
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana Pereira | + 6081 word(s) | 6081 | 2021-06-08 08:32:15 | | | |
| 2 | Bruce Ren | -21 word(s) | 6060 | 2021-06-22 10:28:28 | | | | |
| 3 | Bruce Ren | + 74 word(s) | 6134 | 2021-06-23 10:08:49 | | | | |
| 4 | Bruce Ren | + 4 word(s) | 6064 | 2021-06-23 10:16:02 | | | | |
| 5 | Bruce Ren | + 4 word(s) | 6064 | 2021-06-23 10:18:07 | | |
Video Upload Options
Legionella is responsible for the life-threatening pneumonia commonly known as Legionnaires’ disease or legionellosis. Legionellosis is known to be preventable if proper measures are put into practice. Despite the efforts to improve preventive approaches, Legionella control remains one of the most challenging issues in the water treatment industry. Legionellosis incidence is on the rise and is expected to keep increasing as global challenges become a reality. This puts great emphasis on prevention, which must be grounded in strengthened Legionella management practices. The perpetuation of a water focused monitoring approach and the importance of protozoa and biofilms are bottom-line questions for reliable Legionella real-field surveillance.
Under this scope an integrated monitoring model is proposed to study and control Legionella at water systems, by combining discrete and continuous information about water and biofilm. Although the successful implementation of such model requires a broader discussion across the scientific community and practitioners, this might be a starting point to build more consistent Legionella management strategies that can effectively mitigate legionellosis risks by reinforcing a pro-active Legionella prevention philosophy.
1. Introduction
2. Key Topics That Need to Be Tackled for Effective Legionella Real-Field Prevention
2.1. Legionella a Case of Resilience
2.2. The Ecological Niches of Legionella—Protozoa and Biofilms
2.3. Bottlenecks of Real-Field Legionella Control
2.4. The Scientific Perpetuation of a Water Legionella-Sampling Approach
2.5. Online Biofilm Monitoring—An Unmet Need or an Unexplored Solution?
3. New Pathways to Build an Integrated and Effective Legionella Surveillance Strategy in Water Systems
3.1. An Integrated Monitoring Physical Model for Legionella Study and Control in Real Systems
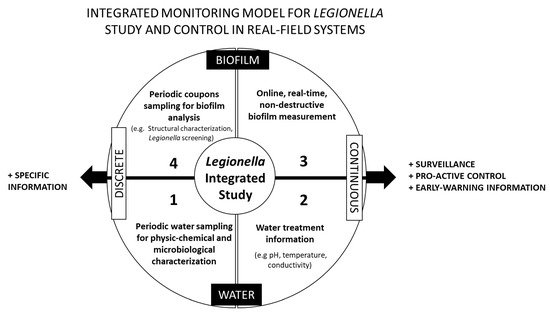
-
1st Set of Information: Water—Discrete Sampling
-
2nd Set of Information: Water—Continuous Monitoring
-
3rd Set of Information: Biofilm—Online Monitoring
-
4th Set of Information: Biofilm—Discrete Sampling
3.2. Representativeness—Worst Case Scenario Conditions
3.3. Final Disclaimer
4. Conclusions
References
- Hughes, E.D.; Swanson, M.S. How Legionella Defend Their Turf. eLife 2019, 8, e48695.
- Abu, A.K.; Amer, A.O. Factors Mediating Environmental Biofilm Formation by Legionella Pneumophila. Front. Cell. Infect. Microbiol. 2018, 8, 38.
- Alarcon Falconi, T.M.; Cruz, M.S.; Naumova, E.N. The Shift in Seasonality of Legionellosis in the USA. Epidemiol. Infect. 2018, 146, 1824–1833.
- Beauté, J. Legionnaires’ Disease in Europe, 2011 to 2015. Eurosurveillance 2017, 22, 1–8.
- European Centre for Disease Prevention and Control. Legionnaires’ Disease—ECDC Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2020.
- Barskey, A.; Lackraj, D.; Tripathi, P.S.; Cooley, L.; Lee, S.; Smith, J.; Edens, C. Legionnaires’ Disease Surveillance Summary Report, United States: 2016–2017; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Shah, P.; Barskey, A.; Binder, A.; Edens, C.; Lee, S.; Smith, J.; Schrag, S.; Whitney, C.; Cooley, L. Legionnaires’ Disease Surveillance Summary Report, United States, 2014–2015; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Buse, H.Y.; Schoen, M.E.; Ashbolt, N.J. Legionellae in Engineered Systems and Use of Quantitative Microbial Risk Assessment to Predict Exposure. Water Res. 2012, 46, 921–933.
