Immunotherapy is a promising therapeutic strategy both for solid and hematologic tumors, such as in Hodgkin (HL) and non-Hodgkin lymphoma (NHL). In particular, immune-checkpoint inhibitors, such as nivolumab and pembrolizumab, are increasingly used for the treatment of refractory/relapsed HL. At the same time, evidence of chimeric antigen receptor (CAR)-T-cell immunotherapy efficacy mostly in NHL is growing. In this setting, the challenge is to identify an appropriate imaging method to evaluate immunotherapy response. The role of 18F-Fluorodeoxyglucose (18F-FDG) positron-emission tomography/computed tomography (PET/CT), especially in early evaluation, is under investigation in order to guide therapeutic strategies, taking into account the possible atypical responses (hyperprogression and pseudoprogression) and immune-related adverse events that could appear on PET images.
1. Introduction
In the last decade, the advent of immunotherapy in clinical practice has represented a keystone in the management of cancer patients, providing new therapeutic opportunities and paving the way for new challenges for oncology. Immunotherapy has revolutionized solid and hematologic tumor treatment with increasing use both in Hodgkin (HL) and non-Hodgkin lymphoma (NHL). In this context, immunotherapy with checkpoint inhibitors such as nivolumab and pembrolizumab, targeted programmed cell death protein 1 (PD-1), represents one of the key pathways most broadly studied.
In particular, classical HL is a peculiar tumor characterized by a vast majority of immune infiltrate, where Hodgkin Reed-Sternberg cells escape immune surveillance through an overexpression of the programmed death 1 ligands (PD-L1). For this reason, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have approved these two anti-PD-1 drugs (nivolumab and pembrolizumab), for the treatment of refractory/relapsed (R/R) HL
[1][2][1,2] with the intent to reactivate the immune system and restore immunity against Hodgkin Reed-Sternberg cells
[3]. Different clinical trials (CHECKMATE 205, KEYNOTE 087) confirm the usefulness of monotherapy with pembrolizumab and nivolumab in HL, with a high overall response and complete response rate, and the interest is now focusing on the possibility of association with chemotherapy
[4][5][4,5]. In patients with R/R HL, studies demonstrated high response rates, with complete response rates in 20% of cases
[2][5][6][2,5,6], as well as a favorable toxicity profile of immune-related adverse events
[7][8][7,8].
At present, there is not the same amount of evidence for NHL. First results in diffuse large B cell lymphoma (DLBCL) are not as encouraging as in HL, probably due to the infrequent expression of PD1/PDL1 (CHECKMATE 139)
[9], but considering some subtypes of DLBCL, such as primary mediastinal B cell lymphoma (PMBCL), in which the expression of PD1/PDL1 is higher, the evidence of checkpoint inhibitor efficacy appears to be stronger (KEYNOTE 013)
[1]. In this setting, chimeric antigen receptor (CAR)-T-cell immunotherapy has shown remarkable efficacy in R/R B-cell malignancies, including DLBCL. However, a substantial fraction of patients will not respond or relapse, without fully knowing the mechanisms leading to CAR-T-cell therapy resistance yet.
Nowadays, the efficacy and safety of these new therapeutic frontiers are a matter of debate and it is essential to individuate which are the adequate tools to be able to fully understand them. In this scenario, a crucial role is played by imaging and, in particular, to 18F-Fluorodeoxyglucose (18F-FDG) positron-emission tomography/computed tomography (PET/CT) is asked whether it could maintain its well-established role in lymphomas, and also for the immunotherapy response assessment. Currently, the literature regarding PET reliability in patients with lymphoma undergoing immunotherapy is still poor, but the preliminary results are encouraging.
2. Evidence Based Medicine of Early 18F-FDG PET/CT during Immunotherapy
2.1. Hodgkin Lymphoma
In the era of PET-guided response-adapted treatment strategies, the role of interim 18F-FDG PET/CT in HL patients treated with cytotoxic chemotherapies is well known and closely correlates with outcome. Whether early 18F-FDG PET/CT also could predict outcome in HL patients treated with anti PD-1 immunotherapy remains to be investigated
[1][2][5][10][11][1,2,5,54,55]. Early 18F-FDG PET/CT could be used to define treatment duration, changing the therapeutic regimen if necessary, or by identifying patients requiring consolidation or by reinforcing the treatment with other agent(s), avoiding unnecessary side effects
[12][56].
