| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magdalena Bryś | + 2427 word(s) | 2427 | 2021-08-04 03:32:49 | | | |
| 2 | Peter Tang | Meta information modification | 2427 | 2021-08-12 04:33:57 | | |
Video Upload Options
Propolis is a natural material that honey bees (Apis mellifera) produce from various botanical sources. The therapeutic activity of propolis, including antibacterial, antifungal, and anti-inflammatory effects, have been known since antiquity. Propolis is a rich source of biologically active compounds, which affect numerous signaling pathways regulating crucial cellular processes. The results of the latest research show that propolis can inhibit proliferation, angiogenesis, and metastasis of cancer cells and stimulate apoptosis. Moreover, it may influence the tumor microenvironment and multidrug resistance of cancers.
1. Introduction
2. Composition of Propolis
|
Compound Name, IUPAC Name; Concentration Used |
Model |
Property |
Chemical Structure |
Reference |
|---|---|---|---|---|
|
Flavonoids, flavanones, flavones and flavonols |
||||
|
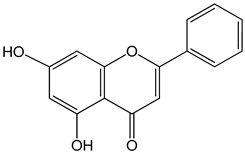
Chrysin (5,7-dihydroxy-2-phenylchromen-4-one) 50 μM 5, 25, 50, 80 µg/mL |
DU145 and PC-3 cells CAL-27 cells |
induction of apoptosis |
|
|
|
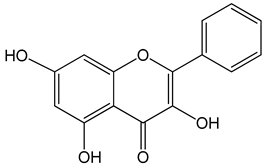
Galangin (3,5,7-trihydroxy-2-phenylchromen-4-one) 0–40 μM 0–40 μM 10, 20 and 30 mg/kg |
mice bearing B16F1 TU212, M4e, HBE, HEP-2 RTE, and HHL-5 cells BALB/c nude mice |
induction of apoptosis induction of apoptosis and inhibition of migration |
|
|
|
Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one) 0–120 μM |
LNCaP cells; mouse BALB/c 3T3 and SVT2 (SV40-transformed BALB/c 3T3) fibroblasts |
inhibition of cell cycle |
|
[3] |
|
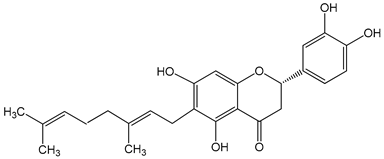
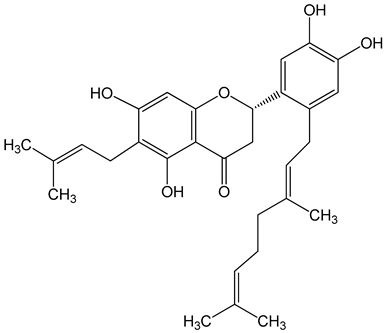
Nymphaeol A/Propolin C ((2S)-2-(3,4-dihydroxyphenyl)-6-[(2E)-3,7-dimethylocta-2,6-dienyl]-5,7-dihydroxy-2,3-dihydrochromen-4-one) 5–20 μM 2.5–20 μM |
A549 cells A549 and HCC827 cells |
anti-angiogenic activity, inhibition of proliferation inhibition of migration and invasion |
|
|
|
Nymphaeol C ((2S)-2-[2-[(2E)-3,7-dimethylocta-2,6-dienyl]-3,4-dihydroxyphenyl]-5,7-dihydroxy-6-(3-methylbut-2-enyl)-2,3-dihydrochromen-4-one) 5–20 μM |
anti-angiogenic activity, inhibition of proliferation |
|
[29] |
|
|
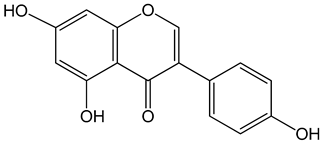