- McClung, R.P.; Roth, D.M.; Vigar, M.; Roberts, V.A.; Kahler, A.M.; Cooley, L.A.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; Yoder, J.S. Waterborne Disease Outbreaks Associated with Environmental and Undetermined Exposures to Water—United States, 2013–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 1222.
- Whiley, H. Legionella Risk Management and Control in Potable Water Systems: Argument for the Abolishment of Routine Testing. Int. J. Environ. Res. Public Health 2016, 14, 12.
- Graham, F.F.; Hales, S.; White, P.S.; Baker, M.G. Review Global Seroprevalence of Legionellosis—A Systematic Review and Meta-Analysis. Sci. Rep. 2020, 10, 1–11.
- Parr, A.; Whitney, E.A.; Berkelman, R.L. Legionellosis on the Rise: A Review of Guidelines for Prevention in the United States. J. Public Health Manag. Pract. 2015, 21, E17–E26.
- Kirschner, A.K.T. Determination of Viable Legionellae in Engineered Water Systems: Do We Find What We Are Looking For? Water Res. 2016, 93, 276–288.
- Koubar, M.; Rodier, M.-H.; Frere, J. Involvement of Minerals in Adherence of Legionella Pneumophila to Surfaces. Curr. Microbiol. 2013, 66, 437–442.
- United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision; Highlights (ST/ESA/SER.A/352); United Nations: New York, NY, USA, 2014.
- Voulvoulis, N. Water Reuse from a Circular Economy Perspective and Potential Risks from an Unregulated Approach. Curr. Opin. Environ. Sci. Heal. 2018, 2, 32–45.
- Walker, J.T. The Influence of Climate Change on Waterborne Disease and Legionella: A Review. Perspect. Public Health 2018, 138, 282–286.
- Passer, J.K.; Danila, R.N.; Laine, E.S.; Como-Sabetti, K.J.; Tang, W.; Searle, K.M. The Association between Sporadic Legionnaires’ Disease and Weather and Environmental Factors, Minnesota, 2011–2018. Epidemiol. Infect. 2020, 148, e156.
- Hicks, L.A.; Rose, C.; Fields, B.; Drees, M.; Engel, J.; Jenkins, P.; Rouse, B.; Blythe, D.; Khalifah, A.P.; Feikin, D.; et al. Increased Rainfall Is Associated with Increased Risk for Legionellosis. Epidemiol. Infect. 2007, 135, 811–817.
- Borella, P.; Guerrieri, E.; Marchesi, I.; Bondi, M.; Messi, P. Water Ecology of Legionella and Protozoan: Environmental and Public Health Perspectives; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 355–380. ISBN 1387-2656.
- Ricketts, K.D.; Joseph, C.; Lee, J.; Wewalka, G. Survey on Legislation Regarding Wet Cooling Systems in European Countries. Euro Surveill. 2008, 13, 18982.
- Fields, B.S.; Benson, R.F.; Besser, R.E. Legionella and Legionnaires’ Disease: 25 Years of Investigation. Clin. Microbiol. Rev. 2002, 15, 506–526.
- Bartram, J.; Chartier, Y.; Lee, J.V.; Bond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; Geneva World Health Organization: Geneva, Switzerland, 2007; Volume 14.
- ECDC. European Technical Guidelines for the Prevention, Control and Investigation, of Infections Caused by Legionella Species. Available online: (accessed on 7 April 2021).
- National Academies of Sciences, Engineering and Medicine. Management of Legionella in Water Systems; The National Academies Press: Washington, DC, USA, 2020; ISBN 978-0-309-49947-7.
- European Union. Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. 2020. Available online: (accessed on 27 May 2021).
- Bentham, R.H. Routine Sampling and the Control of Legionella spp. In Cooling Tower Water Systems. Curr. Microbiol. 2000, 41, 271–275.
- Collins, S.; Walker, J. Comments on Whiley Legionella Risk Management and Control in Potable Water Systems: Argument for the Abolishment of Routine Testing. Int. J. Environ. Res. Public Health 2017, 14, 12. Int. J. Environ. Res. Public Health 2017, 14, 102.
- Young, C.; Smith, D.; Wafer, T.; Crook, B. Rapid Testing and Interventions to Control Legionella Proliferation Following a Legionnaires’ Disease Outbreak Associated with Cooling Towers. Microorganisms 2021, 9, 615.
