
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | András Gy. Németh | + 1168 word(s) | 1168 | 2021-09-13 08:11:35 | | | |
| 2 | Vivi Li | -2 word(s) | 1166 | 2021-09-28 03:56:20 | | |
Video Upload Options
Isothiocyanates (ITCs) are biologically active molecules found in several natural products and pharmaceutical ingredients. Moreover, due to their high and versatile reactivity, they are widely used as intermediates in organic synthesis.This review considers the best practices for the synthesis of ITCs using elemental sulfur, highlighting recent developments. Additionally, we also reveal that in the catalyst‑free reaction of isocyanides and sulfur, two—until this time overlooked and not investigated—different mechanistic pathways exist.
1. Introduction
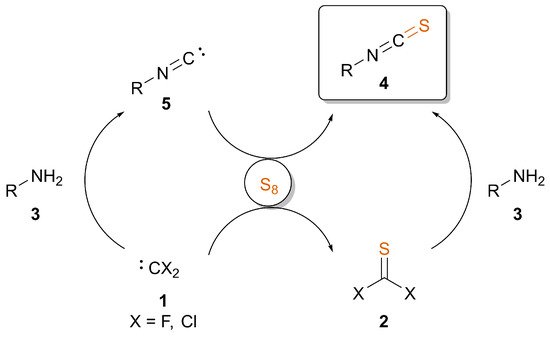
2. Overview and Practical Considerations of the Discussed Methods
Table 1 provides a comparison between the discussed synthetic approaches starting from amines or isocyanides with sulfur. When designing a multistep synthesis plan, depending on the stability of the substrate, one should consider the nature of additives, solvent, temperature and inert conditions if necessary. Generally, reactions involving difluorocarbene or thiocarbonyl fluoride require inert conditions, while isocyanide can be transformed to ITC under less strict conditions. The modification of amines is most effective using PDFA, but in the case of sensible compounds, one may turn to the room temperature approach involving F3CSiMe3 as a carbene source. The presence of potassium fluoride, however, may result in the removal of silyl groups on a complex structure, and a copper catalyst might lead to side coupling reactions and waste containing transition metals. Selenium and tellurium should be handled with care due to toxicity, while Mo or Rh catalysts increase the price and, again, transition metals in the waste. ITC formation from isocyanides, on the other hand, is very effective in the presence of bases. This approach can be performed in a relatively short reaction time compared to the transition metal-catalyzed pathways, even under aqueous conditions. Based on the scope of substrates in the reported methods, one may note that all approaches provide ITCs in good to excellent yields. Challenging derivatives might be trityl ITC, generally obtained in lower yields, presumably because of steric hindrance, and low-molecular weight aliphatic ITCs, such as tert-butyl ITC due to its volatile nature.
Table 1. Summary of methods for ITC synthesis with the application of elemental sulfur
| Ref. | Starting Material |
Additive | Inert Atmosphere |
Solvent | T (°C) |
T (h) |
Yield (%) |
|---|---|---|---|---|---|---|---|
| [48] | Amine | PDFA | Yes | DME | 80 | 0.083 | 21–97 |
| [49] | F3CSiMe3 + KF | Yes | THF | rt | 1–12 | 31–96 | |
| [50] | BrF2CCOOK + 5 mol% CuI, K3PO4 | No | MeCN | 100 | 12 | 38–87 | |
| [51][52] | Isocyanide | 5 mol% Se or 0.02 mol% Te | No | THF | reflux | 0.5–8 | 53–99 |
| [53][54] | “Mo” (X) | No | acetone | reflux | 72 | 61–93 | |
| [55] | “Rh” (X) | Yes | acetone | reflux | 1.5–8 | 83–96 | |
| [42] | 2 eq. NaH | Yes | THF | 40 | 2 | 85 | |
| [41] | 2–5 mol% DBU | No | CyreneTM or GBL |
40 | 4–24 | 34–95 |
3. Conclusions and Outlook
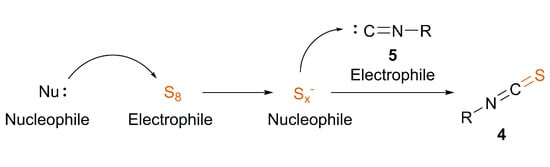
ITCs are a biologically and synthetically relevant functional group, being present in important metabolites, natural products and synthetic intermediates. Their efficient and clean synthesis is of high interest, leading to the appearance of several recent methods. In particular, there are two strategies involving elemental sulfur for the incorporation of the sulfur atom, offering practical and modern approaches. The in situ generation of thiocarbonyl fluoride from difluorocarbene and sulfur provides ITCs with primary amines, or sulfuration of isocyanides may directly lead to ITCs under thermal-, catalytic- or nucleophile-induced conditions. Based on previous literature data and our recent results, we highlighted mechanistic insights into the latter transformation. Besides the conventional nucleophilic carbene and electrophilic sulfur setup, a switched mechanism is also proposed, where the polysulfide anions activated by a nucleophile are able to transform the isocyanide to ITC. This approach offers an efficient, mild and green synthesis of ITCs. We expect that this spotlight on ITC synthesis revealing different mechanistic pathways will inspire further research in the field and open up novel synthetic methodologies due to a deeper understanding.