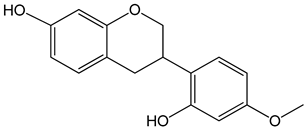
Vestitol (3-(2-hydroxy-4-methoxyphenyl)-3,4-dihydro-2H-chromen-7-ol) 0.37, 3.7, 37, and 370 μM |
HeLa cells |
cytotoxic effect |
|
[31] |
|
Aromatic acids and their derivatives |
||||
|
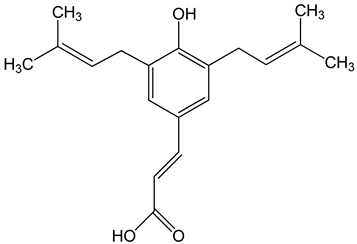
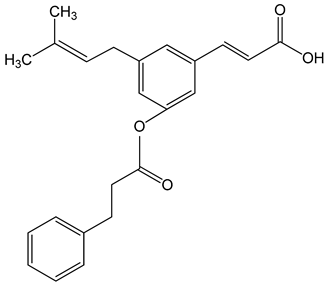
Artepillin C ((E)-3-[4-hydroxy-3,5-bis(3-methylbut-2-enyl)phenyl]prop-2-enoic acid) 250 μM 100 μg/mL 0–150 μM |
HT1080, A549, and U2OS cells BALB/c nude mice AGP-01 and HeLa cells CWR22Rv1 cells |
inhibition of proliferation cytotoxic effect autophagy inhibition |
|
|
|
Baccharin ((1R,3S,4S,6R,9R,13S,15R,16S,19R,20E,22Z,26R,27S,28S)-16-hydroxy-19-[(1R)-1-hydroxyethyl]-6,15,27-trimethylspiro [2,5,11,14,18,25-hexaoxahexacyclo [2 4.2.1.03,9.04,6.09,27.013,15]nonacosa-20,22-diene-28,2′-oxirane]-12,24-dione) 0–150 μM |
CWR22Rv1 cells |
autophagy inhibition |
|
[34] |
|
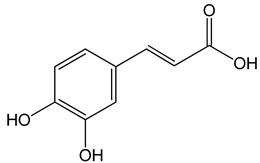
Caffeic acid ((E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid) 50 and 100 μM 65, 130, 190 µg/mL 30 μg/mL, 200 μg/mL 12.5 μM, 1 mM, 50 μM, 100 mg/kg, 20 mg/kg |
MDA-MB-231 cells CAL-27 cells Hep3, SK-Hep1, HepG2 cells |
cell cycle arrest in a dose- and time-dependent manner apoptosis activation inhibition of angiogenesis, apoptosis activation |
|
|
|
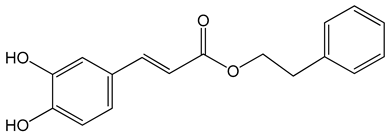
Caffeic acid phenylethyl ester (2-phenylethyl (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate) 0.005–0.1 mg/mL 0.5–500 µM 10 mg/kg/day 15 mg/kg |
AGS, HCT116, HT29, YD15, HSC-4, HN22, MCF-17, MDA-MB-231, MDA-MB-468, A549, HT1080, G361, U2OS, LNCaP, PC-3, DU145, Hep2, SAS, OECM-1, TW01, TW04, SW620, H460 and PANC-1 cells Balb/c nude mice BALB/c AnM-Foxn-1 mice |
inhibition of proliferation, migration and invasion, pro-apoptotic activity anti-metastatic activity |
|
|
|
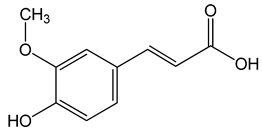
Ferulic acid ((E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoic acid) 50, 100, 150 µg/mL |
CAL-27 cells |
apoptosis activation |
|
[26] |
|
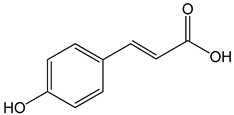
p-coumaric acid ((E)-3-(4-hydroxyphenyl)prop-2-enoic acid) 100 μg/mL 70, 140, 210 µg/mL |
AGP-01 and HeLa cells CAL-27 cells |
cytotoxic effect apoptosis activation |
|
|
|
Other |
||||
|
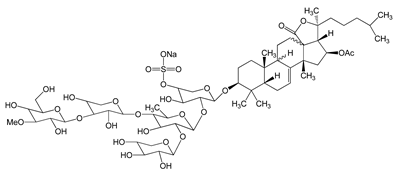