- Lau, H.Y.; Ashbolt, N.J. The Role of Biofilms and Protozoa in Legionella Pathogenesis: Implications for Drinking Water. J. Appl. Microbiol. 2009, 107, 368–378.
- Declerck, P. Biofilms: The Environmental Playground of Legionella Pneumophila. Environ. Microbiol. 2010, 12, 557–566.
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351.
- Coenye, T.; Kjellerup, B.; Stoodley, P.; Bjarnsholt, T. The Future of Biofilm Research—Report on the ‘2019 Biofilm Bash’. Biofilm 2020, 2, 100012.
- Flemming, H.C. Role and Levels of Real-Time Monitoring for Successful Anti-Fouling Strategies-an Overview. Water Sci. Technol. 2003, 47, 1–8.
- Atlas, R.M. Legionella: From Environmental Habitats to Disease Pathology, Detection and Control. Environ. Microbiol. 1999, 1, 283–293.
- Abdel-Nour, M.; Duncan, C.; Low, D.E.; Guyard, C. Biofilms: The Stronghold of Legionella pneumophila. Int. J. Mol. Sci. 2013, 14, 21660–21675.
- Surman, S.; Mortin, G.; Keevil, C.W.; Fitzgeorge, R. Legionella pneumophila Proliferation Is Not Dependent on Intracellular Replication. In Legionella; Marre, R., Kwaik, Y.A., Bartlett, C., Cianciotto, N.P., Fields, B.S., Frosch, M., Hacker, J., Lück, P.C., Eds.; American Society of Microbiology: Washington, DC, USA, 2002; pp. 86–89.
- Schrammel, B.; Cervero-Aragó, S.; Dietersdorfer, E.; Walochnik, J.; Lück, C.; Sommer, R.; Kirschner, A. Differential Development of Legionella Sub-Populations during Short- and Long-Term Starvation. Water Res. 2018, 141, 417–427.
- Wright, J.B. Legionella Biofilms: Their Implications, Study and Control. Biofilms Recent Adv. Study Control. 2000, 17, 291–310.
- Surman, S.B.; Morton, L.H.G.; Keevil, C.W. The Dependence of Legionella Pneumophila on Other Aquatic Bacteria for Survival on R2A Medium. Int. Biodeterior. Biodegrad. 1994, 33, 223–236.
- Cervero-Aragó, S.; Schrammel, B.; Dietersdorfer, E.; Sommer, R.; Lück, C.; Walochnik, J.; Kirschner, A. Viability and Infectivity of Viable but Nonculturable Legionella Pneumophila Strains Induced at High Temperatures. Water Res. 2019, 158, 268–279.
- Alleron, L.; Merlet, N.; Lacombe, C.; Frère, J. Long-Term Survival of Legionella Pneumophila in the Viable but Nonculturable State after Monochloramine Treatment. Curr. Microbiol. 2008, 57, 497–502.
- Epalle, T.; Girardot, F.; Allegra, S.; Maurice-Blanc, C.; Garraud, O.; Riffard, S. Viable but Not Culturable Forms of Legionella Pneumophila Generated After Heat Shock Treatment Are Infectious for Macrophage-Like and Alveolar Epithelial Cells After Resuscitation on Acanthamoeba Polyphaga. Microb. Ecol. 2014, 69, 215–224.
- Shaheen, M.; Scott, C.; Ashbolt, N.J. Long-Term Persistence of Infectious Legionella with Free-Living Amoebae in Drinking Water Biofilms. Int. J. Hyg. Environ. Health 2019, 222, 678–686.
- Dietersdorfer, E.; Kirschner, A.; Schrammel, B.; Ohradanova-Repic, A.; Stockinger, H.; Sommer, R.; Walochnik, J.; Cervero-Aragó, S. Starved Viable but Non-Culturable (VBNC) Legionella Strains Can Infect and Replicate in Amoebae and Human Macrophages. Water Res. 2018, 141, 428–438.
- Declerck, P.; Behets, J.; Delaedt, Y.; Margineanu, A.; Lammertyn, E.; Ollevier, F. Impact of Non-Legionella Bacteria on the Uptake and Intracellular Replication of Legionella Pneumophila in Acanthamoeba Castellanii and Naegleria Lovaniensis. Microb. Ecol. 2005, 50, 536–549.
- Oliva, G.; Sahr, T.; Buchrieser, C. The Life Cycle of L. Pneumophila: Cellular Differentiation Is Linked to Virulence and Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 3.
