
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md Abdul Hannan | + 2288 word(s) | 2288 | 2021-06-10 11:32:55 | | | |
| 2 | Lily Guo | + 1933 word(s) | 4221 | 2021-06-11 03:18:01 | | |
Video Upload Options
Black cumin (Nigella sativa L.), a highly valued nutraceutical herb with a wide array of health benefits, has attracted growing interest from health-conscious individuals, the scientific community, and pharmaceutical industries. The pleiotropic pharmacological effects of black cumin, and its main bioactive component thymoquinone (TQ), have been manifested by their ability to attenuate oxidative stress and inflammation, and to promote immunity, cell survival, and energy metabolism, which underlie diverse health benefits, including protection against metabolic, cardiovascular, digestive, hepatic, renal, respiratory, reproductive, and neurological disorders, cancer, and so on. Furthermore, black cumin acts as an antidote, mitigating various toxicities and drug-induced side effects.
1. Introduction
2. Phytochemical Profiles
3. Benefits of Black Cumin on Human Health and Disease Conditions
3.1. Antioxidant Effects
3.2. Anti-Inflammatory Effects
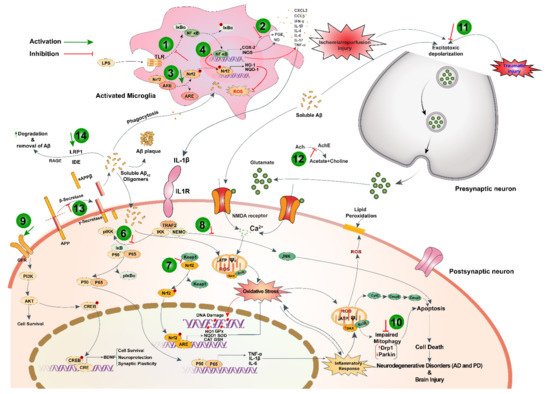
3.3 Protection against Neurological Disorders
Black cumin and TQ have shown their therapeutic promises against a range of neurological conditions, including neurodegenerative disorders (Alzheimer’s disease (AD), and Parkinson’s disease (PD)), ischemic stroke and acute brain injury, anxiety and depression, epilepsy, and schizophrenia (Table 1). Moreover, black cumin and TQ were shown to protect against various chemical-induced neuronal injury in experimental conditions (Table 1). The neuroprotective potentials of black cumin and TQ mostly stem from antioxidative and anti-inflammatory properties [21] (Figure 1).
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) |
References |
|---|---|---|---|
| Neuroinflammation | |||
| TQ (12.5 μM for 24 h) |
LPS/IFNγ or H2O2-activated BV-2 microglial cell | ↓H2O2; ↑GSH; ↑SOD and CAT | [14] |
| TQ (12.5 μM for 24 h) |
LPS/IFNγ or H2O2-activated BV-2 microglial cell | ↑Glutaredoxin-3, biliverdin reductase A, 3-mercaptopyruvate sulfurtransferase, and mitochondrial Lon protease; ↓IL-2, IL-4, IL-6, IL-10, and IL-17a, CFB, CXCL3 and CCL5 | [22] |
| TQ (2.5–10 μM) |
LPS-activated neuroinflammation in BV-2 microglial cell | ↓ROS; ↑LKB1 and AMPK; ↑nuclear accumulation of SIRT1 | [23] |
| Alzheimer’s disease | |||
| TQ (100 nM) |
Aβ1–42-induced neurotoxicity in hiPSC-derived cholinergic neurons | ↑GSH; ↓ROS; ↓synaptic toxicity, attenuate cell death and apoptosis | [24] |
| TQ fraction rich nanoemulsion of seeds (TQRFNE) (250 and 500 mg/kg BW) |
High fat/cholesterol diet-induced neurotoxicity in rats | ↓Aβ40 and Aβ42; ↑APP; ↓PSEN1 and PSEN2; ↓BACE1 and RAGE; ↑IDE and LRP1 | [25] |
| TQ fraction rich nanoemulsion of Nigella seeds (TQRFNE) (250 and 500 mg/kg BW) |
High fat/cholesterol diet-induced neurotoxicity in rats | ↓Memory impairment; ↓lipid peroxidation and soluble Aβ levels; ↑total antioxidant status and antioxidants genes expression | [26] |
| TQ (10, 20, and 40 mg/kg/day p.o. for 14 days) |
Combined AlCl3andD-Gal-induced AD in rats | Improved cognitive deficits; ↓Aβ formation and accumulation; ↓TNF-α and IL-1β; ↓TLRs pathway components; ↓NF-κB and IRF-3 mRNAs | [27] |
| TQ (intragastrically, 20 mg/kg/day once daily for 14 days) |
Combined AlCl3 and D-Gal induced neurotoxicity in rats | ↑ Memory performance; ↑ SOD; ↓TAC; ↓MDA; ↓NO; ↓TNF-α; ↓AChE activity; ↑BDNF and Bcl-2 | [28] |
| TQ (intragastrically, 20 mg/kg/day for 15 days) |
Aβ (1–42) infused rat model of AD | ↓Memory performance (Morris water maze test); ↓IFN-γ; ↑ DCX and MAP2 | [29] |
| Parkinson’s disease | |||
| TQ (100 nM) |
α-Synuclein-induced rat hippocampal and hiPSC-derived neurons | ↑Synaptophysin; ↓synaptic vesicle recycling; ↑spontaneous firing activity | [30] |
| TQ (10 mg/kg BW, 1 week prior to MPTP at 25 mg/kg BW) |
MPTP-induced mouse PD model | ↓MDA; ↑GSH; ↑SOD; ↑CAT; ↓IL-1β and IL-6; ↓TNF-α; ↓COX-2 and iNOS; ↓α-synuclein aggregation | [31] |
| TQ (7.5 and 15 mg/kg/day, p.o.) |