References
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512.
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020, 10, 1614.
- Gupta, P.; Kim, B.; Kim, S.H.; Srivastava, S.K. Molecular targets of isothiocyanates in cancer: Recent advances. Mol. Nutr. Food Res. 2014, 58, 1685–1707.
- Wang, N.; Saidhareddy, P.; Jiang, X. Construction of sulfur-containing moieties in the total synthesis of natural products. Nat. Prod. Rep. 2020, 37, 246–275.
- Hanschen, F.S.; Lamy, E.; Schreiner, M.; Rohn, S. Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angew. Chem. Int. Ed. 2014, 53, 11430–11450.
- Kala, C.; Ali, S.S.; Ahmad, N.; Gilani, S.J.; Khan, N.A. Isothiocyanates: A Review Chandra. Res. J. Pharmacogn. 2018, 5, 71–89.
- Sugiyama, R.; Li, R.; Kuwahara, A.; Nakabayashi, R.; Sotta, N.; Mori, T. Retrograde sulfur flow from glucosinolates to cysteine in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2021, 118, e2017890118.
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013, 2013, 415078.
- Lawson, A.P.; Long, M.J.C.; Coffey, R.T.; Qian, Y.; Weerapana, E.; El Oualid, F.; Hedstrom, L. Naturally occurring isothiocyanates exert anticancer effects by inhibiting deubiquitinating enzymes. Cancer Res. 2015, 75, 5130–5142.
- Dufour, V.; Stahl, M.; Baysse, C. The antibacterial properties of isothiocyanates. Microbiology 2015, 161, 229–243.
- Petri, L.; Szijj, P.A.; Kelemen, Á.; Imre, T.; Gömöry, Á.; Lee, M.T.W.; Hegedus, K.; Ábrányi-Balogh, P.; Chudasama, V.; Keseru, G.M. Cysteine specific bioconjugation with benzyl isothiocyanates. RSC Adv. 2020, 10, 14928–14936.
- Abdeldayem, A.; Raouf, Y.S.; Constantinescu, S.N.; Moriggl, R.; Gunning, P.T. Advances in covalent kinase inhibitors. Chem. Soc. Rev. 2020, 49, 2617–2687.
- Kulkarni, P.M.; Kulkarni, A.R.; Korde, A.; Tichkule, R.B.; Laprairie, R.B.; Denovan-Wright, E.M.; Zhou, H.; Janero, D.R.; Zvonok, N.; Makriyannis, A.; et al. Novel Electrophilic and Photoaffinity Covalent Probes for Mapping the Cannabinoid 1 Receptor Allosteric Site(s). J. Med. Chem. 2016, 59, 44–60.
- Tamura, T.; Hamachi, I. Chemistry for Covalent Modification of Endogenous/Native Proteins: From Test Tubes to Complex Biological Systems. J. Am. Chem. Soc. 2019, 141, 2782–2799.
- Allen, A.D.; Tidwell, T.T. Ketenes and other cumulenes as reactive intermediates. Chem. Rev. 2013, 113, 7287–7342.
- Mukerjee, A.K.; Ashare, R. Isothiocyanates in the Chemistry of Heterocycles. Chem. Rev. 1991, 91, 1–24.
- Norris, B.C.; Bielawski, C.W. Structurally dynamic materials based on bis(N-heterocyclic carbene)s and bis(isothiocyanate)s: Toward reversible, conjugated polymers. Macromolecules 2010, 43, 3591–3593.
- Janczewski, Ł.; Gajda, A.; Gajda, T. Direct, Microwave-Assisted Synthesis of Isothiocyanates. Eur. J. Org. Chem. 2019, 2019, 2528–2532.