- Swanson, M.S.; Hammer, B.K. Legionella Pneumophila Pathogenesis: A Fateful Journey from Amoebae to Macrophages. Annu. Rev. Microbiol. 2000, 54, 567–613.
- Ashbolt, N.J. Environmental (Saprozoic) Pathogens of Engineered Water Systems: Understanding Their Ecology for Risk Assessment and Management. Pathogens 2015, 4, 390–405.
- Rogers, J.; Keevil, C.W. Immunogold and Fluorescein Immunolabelling of Legionella Pneumophila within an Aquatic Biofilm Visualized by Using Episcopic Differential Interference Contrast Microscopy. Appl. Environ. Microbiol. 1992, 58, 2326–2330.
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890.
- Bott, T.R. Industrial Biofouling; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 0080932606.
- Flemming, H.-C.; Wingender, J.; Griegbe, T.; Mayer, C. Physico-Chemical Properties of Biofilms. In Biofilms Recent Advances in their Study and Control; Harwood Academic Publishers: Amsterdam, The Netherland, 2000; Volume 2000, pp. 19–34.
- Watnick, P.; Kolter, R. Biofilm, City of Microbes. J. Bacteriol. 2000, 182, 2675–2679.
- Melo, L.F.; Bott, T.R. Biofouling in Water Systems. Exp. Therm. Fluid Sci. 1997, 14, 375–381.
- Coetser, S.E.; Cloete, T.E. Biofouling and Biocorrosion in Industrial Water Systems. Crit. Rev. Microbiol. 2005, 31, 213–232.
- Flemming, H.-C.C.; Percival, S.L.; Walker, J.T. Contamination Potential of Biofilms in Water Distribution Systems. Water Sci. Technol. Water Supply 2002, 2, 271–280.
- Arndt, H.; Schmidt-Denter, K.; Auer, B.; Weitere, M. Protozoans and Biofilms. In Fossil and Recent Biofilms, A Natural History of Life on Earth; Springer: Dordrecht, The Netherlands, 2003; pp. 173–189. ISBN 978-90-481-6412-7.
- Murga, R.; Forster, T.S.; Brown, E.; Pruckler, J.M.; Fields, B.S.; Donlan, R.M. Role of Biofilms in the Survival of Legionella Pneumophila in a Model Potable-Water System. Microbiology 2001, 147, 3121–3126.
- Declerck, P.; Behets, J.; Margineanu, A.; van Hoef, V.; De Keersmaecker, B.; Ollevier, F. Replication of Legionella Pneumophila in Biofilms of Water Distribution Pipes. Microbiol. Res. 2009, 164, 593–603.
- Van der Kooij, D.; Bakker, G.L.; Italiaander, R.; Veenendaal, H.R.; Wullings, B.A. Biofilm Composition and Threshold Concentration for Growth of Legionella Pneumophila on Surfaces Exposed to Flowing Warm Tap Water without Disinfectant. Appl. Environ. Microbiol. 2017, 83.
- Kuiper, M.W.; Wullings, B.A.; Akkermans, A.D.L.; Beumer, R.R.; Van Der Kooij, D. Intracellular Proliferation of Legionella Pneumophila in Hartmannella Vermiformis in Aquatic Biofilms Grown on Plasticized Polyvinyl Chloride. Appl. Environ. Microbiol. 2004, 70, 6826–6833.
- Wadowsky, R.M.; Yee, R.B. Satellite Growth of Legionella Pneumophila with an Environmental Isolate of Flavobacterium Breve. Appl. Environ. Microbiol. 1983, 46, 1447–1449.
- Taylor, M.; Ross, K.; Bentham, R. Legionella, Protozoa, and Biofilms: Interactions within Complex Microbial Systems. Microb. Ecol. 2009, 58, 538–547.
- Boudarel, H.; Mathias, J.D.; Blaysat, B.; Grédiac, M. Towards Standardized Mechanical Characterization of Microbial Biofilms: Analysis and Critical Review. NPJ Biofilm. Microbiom. 2018, 4.
- Gião, M.S.; Wilks, S.; Azevedo, N.F.; Vieira, M.J.; Keevil, C.W. Incorporation of Natural Uncultivable Legionella Pneumophila into Potable Water Biofilms Provides a Protective Niche against Chlorination Stress. Biofouling 2009, 25, 335–341.