Frondoside A (sodium;[(3R,4R,5R,6S)-6-[(2S,4S,6S,12R,13R,18R)-4-acetyloxy-2,6,13,17,17-pentamethyl-6-(4-methylpentyl)-8-oxo-7-oxapentacyclo[10.8.0.02,9.05,9.013,18]icos-1(20)-en-16-yl]oxy]-5-[(2S,3R,4S,5S,6R)-5-[(2S,3R,4S,5R)-4-[(2S,3R,4S,5R,6R)-3,5-dihydroxy-6-(hydroxymethyl)-4-methoxyoxan-2-yl]oxy-3,5-dihydroxyoxan-2-yl]oxy-4-hydroxy-6-methyl-3-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxyoxan-2-yl]oxy-4-hydroxyoxan-3-yl] sulfate) 0.3–1.2 μM |
A549 cells |
anti-angiogenic activity, inhibition of proliferation |
|
[29] |
|
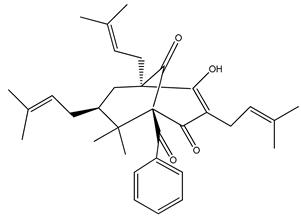
Nemorosone ((1R,5R,7S)-1-benzoyl-4-hydroxy-8,8-dimethyl-3,5,7-tris(3-methylbut-2-enyl)bicyclo[3.3.1]non-3-ene-2,9-dione) 5–50 μM |
HT-29 and THP-1 cells |
inhibition of migration and proliferation |
|
[46] |
3. The Use of Propolis and Its Components in Cancer Therapy
References
- Iqbal, M.; Fan, T.P.; Watson, D.; Alenezi, S.; Saleh, K.; Sahlan, M. Preliminary studies: The potential anti-angiogenic activities of two Sulawesi Island (Indonesia) propolis and their chemical characterization. Heliyon 2019, 5, e01978.
- Zabaiou, N.; Fouache, A.; Trousson, A.; Buñay-Noboa, J.; Marceau, G.; Sapin, V.; Zellagui, A.; Baron, S.; Lahouel, M.; Lobaccaro, J.M.A. Ethanolic extract of Algerian propolis decreases androgen receptor transcriptional activity in cultured LNCaP cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 108–115.
- Zabaiou, N.; Fouache, A.; Trousson, A.; Baron, S.; Zellagui, A.; Lahouel, M.; Lobaccaro, J.A. Biological properties of propolis extracts: Something new from an ancient product. Chem. Phys. Lipids 2017, 207, 214–222.
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.C.; Umsza-Guez, M.A.; Barbosa, J.D.V.; Portela, R.D.; Machado, B.A.S. Propolis: Types, composition, biological activities, and veterinary product patent prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382.
- Stojanović, S.; Najman, S.J.; Bogdanova-Popov, B.; Najman, S.S. Propolis: Chemical composition, biological and pharmacological activity—A Review. Acta Med. Median. 2020, 59, 108–113.
- Alday, E.; Valencia, D.; Garibay-Escobar, A.; Domínguez-Esquivel, Z.; Piccinelli, A.L.; Rastrelli, L.; Monribot-Villanueva, J.; Guerrero-Analco, J.A.; Robles-Zepeda, R.E.; Hernandez, J.; et al. Plant origin authentication of Sonoran Desert propolis: An antiproliferative propolis from a semi-arid region. Sci. Nat. 2019, 106, 25.