Castello and colleagues pointed out a significant reduction in tumor glucose metabolism, expressed by Delta Maximum Standardized Uptake Value (DSUVmax), in responder patients in both early (after 8 weeks) and interim (after 17 weeks) assessment. However, changes in tumor burden metrics, expressed as Delta Metabolic Tumor Volume (DMTV) and Delta Total Lesion Glycolisis (DTLG), were statistically significant only after 17 weeks of treatment. These seemingly opposite results can be explained in part by the changes that occur during the course of immunotherapy within the tumor microenvironment, as pseudoprogression phenomenon. However, it is interesting to underline that by applying the LYRIC criteria, which should have exceeded this limitation, no significant differences were detected in this study
[3].
In other studies, a significant decrease in tumor volume and in tumor glucose metabolism, as well as increases in spleen metabolism were observed in responders at 3 months 18F-FDG PET/CT assessment. In fact, 18F-FDG uptake into healthy spleen tissue appears significantly increased in responders, suggesting a favorable immunological reconstitution
[13][24]. In 78% of patients with objective imaging responses at 3 months, the clinical benefit lasted longer than one year, demonstrating that imaging management strategies are feasible and that early evaluation with 18F-FDG PET/CT is a useful tool
[14][50].
A key point emerged about the significance of pseudoprogression detected by early 18F-FDG PET/CT, with regards to its correlation with the following detection of a real progression. Progressive disease, based upon standard criteria, at an early time-point in patients with R/R HL treated with anti PD-1 may be considered to carry a high risk of being “true” progression rather than pseudo-progression. In fact, studies comparing immune-related LYRIC criteria to conventional response criteria, pointed out that patients classified as indeterminate response (IR) by LYRIC criteria at the early assessment were subsequently confirmed as having true progression of metabolic disease (PMD) at the late evaluation
[15][46]. As shown in the cohort studied from Chen et al., a trend towards worse overall survival (OS) was present in patients with type 2 IR according to LYRIC criteria
[16][47]. Recently, a similar conclusion was carried out by Mokrane et al. who hypothesized that the “wait-and-see” strategy recommended in other tumor types does not seem applicable at an early time point assessment in patients with R/R HL treated with immunotherapy
[8]. The authors support the idea that progressive disease at primary evaluation with CT or 18F-FDG PET/CT at an early stage should be considered at high risk of being a true progressive disease. Comparing both imaging modalities, 18F-FDG PET/CT detected progressive disease in patients more frequently than CT alone did. This may be explained by the ability of functional imaging to depict “viable” HL lesions with high glucose consumption before anatomic progression
[17][57]. Although previous studies demonstrated that 18F-FDG PET/CT tended to upstage up to 40% of patients at baseline evaluation compared with CT alone
[8][18][19][8,58,59]. However, the clinical value of the complete metabolic response in patients with R/R HL treated with immunotherapy remains controversial. Some reports suggest that anti-PD-1 could be stopped in patients achieving a complete metabolic response
[20][60], while in the case of a partial response, more aggressive strategies may be required
[13][21][24,61]. Others contest that a complete metabolic response can predict a clinical benefit
[4][22][4,62] without defining the timing of immunotherapy treatment ending. Regardless of time assessment, response-adapted treatment strategies in patients with R/R HL treated with immunotherapy should take into account that early assessment with 18F-FDG PET/CT outperforms CT in identifying patients who achieve a metabolic response
[14][50]. In conclusion, complete responders at either primary CT or PET/CT assessments experience a 2-year OS excellent probability and clinicians could consider a treatment de-escalation. Moreover, the major incremental value of 18F-FDG PET/CT is to help detect earlier and higher rates of complete response. Finally, a progressive disease at either primary CT or PET/CT assessment carries a poorer prognosis, as well as a higher risk of being a true progressive disease rather than pseudoprogression, in this case clinicians could consider treatment escalation/association.
In is described a rapresentative case of early 18F-FDG PET/CT evaluation in a classic HL patients trated with nivolumab.