- Wright, J.B.; Ruseska, I.; Costerton, J.W. Decreased Biocide Susceptibility of Adherent Legionella Pneumophila. J. Appl. Bacteriol. 1991, 71, 531–538.
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193.
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of Bacterial Biofilms to Disinfectants: A Review. Biofouling 2011, 27, 1017–1032.
- McCoy, W.F.; Downes, E.L.; Leonidas, L.F.; Cain, M.F.; Sherman, D.L.; Chen, K.; Devender, S.; Neville, M.J. Inaccuracy in Legionella Tests of Building Water Systems Due to Sample Holding Time. Water Res. 2012, 46, 3497–3506.
- Flemming, H.-C. Microbial Biofouling: Unsolved Problems, Insufficient Approaches, and Possible Solutions. In Biofilm Highlights; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 81–110. ISBN 978-3-642-19939-4.
- Bonadonna, L.; Briancesco, R.; Libera, S.D.; Lacchetti, I.; Paradiso, R.; Semproni, M. Microbial Characterization of Water and Biofilms in Drinking Water Distribution Systems at Sport Facilities. Cent. Eur. J. Public Health 2009, 17, 99–102.
- Kirschner, A.K.T.; Rameder, A.; Schrammel, B.; Indra, A.; Farnleitner, A.H.; Sommer, R. Development of a New CARD-FISH Protocol for Quantification of Legionella Pneumophila and Its Application in Two Hospital Cooling Towers. J. Appl. Microbiol. 2012, 112, 1244–1256.
- Collins, S.; Stevenson, D.; Walker, J.; Bennett, A. Evaluation of Legionella Real-Time PCR against Traditional Culture for Routine and Public Health Testing of Water Samples. J. Appl. Microbiol. 2017, 122, 1692–1703.
- Fisher, K.E.; Wickenberg, L.P.; Leonidas, L.F.; Ranz, A.A.; Habib, M.A.; Buford, R.M.; McCoy, W.F. Next Day Legionella PCR: A Highly Reliable Negative Screen for Legionella in the Built Environment. J. Water Health 2020, 18, 345–357.
- Olabarria, G.; Eletxigerra, U.; Rodriguez, I.; Bilbao, A.; Berganza, J.; Merino, S. Highly Sensitive and Fast Legionella Spp. in Situ Detection Based on a Loop Mediated Isothermal Amplification Technique Combined to an Electrochemical Transduction System. Talanta 2020, 217, 121061.
- De Filippis, P.; Mozzetti, C.; Messina, A.; D’Alò, G.L. Prevalence of Legionella in Retirement Homes and Group Homes Water Distribution Systems. Sci. Total Environ. 2018, 643, 715–724.
- Garner, E.; McLain, J.; Bowers, J.; Engelthaler, D.M.; Edwards, M.A.; Pruden, A. Microbial Ecology and Water Chemistry Impact Regrowth of Opportunistic Pathogens in Full-Scale Reclaimed Water Distribution Systems. Environ. Sci. Technol. 2018, 52, 9056–9068.
- Strathmann, M.; Mittenzwey, K.-H.; Sinn, G.; Papadakis, W.; Flemming, H.-C. Simultaneous Monitoring of Biofilm Growth, Microbial Activity, and Inorganic Deposits on Surfaces with an in Situ, Online, Real-Time, Non-Destructive, Optical Sensor. Biofouling 2013, 29, 573–583.
- Janknecht, P.; Melo, L.F. Online Biofilm Monitoring. Rev. Environ. Sci. Biotechnol. 2003, 2, 269–283.
- Nivens, D.E.; Palmer, R.J.; White, D.C. Continuous Nondestructive Monitoring of Microbial Biofilms: A Review of Analytical Techniques. J. Ind. Microbiol. 1995, 15, 263–276.
- Flemming, H.C. Biofouling and Me: My Stockholm Syndrome with Biofilms. Water Res. 2020, 173, 115576.
- Walker, J.T.; McDermott, P. Confirming the Presence of Legionella Pneumophila in Your Water System: A Review of Current Legionella Testing Methods. J. AOAC Int. 2021.
- LeChevallier, M.W. Guidance on Developing a Legionella Pneumophila Monitoring Program for Utility Distribution Systems. Health. Educ. Public. Health 2021, 4, 369–376.
- Shaheen, M.; Ashbolt, N.J. Differential Bacterial Predation by Free-Living Amoebae May Result in Blooms of Legionella in Drinking Water Systems. Microorganisms 2021, 9, 174.
