Isothiocyanates (ITCs) are biologically active molecules found in several natural products and pharmaceutical ingredients. Moreover, due to their high and versatile reactivity, they are widely used as intermediates in organic synthesis.This review considers the best practices for the synthesis of ITCs using elemental sulfur, highlighting recent developments. Additionally, we also reveal that in the catalyst‑free reaction of isocyanides and sulfur, two—until this time overlooked and not investigated—different mechanistic pathways exist.
- isothiocyanates
- elemental sulfur
- carbenes
- isocyanides
Introduction
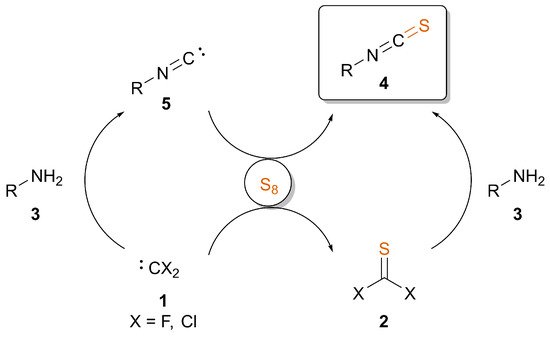
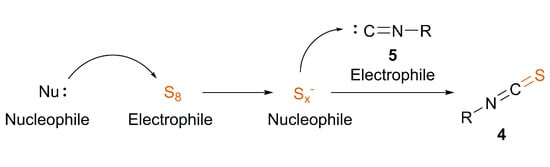
Overview and Practical Considerations of the Discussed Methods
Table 1 provides a comparison between the discussed synthetic approaches starting from amines or isocyanides with sulfur. When designing a multistep synthesis plan, depending on the stability of the substrate, one should consider the nature of additives, solvent, temperature and inert conditions if necessary. Generally, reactions involving difluorocarbene or thiocarbonyl fluoride require inert conditions, while isocyanide can be transformed to ITC under less strict conditions. The modification of amines is most effective using PDFA, but in the case of sensible compounds, one may turn to the room temperature approach involving F3CSiMe3 as a carbene source. The presence of potassium fluoride, however, may result in the removal of silyl groups on a complex structure, and a copper catalyst might lead to side coupling reactions and waste containing transition metals. Selenium and tellurium should be handled with care due to toxicity, while Mo or Rh catalysts increase the price and, again, transition metals in the waste. ITC formation from isocyanides, on the other hand, is very effective in the presence of bases. This approach can be performed in a relatively short reaction time compared to the transition metal-catalyzed pathways, even under aqueous conditions. Based on the scope of substrates in the reported methods, one may note that all approaches provide ITCs in good to excellent yields. Challenging derivatives might be trityl ITC, generally obtained in lower yields, presumably because of steric hindrance, and low-molecular weight aliphatic ITCs, such as tert-butyl ITC due to its volatile nature.
Table 1. Summary of methods for ITC synthesis with the application of elemental sulfur
|
Ref. |
Starting Material |
Additive |
Atmosphere |
Solvent |
T (°C) |
T (h) |
Yield (%) |
|
[56] |
Amine |
PDFA |
Yes |
DME |
80 |
0.083 |
21–97 |
|
[57] |
F3CSiMe3 + KF |
Yes |
THF |
rt |
1–12 |
31–96 |
|
|
[62] |
BrF2CCOOK + 5 mol% CuI, K3PO4 |
No |
MeCN |
100 |
12 |
38–87 |
|
|
[68,69] |
Isocyanide |
5 mol% Se or 0.02 mol% Te |
No |
THF |
reflux |
0.5–8 |
53–99 |
|
[70,71] |
“Mo” (X) |
No |
acetone |
reflux |
72 |
61–93 |
|
|
[72] |
“Rh” (X) |
Yes |
acetone |
reflux |
1.5–8 |
83–96 |
|
|
[42] |
2 eq. NaH |
Yes |
THF |
40 |
2 |
85 |
|
|
[41] |
2–5 mol% DBU |
No |
CyreneTM or GBL |
40 |
4–24 |
34–95 |
Conclusions and Outlook
ITCs are a biologically and synthetically relevant functional group, being present in important metabolites, natural products and synthetic intermediates. Their efficient and clean synthesis is of high interest, leading to the appearance of several recent methods. In particular, there are two strategies involving elemental sulfur for the incorporation of the sulfur atom, offering practical and modern approaches. The in situ generation of thiocarbonyl fluoride from difluorocarbene and sulfur provides ITCs with primary amines, or sulfuration of isocyanides may directly lead to ITCs under thermal-, catalytic- or nucleophile-induced conditions. Based on previous literature data and our recent results, we highlighted mechanistic insights into the latter transformation. Besides the conventional nucleophilic carbene and electrophilic sulfur setup, a switched mechanism is also proposed, where the polysulfide anions activated by a nucleophile are able to transform the isocyanide to ITC. This approach offers an efficient, mild and green synthesis of ITCs. We expect that this spotlight on ITC synthesis revealing different mechanistic pathways will inspire further research in the field and open up novel synthetic methodologies due to a deeper understanding.
This entry is adapted from the peer-reviewed paper 10.3390/catal11091081