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs. human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174.
- Doğan, H.; Silici, S.; Ozcimen, A.A. Biological Effects of Propolis on Cancer. Turk. J. Agric. Food Sci. Technol. 2020, 8, 573.
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159.
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047.
- Martinello, M.; Mutinelli, F. Antioxidant activity in bee products: A review. Antioxidants 2021, 10, 71.
- Ripari, N.; Sartori, A.A.; da Silva Honorio, M.; Conte, F.L.; Tasca, K.I.; Santiago, K.B.; Sforcin, J.M. Propolis antiviral and immunomodulatory activity: A review and perspectives for COVID-19 treatment. J. Pharm. Pharmacol. 2021, 73, 281–299.
- Franchin, M.; Freires, I.A.; Lazarini, J.G.; Nani, B.D.; da Cunha, M.G.; Colón, D.F.; de Alencar, S.M.; Rosalen, P.L. The use of Brazilian propolis for discovery and development of novel anti-inflammatory drugs. Eur. J. Med. Chem. 2018, 153, 49–55.
- de Mendonça, I.C.G.; de Moraes Porto, I.C.C.; do Nascimento, T.G.; de Souza, N.S.; dos Santos Oliveira, J.M.; dos Santos Arruda, R.E.; Mousinho, K.C.; dos Santos, A.F.; Basílio-Júnior, I.D.; Parolia, A.; et al. Brazilian red propolis: Phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complement. Altern. Med. 2015, 15, 357.
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703.
- Zulhendri, F.; Felitti, R.; Fearnley, J.; Ravalia, M. The use of propolis in dentistry, oral health, and medicine: A review. J. Oral Biosci. 2021.
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The ethanol extract of polish propolis exhibits anti-proliferative and/or pro-apoptotic effect on HCT 116 colon cancer and Me45 Malignant melanoma cells in vitro conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212.
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant potential of propolis, bee pollen, and royal jelly: Possible medical application. Oxid. Med. Cell. Longev. 2018, 2018.
- Mora, D.P.P.; Santiago, K.B.; Conti, B.J.; de Oliveira Cardoso, E.; Conte, F.L.; Oliveira, L.P.G.; de Assis Golim, M.; Uribe, J.F.C.; Gutiérrez, R.M.; Buitrago, M.R.; et al. The chemical composition and events related to the cytotoxic effects of propolis on osteosarcoma cells: A comparative assessment of Colombian samples. Phyther. Res. 2019, 33, 591–601.
- De Oliveira Reis, J.H.; de Abreu Barreto, G.; Cerqueira, J.C.; dos Anjos, J.P.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; MacHado, B.A.S. Evaluation of the antioxidant profile and cytotoxic activity of red propolis extracts from different regions of northeastern Brazil obtained by conventional and ultrasoundassisted extraction. PLoS ONE 2019, 14, e0219063.
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical composition and antioxidant activity of propolis prepared in different forms and in different solvents useful for finished products. Foods 2018, 7, 41.
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; Cunha, S.; Danert, C.; Eberlin, M.N.; Falcão, I.; Isla, M.I.; Inés, M.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2019, 8839, 1–49.
- dos Santos, D.A.; Munari, F.M.; da Silva Frozza, C.O.; Moura, S.; Barcellos, T.; Henriques, J.A.P.; Roesch-Ely, M. Brazilian red propolis extracts: Study of chemical composition by ESI-MS/MS (ESI+) and cytotoxic profiles against colon cancer cell lines. Biotechnol. Res. Innov. 2019, 3, 120–130.
- Noureddine, H.; Hage-Sleiman, R.; Wehbi, B.; Fayyad-Kazan, A.H.; Hayar, S.; Traboulssi, M.; Alyamani, O.A.; Faour, W.H.; ElMakhour, Y. Chemical characterization and cytotoxic activity evaluation of Lebanese propolis. Biomed. Pharmacother. 2017, 95, 298–307.