- Valster, R.M.; Wullings, B.A.; van der Kooij, D. Detection of Protozoan Hosts for Legionella Pneumophila in Engineered Water Systems by Using a Biofilm Batch Test. Appl. Environ. Microbiol. 2010, 76, 7144–7153.
- Nisar, M.A.; Ross, K.E.; Brown, M.H.; Bentham, R.; Whiley, H. Legionella Pneumophila and Protozoan Hosts: Implications for the Control of Hospital and Potable Water Systems. Pathogens 2020, 9, 286.
- Cunliffe, D.; Bartram, J.; Briand, E.; Chartier, Y.; Colbourne, J.; Drury, D.; Lee, J.; Schaefer, B.; Surman-Lee, S. Water Safety in Buildings; World Health Organization: Geneva, Switzerland, 2011.
- Coniglio, M.A.; Ferrante, M.; Yassin, M.H. Preventing Healthcare-Associated Legionellosis: Results after 3 Years of Continuous Disinfection of Hot Water with Monochloramine and an Effective Water Safety Plan. Int. J. Environ. Res. Public Health 2018, 15, 1594.
- Whiley, H.; Hinds, J.; Xi, J.; Bentham, R. Real-Time Continuous Surveillance of Temperature and Flow Events Presents a Novel Monitoring Approach for Hospital and Healthcare Water Distribution Systems. Int. J. Environ. Res. Public Health 2019, 16, 1332.
- Flemming, H.-C.; Griebe, T.; Schaule, G. Antifouling Strategies in Technical Systems—A Short Review. Water Sci. Technol. 1996, 34, 517–524.
- Pereira, A.; Melo, L.F.; Martins, J.; Freire, M. Fouling and Cleaning Monitoring Using the MSS—Industrial Perspective. In Proceedings of the Heat exchangers fouling and cleaning, Schladming, Austria, 14–19 June 2009.
- Pereira, A.; Mendes, J.; Melo, L.F. Using Nanovibrations to Monitor Biofouling. Biotechnol. Bioeng. 2008, 99, 1407–1415.
- Pavanello, G.; Faimali, M.; Pittore, M.; Mollica, A.; Mollica, A.; Mollica, A. Exploiting a New Electrochemical Sensor for Biofilm Monitoring and Water Treatment Optimization. Water Res. 2011, 45, 1651–1658.
- Bierganns, P.; Beardwood, E. A New and Novel Abiotic-Biotic Fouling Sensor for Aqueous Systems. In Proceedings of the Heat Exchanger Fouling and Cleaning, Aranjuez, Spain, 11–16 June 2017.
- Gomes, I.B.; Simões, M.; Simões, L.C. An Overview on the Reactors to Study Drinking Water Biofilms. Water Res. 2014, 62, 63–87.
- Deines, P.; Sekar, R.; Husband, P.S.; Boxall, J.B.; Osborn, A.M.; Biggs, C.A. A New Coupon Design for Simultaneous Analysis of in Situ Microbial Biofilm Formation and Community Structure in Drinking Water Distribution Systems. Appl. Microbiol. Biotechnol. 2010, 87, 749–756.
- Pereira, M.O.; Vieira, M.J.; Beleza, V.M.; Melo, L.F. Comparison of Two Biocides—Carbamate and Glutaraldehyde—In the Control of Fouling in Pulp and Paper Industry. Environ. Technol. 2001, 22, 781–790.
- Teodósio, J.S.; Silva, F.C.; Moreira, J.M.R.; Simões, M.; Melo, L.F.; Alves, M.A.; Mergulhão, F.J. Flow Cells as Quasi-Ideal Systems for Biofouling Simulation of Industrial Piping Systems. Biofouling 2013, 29, 953–966.
- Ginige, M.P.; Garbin, S.; Wylie, J.; Krishna, K.C.B. Effectiveness of Devices to Monitor Biofouling and Metals Deposition on Plumbing Materials Exposed to a Full-Scale Drinking Water Distribution System. PLoS ONE 2017, 12, e0169140.
- Aggarwal, S.; Stewart, P.S.; Hozalski, R.M. Biofilm Cohesive Strength as a Basis for Biofilm Recalcitrance: Are Bacterial Biofilms Overdesigned? Microbiol. Insights 2016, 8, 29–32.
- Picioreanu, C.; van Loosdrecht, M.; Heijnen, S. Modelling and Predicting Biofilm Structure; Cambridge University Press: Cambridge, UK, 1999; pp. 129–166. ISBN 9780521793025.