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119.
- Sun, N.; Li, B.; Shao, J.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. A general and facile one-pot process of isothiocyanates from amines under aqueous conditions. Beilstein J. Org. Chem. 2012, 8, 61–70.
- Fu, Z.; Yuan, W.; Chen, N.; Yang, Z.; Xu, J. Na2S2O8-mediated efficient synthesis of isothiocyanates from primary amines in water. Green Chem. 2018, 20, 4484–4491.
- Bassetto, M.; Ferla, S.; Pertusati, F.; Kandil, S.; Westwell, A.D.; Brancale, A.; Mcguigan, C. Design and synthesis of novel bicalutamide and enzalutamide derivatives as antiproliferative agents for the treatment of prostate cancer. Eur. J. Med. Chem. 2016, 118, 230–243.
- Kim, S.; Yi, K.Y. Di-2-pyridyl thionocarbonate. A new reagent for the preparation of isothiocyanates and carbodiimides. Tetrahedron Lett. 1985, 26, 1661–1664.
- Larsen, C.; Steliou, K.; Harpp, D.N. Thiocarbonyl Transfer Reagents. J. Org. Chem. 1978, 43, 337–339.
- Wong, R.; Dolman, S.J. Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts. J. Org. Chem. 2007, 72, 3969–3971.
- Nath, J.; Ghosh, H.; Yella, R.; Patel, B.K. Molecular Iodine Mediated Preparation of Isothiocyanates from Dithiocarbamic Acid Salts. Eur. J. Org. Chem. 2009, 1849–1851.
- Zhang, X.; Lee, Y.K.; Kelley, J.A.; Burke, T.R. Preparation of Aryl Isothiocyanates via Protected Phenylthiocarbamates and Application to the Synthesis of Caffeic Acid (4-Isothiocyanato) phenyl Ester Isothiocyanates have been widely used in organic thetic isothiocyanates have been reported to exhibit. J. Org. Chem. 2000, 65, 6237–6240.
- Li, Z.; Ma, H.; Han, C.; Xi, H.; Meng, Q.; Chen, X.; Sun, X. Synthesis of Isothiocyanates by Reaction of Amines with Phenyl Chlorothiono- formate via One-Pot or Two-Step Process. Synthesis 2013, 45, 1667–1674.
- Rong, H.J.; Chen, T.; Xu, Z.G.; Su, T.D.; Shang, Y.; Wang, Y.Q.; Yang, C.F. 4-Dimethylaminopyridine-catalyzed synthesis of isothiocyanates from amines and carbon disulfide. Tetrahedron Lett. 2021, 68, 152868.
- Reagent, D.; Janczewski, Ł.; Kreigel, D. Synthesis of Isothiocyanates Using DMT/NMM/TsO—As a New Desulfurization Reagent. Molecules 2021, 26, 2740.
- Baumann, M.; Baxendale, I.R. The rapid generation of isothiocyanates in flow. Beilstein J. Org. Chem. 2013, 9, 1613–1619.
- Tanaka, S.; Uemura, S.; Okano, M. The Thallium(I) Salt-catalyzed Formation of Isothiocyanates form Isocyanides and Disulfides. Bull. Chem. Soc. Jpn. 1977, 50, 2785–2788.
- Eschliman, K.; Bossmann, S.H. Synthesis of Isothiocyanates: An Update. Synthesis 2019, 51, 1746–1752.
- Boyer, J.H.; Ramakrishnan, V.T. Sulfurization of Isocyanides. J. Org. Chem. 1972, 37, 1360–1364.
- Reisfen, M. Zur Reaktion von Amidacetalen rnit Heterocumulenen. Chem. Ber. 1977, 110, 37–48.
- Cunico, R.F.; Maity, B.C. Direct Carbamoylation of Alkenyl Halides. Org. Lett. 2003, 5, 4947–4949.
- Huang, J.; Schanz, H.J.; Stevens, E.D.; Nolan, S.P.; Capps, K.B.; Bauer, A.; Hoff, C.D. Structural and solution calorimetric studies of sulfur binding to nucleophilic carbenes. Inorg. Chem. 2000, 39, 1042–1045.
- Sharma, S. Isothiocyanates in Heterocyclic Synthesis. Sulf. Rep. 1989, 8, 327–454.
- Kowaoka, Y. Studies of Rubber Vulcanization Accelerators, V. J. Soc. Chem. Ind. Jpn. Suppl. 1940, 43, 53–57.