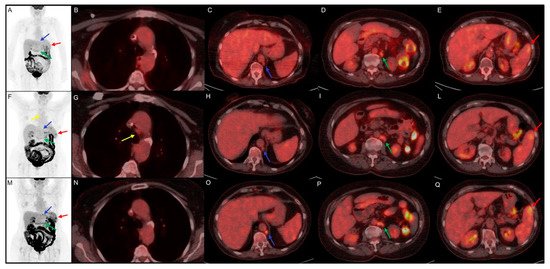
Figure 1. Clinical case of a 75 year-old female with history of classic Hodgkin Lymphoma firstly diagnosed in August 2014. She underwent chemotherapy with ABVD and, in September 2017, with brentuximab and R-bendamustine for disease relapse. In June 2019, the disease persistence induced hematologists to choose an off-label treatment with nivolumab. (A–E) 18F-FDG PET/CT performed before the start of immunotherapy, documented disease localized in para-aortic (blue arrow, SUVmax 4.5) and lombo-aortic (green arrow, SUVmax 5.5) lymph nodes and splenic lesions (red arrow, SUVmax 4.7). (F–L) Early 18F-FDG PET/CT performed 3 months after the start of the immunotreatment, indicate a dissociated response with decreasing SUVmax in para-aortic (blue arrow, SUVmax 2.5) and lombo-aortic (green arrow, SUVmax 4.5) lymph nodes but increased 18F-FDG uptake in splenic lesions (red arrow, SUVmax 5.7) and the appearance of a mediastinal adenopathy (yellow arrow, SUVmax 2.4). (M–Q) Interim 18F-FDG PET/CT, performed 6 months after the start of nivolumab, documented an increased uptake in para-aortic (blue arrow, SUVmax 6.9), lombo-aortic (green arrow, SUVmax 5.3) lymph nodes and in splenic lesions (red arrow, SUVmax 6.5). The mediastinal adenopathy previously detected showed no FDG uptake.
The immunotherapy was well tolerated and is still ongoing; at the last clinical follow-up, the patient referred to a comparison of asthenia and sweating, probably due to a disease progression. In this clinical case the first interim evaluation showed a real disease progression, which was confirmed by the subsequent 18F-FDG PET/CT and by clinical worsening. The pathological meaning of mediastinal adenopathy at early evaluation 18F-FDG PET/CT remains doubtful for the possible relation with frequent inflammatory activation during immunotherapy.
2.2. Non Hodgkin Lymphoma
As mentioned above, the role of immunotherapy in NHL is still under evaluation, especially due to heterogeneous biological features of various subtypes, among which only few showed encouraging results (). In 18 R/R PMBL patients with frequent 9p24.1 alteration, recruited in KEYNOTE 013, an ORR of 41% was achieved with 2 patients obtaining a CR
[1][23][24][1,63,64].
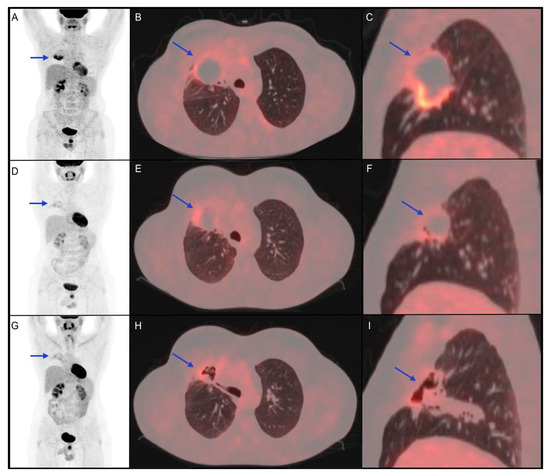
Figure 2. Clinical case of a 34-year-old male patient affected by diffuse large B-cell lymphoma diagnosed with biopsy of mediastinal mass, with right lung involvement, in June 2016. He underwent radio-chemotherapy with R-CHOP, R-DHAP and R-IEV with a subsequent stem cell transplant in June 2017. In March 2018, the persistence of the right lung lesion led haematologists to choose a following therapeutic option immune checkpoint inhibitor (pembrolizumab). (A–C) 18F-FDG PET/CT performed before the start of immunotherapy shows a metabolically active right lung lesion (blue arrow, SUVmax 8.7). (D–F) 18F-FDG PET/CT performed 5 months after the start of immuno-treatment shows partial metabolic response of the known lesion with a reduction of SUVmax (blue arrow, SUVmax 4.2). (G–I) 18F-FDG PET/CT 4 months after the end of immunotherapy shows further metabolic reduction (SUVmax 3.5) of the lung lesion, which appeared excavated on CT coregistered to PET images. The patient was followed-up with stable disease until December 2020, when progression disease was documented.
In this clinical case, the partial response showed by interim 18F-FDG PET/CT, confirmed at the post-treatment metabolic evaluation, was found to be prognostically reliable, considering the 2-year PFS.
However, because only a small subset of NHL patients has PD-L1/L2 expression, current investigations in NHL are therefore focusing on targeting other checkpoints, with an increasing interest in the use of novel CAR-T cell therapies. Even for this kind of therapy, the problem concerning tumor site inflammation and false-positive results exists and makes difficult 18F-FDG PET/CT response assessment. The pseudoprogression observed with CAR-T cell therapy was more rapid and significant compared with that of checkpoint inhibitors, which could be secondary to the different malignancy types treated with each therapy, but also may reflect a greater efficacy of CAR-T cell therapy
[25][51].
