
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristina Ferrari | + 2630 word(s) | 2630 | 2021-04-26 10:23:43 | | | |
| 2 | Lily Guo | Meta information modification | 2630 | 2021-05-24 04:28:45 | | |
Video Upload Options
Immunotherapy is a promising therapeutic strategy both for solid and hematologic tumors, such as in Hodgkin (HL) and non-Hodgkin lymphoma (NHL). In particular, immune-checkpoint inhibitors, such as nivolumab and pembrolizumab, are increasingly used for the treatment of refractory/relapsed HL. At the same time, evidence of chimeric antigen receptor (CAR)-T-cell immunotherapy efficacy mostly in NHL is growing. In this setting, the challenge is to identify an appropriate imaging method to evaluate immunotherapy response. The role of 18F-Fluorodeoxyglucose (18F-FDG) positron-emission tomography/computed tomography (PET/CT), especially in early evaluation, is under investigation in order to guide therapeutic strategies, taking into account the possible atypical responses (hyperprogression and pseudoprogression) and immune-related adverse events that could appear on PET images.
1. Introduction
2. Evidence Based Medicine of Early 18F-FDG PET/CT during Immunotherapy
2.1. Hodgkin Lymphoma
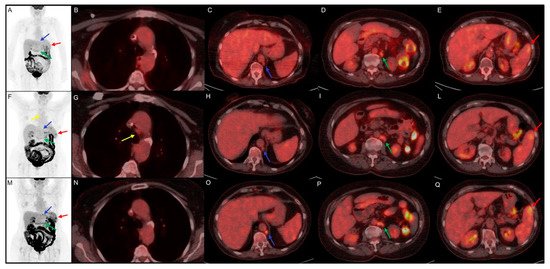
2.2. Non Hodgkin Lymphoma
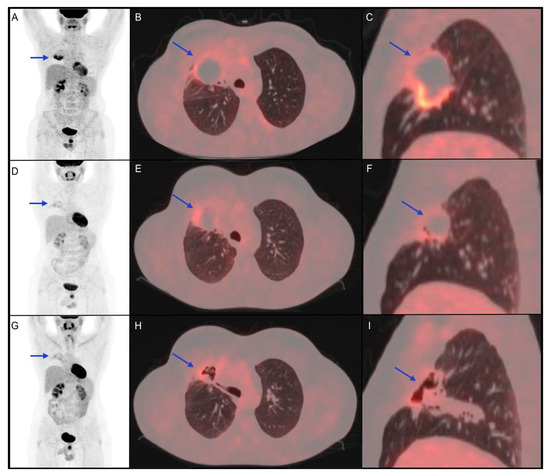
2.3. Role of 18F-FDG PET/CT in IrAEs
References
- Armand, P.; Shipp, M.A.; Ribrag, V.; Michot, J.M.; Zinzani, P.L.; Kuruvilla, J.; Snyder, E.S.; Ricart, A.D.; Balakumaran, A.; Rose, S.; et al. Programmed death-1 blockade with pembrolizumab in patients with classical hodgkin lymphoma after brentuximab vedotin failure. J. Clin. Oncol. 2016, 34, 3733–3739.
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2015, 372, 311–319.
- Castello, A.; Grizzi, F.; Qehajaj, D.; Rahal, D.; Lutman, F.; Lopci, E. 18 F-FDG PET/CT for response assessment in Hodgkin lymphoma undergoing immunotherapy with checkpoint inhibitors. Leuk. Lymphoma 2019, 60, 367–375.
- Armand, P.; Engert, A.; Younes, A.; Fanale, M.; Santoro, A.; Zinzani, P.L.; Timmerman, J.M.; Collins, G.P.; Ramchandren, R.; Cohen, J.B.; et al. Nivolumab for relapsed/refractory classic hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: Extended follow-up of the multicohort single-arm phase II checkmate 205 trial. J. Clin. Oncol. 2018, 36, 1428–1439.
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132.
- Kostakoglu, L.; Gallamini, A. Interim 18F-FDG PET in hodgkin lymphoma: Would PET-adapted clinical trials lead to a paradigm shift? J. Nucl. Med. 2013, 54, 1082–1093.
- Kasamon, Y.L.; De Claro, R.A.; Wang, Y.; Shen, Y.L.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Nivolumab for the Treatment of Relapsed or Progressive Classical Hodgkin Lymphoma. Oncologist 2017, 22, 585–591.
- Mokrane, F.Z.; Chen, A.; Schwartz, L.H.; Morschhauser, F.; Stamatoullas, A.; De Colella, J.M.S.; Vercellino, L.; Casasnovas, O.; Chauchet, A.; Delmer, A.; et al. Performance of CT compared with 18F-FDG PET in predicting the efficacy of nivolumab in relapsed or Refractory Hodgkin lymphoma. Radiology 2020, 295, 651–661.
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.M.; Reddy, N.; et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J. Clin. Oncol. 2019, 37, 481–489.