- Davis, R.E. Nucleophilic Displacement Reactions at the Sulfur-Sulfur Bond. In Survey of Progress in Chemistry: Volume 2; Scott, A.F., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1964; Volume 2, pp. 189–238.
- Nickisch, R.; Conen, P.; Gabrielsen, S.M.; Meier, M.A.R. A more sustainable isothiocyanate synthesis by amine catalyzed sulfurization of isocyanides with elemental sulfur. RSC Adv. 2021, 11, 3134–3142.
- Németh, A.G.; Keserű, G.M.; Ábrányi-Balogh, P. A novel three-component reaction between isocyanides, alcohols or thiols and elemental sulfur: A mild, catalyst-free approach towards O -thiocarbamates and dithiocarbamates. Beilstein J. Org. Chem. 2019, 15, 1523–1533.
- Németh, A.G.; Szabó, R.; Domján, A.; Keserű, G.M.; Ábrányi-Balogh, P. Chromatography-free multicomponent synthesis of thioureas enabled by aqueous solution of elemental sulfur. ChemistryOpen 2020, 10, 16–27.
- Németh, A.G.; Szabó, R.; Orsy, G.; Mándity, I.M.; Keserű, G.M.; Ábrányi-Balogh, P. Continuous-Flow Synthesis of Thioureas, Enabled by Aqueous Polysulfide Solution. Molecules 2021, 26, 303.
- Németh, A.G.; Marlok, B.; Domján, A.; Gao, Q.; Han, X.; Keserű, G.M.; Ábrányi-Balogh, P. Convenient multicomponent one-pot synthesis of 2-iminothiazolines and 2-aminothiazoles using elemental sulfur under aqueous conditions. Eur. J. Org. Chem. 2021, 28–33.
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Three-component reaction between isocyanides, aliphatic amines and elemental sulfur: Preparation of thioureas under mild conditions with complete atom economy. Synthesis 2014, 46, 3172–3179.
- Steudel, R.; Chivers, T. The role of polysulfide dianions and radical anions in the chemical, physical and biological sciences, including sulfur-based batteries. Chem. Soc. Rev. 2019, 48, 3279–3319.
- Yu, J.; Lin, J.H.; Xiao, J.C. Reaction of Thiocarbonyl Fluoride Generated from Difluorocarbene with Amines. Angew. Chem. Int. Ed. 2017, 56, 16669–16673.
- Zhen, L.; Fan, H.; Wang, X.; Jiang, L. Synthesis of thiocarbamoyl fluorides and isothiocyanates using CF3SiMe3 and elemental sulfur or AgSCF3 and KBr with amines. Org. Lett. 2019, 21, 2106–2110.
- Feng, W.; Zhang, X.G. Organophosphine-free copper-catalyzed isothiocyanation of amines with sodium bromodifluoroacetate and sulfur. Chem. Commun. 2019, 55, 1144–1147.
- Fujiwara, S.; Shin-Ike, T.; Sonoda, N.; Aoki, M.; Okada, K.; Miyoshi, N.; Kambe, N. Novel selenium catalyzed synthesis of isothiocyanates from isocyanides and elemental sulfur. Tetrahedron Lett. 1991, 32, 3503–3506.
- Fujiwara, S.; Shin-Ike, T.; Okada, K.; Aoki, M.; Kambe, N.; Sonoda, N. A marvelous catalysis of tellurium in the formation of isothiocyanates from isocyanides and sulfur. Tetrahedron Lett. 1992, 33, 7021–7024.
- Adam, W.; Bargon, R.M.; Bosio, S.G.; Schenk, W.A.; Stalke, D. Direct Synthesis of Isothiocyanates from Isonitriles by Molybdenum-Catalyzed Sulfur Transfer with Elemental Sulfur. J. Org. Chem. 2002, 67, 7037–7041.
- Farrell, W.S.; Zavalij, P.Y.; Sita, L.R. Catalytic Production of Isothiocyanates via a Mo(II)/Mo(IV) Cycle for the “Soft” Sulfur Oxidation of Isonitriles. Organometallics 2016, 35, 2361–2366.
- Arisawa, M.; Ashikawa, M.; Suwa, A.; Yamaguchi, M. Rhodium-catalyzed synthesis of isothiocyanate from isonitrile and sulfur. Tetrahedron Lett. 2005, 46, 1727–1729.