- Ryu, S.; Lim, W.; Bazer, F.W.; Song, G. Chrysin induces death of prostate cancer cells by inducing ROS and ER stress. J. Cell. Physiol. 2017, 232, 3786–3797.
- Celinska-Janowicz, K.; Zareba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Palka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336.
- Benguedouar, L.; Lahouel, M.; Gangloff, S.C.; Durlach, A.; Grange, F.; Bernard, P.; Antonicelli, F. Ethanolic extract of Algerian propolis and galangin decreased murine melanoma tumor progression in mice. Anti Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2016, 16, 1172–1183.
- Wang, H.X.; Tang, C. Galangin suppresses human laryngeal carcinoma via modulation of caspase-3 and AKT signaling pathways. Oncol. Rep. 2017, 38, 703–714.
- Nguyen, H.X.; Nguyen, M.T.T.; Nguyen, N.T.; Awale, S. Chemical constituents of propolis from Vietnamese Trigona minor and Their antiausterity activity against the PANC-1 human pancreatic cancer cell line. J. Nat. Prod. 2017, 80, 2345–2352.
- Pai, J.T.; Lee, Y.C.; Chen, S.Y.; Leu, Y.L.; Weng, M.S. Propolin C inhibited migration and invasion via suppression of EGFR-mediated epithelial-to-mesenchymal transition in human lung cancer cells. Evid. Based Complement. Altern. Med. 2018, 2018, 7202548.
- Nani, B.D.; Franchin, M.; Lazarini, J.G.; Freires, I.A.; da Cunha, M.G.; Bueno-Silva, B.; de Alencar, S.M.; Murata, R.M.; Rosalen, P.L. Isoflavonoids from Brazilian red propolis down-regulate the expression of cancer-related target proteins: A pharmacogenomic analysis. Phytother. Res. 2018, 32, 750–754.
- Bhargava, P.; Grover, A.; Nigam, N.; Kaul, A.; Doi, M.; Ishida, Y.; Kakuta, H.; Kaul, S.C.; Terao, K.; Wadhwa, R. Anticancer activity of the supercritical extract of Brazilian green propolis and its active component, artepillin C: Bioinformatics and experimental analyses of its mechanisms of action. Int. J. Oncol. 2018, 52, 925–932.
- Arruda, C.; Ribeiro, V.P.; Mejía, J.A.A.; Almeida, M.O.; Goulart, M.O.; Candido, A.C.B.B.; dos Santos, R.A.; Magalhães, L.G.; Martins, C.H.G.; Bastos, J.K. Green propolis: Cytotoxic and leishmanicidal activities of artepillin C, p-Coumaric Acid, and their degradation products. Rev. Bras. Farmacogn. 2020, 30, 169–176.
- Endo, S.; Hoshi, M.; Matsunaga, T.; Inoue, T.; Ichihara, K.; Ikari, A. Autophagy inhibition enhances anticancer efficacy of artepillin C, a cinnamic acid derivative in Brazilian green propolis. Biochem. Biophys. Res. Commun. 2018, 497, 437–443.
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzebska-Stojko, Z.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two components of propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules 2017, 22, 1554.
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 3–5.
- Ren, X.; Liu, J.; Hu, L.; Liu, Q.; Wang, D.; Ning, X. Caffeic acid phenethyl ester inhibits the proliferation of HEp2 cells by regulating Stat3/Plk1 pathway and inducing S phase arrest. Biol. Pharm. Bull. 2019, 42, 1689–1693.
- Gajek, G.; Marciniak, B.; Lewkowski, J.; Kontek, R. Antagonistic effects of CAPE (a Component of Propolis) on the cytotoxicity and genotoxicity of irinotecan and SN38 in Human gastrointestinal cancer cells in vitro. Molecules 2020, 25, 658.