- Haverkos, B.M.; Abbott, D.; Hamadani, M.; Armand, P.; Flowers, M.E.; Merryman, R.; Kamdar, M.; Kanate, A.S.; Saad, A.; Mehta, A.; et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: High response rate but frequent GVHD. Blood 2017, 130, 221–228.
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294.
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019, 134, 1144–1153.
- Dercle, L.; Seban, R.D.; Lazarovici, J.; Schwartz, L.H.; Houot, R.; Ammari, S.; Danu, A.; Edeline, V.; Marabelle, A.; Ribrag, V.; et al. 18 F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with hodgkin lymphoma treated by Anti–Programmed death 1 immune checkpoint inhibitor. J. Nucl. Med. 2018, 59, 15–24.
- Dercle, L.; Ammari, S.; Seban, R.D.; Schwartz, L.H.; Houot, R.; Labaied, N.; Mokrane, F.Z.; Lazarovici, J.; Danu, A.; Marabelle, A.; et al. Kinetics and nadir of responses to immune checkpoint blockade by anti-PD1 in patients with classical Hodgkin lymphoma. Eur. J. Cancer 2018, 91, 136–144.
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.F.; Armand, P. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016, 128, 2489–2496.
- Chen, A.; Mokrane, F.Z.; Schwartz, L.H.; Morschhauser, F.; Stamatoullas, A.; Schiano de Colella, J.M.; Vercellino, L.; Casasnovas, O.; Chauchet, A.; Delmer, A.; et al. Early 18F-FDG PET/CT Response Predicts Survival in Relapsed or Refractory Hodgkin Lymphoma Treated with Nivolumab. J. Nucl. Med. 2020, 61, 649–654.
- Isohashi, K.; Tatsumi, M.; Higuchi, I.; Inoue, A.; Nakajo, K.; Ishikawa, J.; Shimosegawa, E.; Kanakura, Y.; Nakamura, H.; Hatazawa, J. 18F-FDG-PET in patients with malignant lymphoma having long-term follow-up: Staging and restaging, and evaluation of treatment response and recurrence. Ann. Nucl. Med. 2008, 22, 795–802.
- Barrington, S.F.; Kirkwood, A.A.; Franceschetto, A.; Fulham, M.J.; Roberts, T.H.; Almquist, H.; Brun, E.; Hjorthaug, K.; Viney, Z.N.; Pike, L.C.; et al. PET-CT for staging and early response: Results from the Response-Adapted Therapy in Advanced Hodgkin Lymphoma study. Blood 2016, 127, 1531–1538.
- Tatsumi, M.; Cohade, C.; Nakamoto, Y.; Fishman, E.K.; Wahl, R.L. Direct comparison of FDG PET and CT findings in patients with lymphoma: Initial experience. Radiology 2005, 237, 1038–1045.
- Manson, G.; Herbaux, C.; Brice, P.; Bouabdallah, K.; Stamatoullas, A.; Schiano, J.M.; Ghesquieres, H.; Dercle, L.; Houot, R. Prolonged remissions after anti-PD-1 discontinuation in patients with Hodgkin lymphoma. Blood 2018, 131, 2856–2859.
- Rossi, C.; Gilhodes, J.; Maerevoet, M.; Herbaux, C.; Morschhauser, F.; Brice, P.; Garciaz, S.; Borel, C.; Ysebaert, L.; Obéric, L.; et al. Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory hodgkin lymphoma: A series from lysa centers. Am. J. Hematol. 2018, 93, 1042–1049.
- Ansell, S.M. Immunotherapy in Hodgkin Lymphoma: The Road Ahead. Trends Immunol. 2019, 40, 380–386.
- Xie, W.; Medeiros, L.J.; Li, S.; Yin, C.C.; Khoury, J.D.; Xu, J. PD-1/PD-L1 Pathway and Its Blockade in Patients with Classic Hodgkin Lymphoma and Non-Hodgkin Large-Cell Lymphomas. Curr. Hematol. Malig. Rep. 2020, 15, 372–381.
- Merryman, R.W.; Armand, P.; Wright, K.T.; Rodig, S.J. Checkpoint blockade in Hodgkin and non-Hodgkin lymphoma. Blood Adv. 2017.
- Wang, J.; Hu, Y.; Yang, S.; Wei, G.; Zhao, X.; Wu, W.; Zhang, Y.; Zhang, Y.; Chen, D.; Wu, Z.; et al. Role of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Predicting the Adverse Effects of Chimeric Antigen Receptor T Cell Therapy in Patients with Non-Hodgkin Lymphoma. Biol. Blood Marrow Transplant. 2019, 25, 1092–1098.
- Derlin, T.; Schultze-Florey, C.; Werner, R.A.; Möhn, N.; Skripuletz, T.; David, S.; Beutel, G.; Eder, M.; Ross, T.L.; Bengel, F.M.; et al. 18F-FDG PET/CT of off-target lymphoid organs in CD19-targeting chimeric antigen receptor T-cell therapy for relapsed or refractory diffuse large B-cell lymphoma. Ann. Nucl. Med. 2020, 35.