Considering the significance of early PET evaluation for outcome prediction, recently Derlin and colleagues found that, for achieving remission, an early metabolic response at PET was required
[26][53]. In a pilot study including patients who performed 18F-FDG PET/CT scans before and 1 month after CAR-T-cell therapy, Shah et al. demonstrated that all patients that did not reach a complete response subsequently relapsed
[27][52]. On the other hand, patients who achieved a complete metabolic response, with no residual MTV, showed a long-term remission of their disease over 2 years after treatment. Therefore, non-responders with clear signs of early progression could benefit from a quick change in therapy at 1-month PET/CT
[27][52]. Indeed, Imber et al. reported that in the majority of patients with post-CAR-T progression, salvage radiation therapy had to be delivered to FDG-avid sites in pre-CAR-T PET
[28][65]. Patients with an unfavorable outcome demonstrated a significantly higher decrease of glucose metabolism in both spleen and lymph node between baseline and early 18F-FDG PET/CT. This finding can be explained by low CAR-T expansion and survival after migration to spleen and lymph nodes following the intravenous injection, as observed in preclinical rodent models or by depletion of off-target B cells, disrupting crucial immune networks for anti-tumor response
[29][30][66,67]. However, this mechanism needs more evidence to be confirmed.
Immunological mechanisms may also contribute to toxicity, for example with the cytokine release syndrome (CRS), caused by cytokines produced by both activated CAR-T cells and other immune cells
[31][32][68,69], and the immune effector cell-associated neurotoxicity syndrome (ICANS)
[27][26][33][52,53,70]. In terms of adverse effects, Whang et al. found that higher disease burden, measured by MTV and TLG, was associated with more severe CRS
[25][34][35][51,71,72]. Concerning acute toxicity, Derlin et al. found that higher lymphoma cell metabolism (SUVmax) was associated with neurotoxicity. Importantly, SUVmax is directly related to the Ki-67 proliferation index in DLBCL
[36][73], indicating that patients with high proliferation lymphoma may be particularly prone to develop adverse effects. Of note, Rubin et al. reported cortical and sub-cortical 18F-FDG PET/CT hypometabolism in patients with neurological toxicities
[37][74].
2.3. Role of 18F-FDG PET/CT in IrAEs
Immune-related adverse event (IrAE) is a frequent occurrence in immunotherapy, although its precise pathophysiology remains unclear. It can be explained through enhancing antitumor immune response by ICIs that can alter immunologic homeostasis up to break self-tolerance and develop autoimmune disease, virtually involving any organ
[38][75]. IrAE can occur at any time during ICI therapy, but is common within the first 3 months
[39][76]. Since not all IrAEs exhibit clinical signs and symptoms, 18F-FDG PET/CT could provide more information on IrAEs, even before they become manifest. Furthermore, it is debated whether IrAEs may be a favorable prognostic marker for immunotherapy, because they may reflect the antitumor immune activation.
Several evidences indicated that IrAEs are associated with a higher response rate, although this is still controversial
[40][41][34,77]. For example, diffuse 18F-FDG uptake in thyroid or autoimmune thyroiditis has been reported to predict a favorable outcome in DLBCL, treated with rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP)
[42][78]. Similar conclusions were reached in other studies, in which patients who developed imaging signs on PET/CT of at least one IrAE (most frequently colitis and arthritis) had a significantly longer progression-free survival (PFS) than those without irAEs
[43][79]. For this reason, it is important to report immune-related findings, even if they are not necessarily associated with clinically significant IrAE.
In the approach to metabolic imaging with 18F-FDG PET/CT, another early sign of immune activity is the inversion of the liver-to-spleen ratio (normally > 1), possibly reflecting the immune activation preceding T cell proliferation, but also the reactive nodes in the drainage basin of the primary tumor, which could be wrongly diagnosed as cancerous lymph nodes
[44][45][80,81]. Moreover, IrAEs often require immunosuppressive treatment, which increases the risk of developing infections
[46][82].
Because the autoimmune/inflammatory process appears as diffuse increased FDG-metabolism, differentiating IrAEs from metastases or tumor progression is needed in PET images interpretation. For this purpose, clinical symptoms, laboratory parameters, CT-coregistered to PET images or complementary specific imaging methods could be helpful. For example, pneumonitis, a common IrAE, appears at PET/CT with different intensities of FDG-uptake associated with reactive mediastinal lymphadenopathy and possibly pleural effusions. CT specific imaging patterns (ground-glass opacities and consolidations) can clarify the diagnostic doubt
[39][76].