- Yu, H.J.; Shin, J.A.; Yang, I.H.; Won, D.H.; Ahn, C.H.; Kwon, H.J.; Lee, J.S.; Cho, N.P.; Kim, E.C.; Yoon, H.J.; et al. Apoptosis induced by caffeic acid phenethyl ester in human oral cancer cell lines: Involvement of Puma and Bax activation. Arch. Oral Biol. 2017, 84, 94–99.
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular characterization and enhancement of anticancer activity of caffeic acid phenethyl ester by γ cyclodextrin. J. Cancer 2016, 7, 1755–1771.
- Tseng, J.C.; Lin, C.Y.; Su, L.C.; Fu, H.H.; Der Yang, S.; Chuu, C.P. CAPE suppresses migration and invasion of prostate cancer cells via activation of non-canonical Wnt signaling. Oncotarget 2016, 7, 38010–38024.
- Chung, L.C.; Chiang, K.C.; Feng, T.H.; Chang, K.S.; Chuang, S.T.; Chen, Y.J.; Tsui, K.H.; Lee, J.C.; Juang, H.H. Caffeic acid phenethyl ester upregulates N-myc downstream regulated gene 1 via ERK pathway to inhibit human oral cancer cell growth in vitro and in vivo. Mol. Nutr. Food Res. 2017, 61, 1–30.
- Chiang, K.C.; Yang, S.W.; Chang, K.P.; Feng, T.H.; Chang, K.S.; Tsui, K.H.; Shin, Y.S.; Chen, C.C.; Chao, M.; Juang, H.H. Caffeic acid phenethyl ester induces N-myc downstream regulated gene 1 to inhibit cell proliferation and invasion of human nasopharyngeal cancer cells. Int. J. Mol. Sci. 2018, 19, 1397.
- Fraser, S.P.; Hemsley, F.; Djamgoz, M.B.A. Caffeic acid phenethyl ester: Inhibition of metastatic cell behaviours via voltage-gated sodium channel in human breast cancer in vitro. Int. J. Biochem. Cell Biol. 2016, 71, 111–118.
- Duan, J.; Xiaokaiti, Y.; Fan, S.; Pan, Y.; Li, X.; Li, X. Direct interaction between caffeic acid phenethyl ester and human neutrophil elastase inhibits the growth and migration of PANC-1 cells. Oncol. Rep. 2017, 37, 3019–3025.
- Frión-Herrera, Y.; Gabbia, D.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Nemorosone inhibits the proliferation and migration of hepatocellular carcinoma cells. Life Sci. 2019, 235, 116817.
- De Giffoni De Carvalho, J.T.; Da Silva Baldivia, D.; Leite, D.F.; De Araújo, L.C.A.; De Toledo Espindola, P.P.; Antunes, K.A.; Rocha, P.S.; De Picoli Souza, K.; Dos Santos, E.L. Medicinal plants from Brazilian Cerrado: Antioxidant and anticancer potential and protection against chemotherapy toxicity. Oxid. Med. Cell. Longev. 2019, 2019.
- Motawi, T.K.; Abdelazim, S.A.; Darwish, H.A.; Elbaz, E.M.; Shouman, S.A. Modulation of Tamoxifen Cytotoxicity by Caffeic Acid Phenethyl Ester in MCF-7 Breast Cancer Cells. Oxid. Med. Cell. Longev. 2016, 2016.
- Sameni, H.R.; Yosefi, S.; Alipour, M.; Pakdel, A.; Torabizadeh, N.; Semnani, V.; Bandegi, A.R. Co-administration of 5FU and propolis on AOM/DSS induced colorectal cancer in BALB-c mice. Life Sci. 2021, 276, 119390.
- Wang, C.C.; Wang, Y.X.; Yu, N.Q.; Hu, D.; Wang, X.Y.; Chen, X.G.; Liao, Y.W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian green propolis extract synergizes with protoporphyrin IX-mediated photodynamic therapy via enhancement of intracellular accumulation of protoporphyrin IX and attenuation of NF-κB and COX-2. Molecules 2017, 22, 732.