- Shah, N.N.; Nagle, S.J.; Torigian, D.A.; Farwell, M.D.; Hwang, W.T.; Frey, N.; Nasta, S.D.; Landsburg, D.; Mato, A.; June, C.H.; et al. Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor—T-cell therapy in B-cell non-Hodgkin lymphomas. Cytotherapy 2018, 20, 1415–1418.
- Imber, B.S.; Sadelain, M.; DeSelm, C.; Batlevi, C.; Brentjens, R.J.; Dahi, P.B.; Giralt, S.; Park, J.H.; Sauter, C.; Scordo, M.; et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br. J. Haematol. 2020, 190, 45–51.
- Weist, M.R.; Starr, R.; Aguilar, B.; Chea, J.; Miles, J.K.; Poku, E.; Gerdts, E.; Yang, X.; Priceman, S.J.; Forman, S.J.; et al. PET of adoptively transferred chimeric antigen receptor T Cells with 89Zr-Oxine. J. Nucl. Med. 2018, 59, 1531–1537.
- Mulazzani, M.; Fräßle, S.P.; Von Mücke-Heim, I.; Langer, S.; Zhou, X.; Ishikawa-Ankerhold, H.; Leube, J.; Zhang, W.; Dötsch, S.; Svec, M.; et al. Long-term in vivo microscopy of CAR T cell dynamics during eradication of CNS lymphoma in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 24275–24284.
- Cheng, J.; Zhao, L.; Zhang, Y.; Qin, Y.; Guan, Y.; Zhang, T.; Liu, C.; Zhou, J. Understanding the Mechanisms of Resistance to CAR T-Cell Therapy in Malignancies. Front. Oncol. 2019, 9, 1237.
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 2017, 35, 1803–1813.
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638.
- Giavridis, T.; Van Der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade letter. Nat. Med. 2018, 24, 731–738.
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 2018, 24, 739–748.
- Wu, X.; Pertovaara, H.; Korkola, P.; Vornanen, M.; Eskola, H.; Kellokumpu-Lehtinen, P.L. Glucose metabolism correlated with cellular proliferation in diffuse large B-cell lymphoma. Leuk. Lymphoma 2012, 53, 400–405.
- Rubin, D.B.; Danish, H.H.; Ali, A.B.; Li, K.; Larose, S.; Monk, A.D.; Cote, D.J.; Spendley, L.; Kim, A.H.; Robertson, M.S.; et al. Neurological toxicities associated with chimeric antigen receptor T-cell therapy. Brain 2019, 142, 1334–1348.
- Makuku, R.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. Current and Future Perspectives of PD-1/PDL-1 Blockade in Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 1–15.
- Costa, L.B.; Queiroz, M.A.; Barbosa, F.G.; Nunes, R.F.; Zaniboni, E.C.; Ruiz, M.M.; Jardim, D.; Marin, J.F.G.; Cerri, G.G.; Buchpiguel, C.A. Reassessing patterns of response to immunotherapy with pet: From morphology to metabolism. Radiographics 2021, 41, 120–143.
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152.
- Nobashi, T.; Baratto, L.; Reddy, S.A.; Srinivas, S.; Toriihara, A.; Hatami, N.; Yohannan, T.K.; Mittra, E. Predicting Response to Immunotherapy by Evaluating Tumors, Lymphoid Cell-Rich Organs, and Immune-Related Adverse Events Using FDG-PET/CT. Clin. Nucl. Med. 2019, 44, e272–e279.
- Petersen, H.; Holdgaard, P.C.; Madsen, P.H.; Knudsen, L.M.; Gad, D.; Gravergaard, A.E.; Rohde, M.; Godballe, C.; Engelmann, B.E.; Bech, K.; et al. FDG PET/CT in cancer: Comparison of actual use with literature-based recommendations. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 695–706.
- Sachpekidis, C.; Kopp-Schneider, A.; Hakim-Meibodi, L.; Dimitrakopoulou-Strauss, A.; Hassel, J.C. 18 F-FDG PET/CT longitudinal studies in patients with advanced metastatic melanoma for response evaluation of combination treatment with vemurafenib and ipilimumab. Melanoma Res. 2019, 29, 178–186.
- Aide, N.; Hicks, R.J.; Le Tourneau, C.; Lheureux, S.; Fanti, S.; Lopci, E. FDG PET/CT for assessing tumour response to immunotherapy: Report on the EANM symposium on immune modulation and recent review of the literature. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 238–250.
- Pektor, S.; Hilscher, L.; Walzer, K.C.; Miederer, I.; Bausbacher, N.; Loquai, C.; Schreckenberger, M.; Sahin, U.; Diken, M.; Miederer, M. In vivo imaging of the immune response upon systemic RNA cancer vaccination by FDG-PET. EJNMMI Res. 2018, 8.
- Maschmeyer, G.; De Greef, J.; Mellinghoff, S.C.; Nosari, A.; Thiebaut-Bertrand, A.; Bergeron, A.; Franquet, T.; Blijlevens, N.M.A.; Maertens, J.A. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia 2019, 33, 844–862.




