The Conserved Sex Regulator DMRT1 Recruits SOX9 in Sexual Cell Fate Reprogramming. Nucleic Acids Res. 2021, 49, 6144–6164.
- Ottolenghi, C.; Pelosi, E.; Tran, J.; Colombino, M.; Douglass, E.; Nedorezov, T.; Cao, A.; Forabosco, A.; Schlessinger, D. Loss of Wnt4 and Foxl2 Leads to Female-to-Male Sex Reversal Extending to Germ Cells. Hum. Mol. Genet. 2007, 16, 2795–2804.
- Pannetier, M.; Chassot, A.-A.; Chaboissier, M.-C.; Pailhoux, E. Involvement of FOXL2 and RSPO1 in Ovarian Determination, Development, and Maintenance in Mammals. Sex. Dev. 2016, 10, 167–184.
- Georges, A.; L’Hôte, D.; Todeschini, A.L.; Auguste, A.; Legois, B.; Zider, A.; Veitia, R.A. The Transcription Factor FOXL2 Mobilizes Estrogen Signaling to Maintain the Identity of Ovarian Granulosa Cells. eLife 2014, 3, e04207.
- Blount, A.L.; Schmidt, K.; Justice, N.J.; Vale, W.W.; Fischer, W.H.; Bilezikjian, L.M. FoxL2 and Smad3 Coordinately Regulate Follistatin Gene Transcription*. J. Biol. Chem. 2009, 284, 7631–7645.
- Kashimada, K.; Pelosi, E.; Chen, H.; Schlessinger, D.; Wilhelm, D.; Koopman, P. FOXL2 and BMP2 Act Cooperatively to Regulate Follistatin Gene Expression during Ovarian Development. Endocrinology 2011, 152, 272–280.
- Pisarska, M.D.; Kuo, F.-T.; Bentsi-Barnes, I.K.; Khan, S.; Barlow, G.M. LATS1 Phosphorylates Forkhead L2 and Regulates Its Transcriptional Activity. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E101–E109.
- Nicol, B.; Grimm, S.A.; Chalmel, F.; Lecluze, E.; Pannetier, M.; Pailhoux, E.; Dupin-De-Beyssat, E.; Guiguen, Y.; Capel, B.; Yao, H.H.-C. RUNX1 Maintains the Identity of the Fetal Ovary through an Interplay with FOXL2. Nat. Commun. 2019, 10, 5116.
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E.; et al. Mutation of FOXL2 in Granulosa-Cell Tumors of the Ovary. N. Engl. J. Med. 2009, 360, 2719–2729.
- Fang, X.; Ni, N.; Gao, Y.; Vincent, D.F.; Bartholin, L.; Li, Q. A Novel Mouse Model of Testicular Granulosa Cell Tumors. Mol. Hum. Reprod. 2018, 24, 343–356.
- Färkkilä, A.; Haltia, U.-M.; Tapper, J.; McConechy, M.K.; Huntsman, D.G.; Heikinheimo, M. Pathogenesis and Treatment of Adult-Type Granulosa Cell Tumor of the Ovary. Ann. Med. 2017, 49, 435–447.
- Onder, S.; Hurdogan, O.; Bayram, A.; Yilmaz, I.; Sozen, H.; Yavuz, E. The Role of FOXL2, SOX9, and β-Catenin Expression and DICER1 Mutation in Differentiating Sex Cord Tumor with Annular Tubules from Other Sex Cord Tumors of the Ovary. Virchows Arch. 2021.