- Darvishi, N.; Yousefinejad, V.; Akbari, M.E.; Abdi, M.; Moradi, N.; Darvishi, S.; Mehrabi, Y.; Ghaderi, E.; Jamshidi-Naaeini, Y.; Ghaderi, B.; et al. Antioxidant and anti-inflammatory effects of oral propolis in patients with breast cancer treated with chemotherapy: A Randomized controlled trial. J. Herb. Med. 2020, 23, 100385.
- Ebeid, S.A.; Abd El Moneim, N.A.; El-Benhawy, S.A.; Hussain, N.G.; Hussain, M.I. Assessment of the radioprotective effect of propolis in breast cancer patients undergoing radiotherapy. New perspective for an old honey bee product. J. Radiat. Res. Appl. Sci. 2016, 9, 431–440.
- Kuo, C.C.; Wang, R.H.; Wang, H.H.; Li, C.H. Meta-analysis of randomized controlled trials of the efficacy of propolis mouthwash in cancer therapy-induced oral mucositis. Support. Care Cancer 2018, 26, 4001–4009.
- Piredda, M.; Facchinetti, G.; Biagioli, V.; Giannarelli, D.; Armento, G.; Tonini, G.; De Marinis, M.G. Propolis in the prevention of oral mucositis in breast cancer patients receiving adjuvant chemotherapy: A pilot randomised controlled trial. Eur. J. Cancer Care 2017, 26.
- Akhavan-Karbassi, M.H.; Yazdi, M.F.; Ahadian, H.; Sadr-Abad, M.J. Randomized double-blind placebo-controlled trial of propolis for oral mucositis in patients receiving chemotherapy for head and neck cancer. Asian Pac. J. Cancer Prev. 2016, 17, 3611–3614.
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.R.; Prasad, N.R. Phytochemicals reverse P-glycoprotein mediated multidrug resistance via signal transduction pathways. Biomed. Pharmacother. 2021, 139, 111632.
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348.
- Sritharan, S.; Sivalingam, N. A comprehensive review on time-tested anticancer drug doxorubicin. Life Sci. 2021, 278, 119527.
- Frión-Herrera, Y.; Gabbia, D.; Díaz-García, A.; Cuesta-Rubio, O.; Carrara, M. Chemosensitizing activity of Cuban propolis and nemorosone in doxorubicin resistant human colon carcinoma cells. Fitoterapia 2019, 136, 104173.
- Kebsa, W.; Lahouel, M.; Rouibah, H.; Zihlif, M.; Ahram, M.; Abu-Irmaileh, B.; Mustafa, E.; Al-Ameer, H.J.; Al Shhab, M. Reversing Multidrug Resistance in Chemo-resistant Human Lung Adenocarcinoma (A549/DOX) Cells by Algerian Propolis Through Direct Inhibiting the P-gp Efflux-pump, G0/G1 Cell Cycle Arrest and Apoptosis Induction. Anticancer Agents Med. Chem. 2018, 18, 1330–1337.
- Banzato, T.P.; Gubiani, J.R.; Bernardi, D.I.; Nogueira, C.R.; Monteiro, A.F.; Juliano, F.F.; De Alencar, S.M.; Pilli, R.A.; De Lima, C.A.D.; Longato, G.B.; et al. Antiproliferative Flavanoid Dimers Isolated from Brazilian Red Propolis. J. Nat. Prod. 2020, 83, 1784–1793.
- Nyman, G.; Oldberg Wagner, S.; Prystupa-Chalkidis, K.; Ryberg, K.; Hagvall, L. Contact allergy in western sweden to propolis of four different origins. Acta Derm. Venereol. 2020, 100, 1–5.
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid. Based Complement. Altern. Med. 2013, 2013.