Rotenone-induced rat PD model | ↓Oxidative stress; ↑Parkin; ↓ Drp1; ↑dopamine; ↑TH levels | [32] |
| Ischemic stroke | |||
| Hydroalcoholic seed extract (20 mg/kg BW) |
Global ischemia in rats | ↓Brain edema and infarct volume; ↑VEGF, HIF and MMP9 | [33] |
| TQ | Stroke-prone spontaneously hypertensive rats | ↓Chemoattractant protein-1, Cox-2, IL-1β, and IL-6 | [34] |
| Traumatic brain injury | |||
| TQ (5 mg/kg/day for seven days) |
Feeney’s falling weight-induced moderate head trauma | ↑Neuron densities; ↓MDA | [35] |
| Anxiety and Depression | |||
| Ethanolic seed extract | Chronic stress-induced depression model | ↓NO | [36] |
| TQ-loaded solid lipid nanoparticle (20 mg/kg, p.o.) and TQ (20 mg/kg, p.o.) |
Chronic stress-induced depression model | ↓IL-6, TNFα; ↑BDNF; ↑5-HT; ↑IDO | [37] |
| NSO (0.2 mL/kg for 20 days) |
Stress-induced depression model | ↑Memory performance (FST) | [38] |
| Hydroalcoholic seed extract (200 and 400 mg/kg) |
Stress-induced depression and anxiety model | ↑Anxiolytic (Open field and elevated plus-maze test); ↓depression (FST) | [39] |
| Epilepsy | |||
| Ethanolic seed extract (400 mg/kg/day, p.o.) | PTZ-induced kindling mode | ↓Kindling development; ↑memory performance (Morris water maze test); ↓LTP | [40] |
| NSO (400 and 600 mg/kg BW) | Electroshock-induced seizures | ↑Anticonvulsant activity | [41] |
| TQ (10 mg/kg, i.p) |
Lithium chloride and pilocarpine-induced seizure | ↑Memory performance; ↑SOD; ↑Nrf2, HO-1 | [42] |
| TQ (10 mg/kg, i.p) |
Lithium chloride and pilocarpine-induced seizure | ↑Memory performance; ↓COX-2, TNF-α and NF-κB | [43] |
| Hydroalcoholic seed extract (200 and 400 mg/kg for 5 days) |
PTZ-induced seizure model | ↑Memory performance (Morris water maze and passive avoidance test); ↑ total thiol; ↓MDA | [44] |
| Schizophrenia | |||
| TQ (20 mg/kg, daily for 28 days, i.p.) |
Mice model of schizophrenia (haloperidol-induced catalepsy and apomorphine-induced climbing behavior) |
Anti-amnesic effect; ↓AChE activity; ↓ TBARS; ↑GSH and catalase; ↑dopamine level | [45] |
| Miscellaneous effects | |||
| Chemical-induced toxicity | |||
| TQ (5 mg/kg, i.p. for 11days) |
Acrylamide-induced neurotoxicity in rats | Improved gait abnormalities; ↑GSH; ↓MDA;↓caspases 3 and 9, and Bax/Bcl-2, pP38/P38 and pJNK/JNK; ↓pERK/ERK; restore BBB integrity | [46] |
| TQ (5 and 10 mg/kg, i.p., for 11 days) |
Acrylamide-Induced Peripheral Nervous System Toxicity in rats | Improved gait abnormalities; ↑GSH and ↓MDA;↓caspases 3 and 9, and Bax/Bcl-2, pP38/P38 and pJNK/JNK; ↓pERK/ERK | [47] |
| TQ (10 µM and 20 µM) |
Arsenic-induced cytotoxicity in SH-SY5Y cells | Promotes DNA repairing; ↓ROS, balanced transmembrane potential; ↓ Bax and PARP-1, and ↑Bcl-2 | [48] |
| TQ (5 mg/kg/day, for 3 days, p.o.) |
Arsenic-induced hippocampal toxicity in rats | Improve anxiety behavior (Open field test and elevated plus maze); ↑GSH and SOD; ↓DNA damage; ↓TNF-α and INF-γ | [49] |
| TQ (2.5 and 5 mg/kg BW, for 8 days, p.o.) |
Arsenic-induced hippocampal toxicity in Wistar rats | ↑Δψm | [50] |
| NSO (1 mL/kg BW for 7 days) |
Dichlorvos-induced oxidative and neuronal damage in rats | ↓Vacuolation in the frontal and cerebellar cortices;↑TAC and GSH↓ROS | [51] |
| Radiotoxicity | |||
| TQ | Radiation-induced oxidative stress in brain tissue | ↑Antioxidant enzymes | [52] |
3.4 Anti-Cancer Effects
Black cumin and its compounds are widely known for their potent anticancer actions. Accumulating evidence suggests that chemical constituents of black cumin seeds are chemopreventive and potent in inhibiting cell proliferation and provoking apoptosis (Table 2). In a recent study, administration of black cumin seed ethanolic extract (250 mg/kg; p.o. for 5 days) was reported to attenuate diethylnitrosamine (DENA)-induced liver carcinogenesis and reduce serum AFP and VEGF levels and liver HGFβ protein in rats [54].
| Treatment with Doses | Experimental Model | Major Findings (Including Molecular Changes) |
References |
|---|---|---|---|
| Seeds incorporated silver nanoparticles (NS-AgNP) (25–200 µg/mL) |
Human breast cancer cell line (HCC-712) | Dose-dependent cytotoxicity; ↓cell density | [55] |
| Aqueous seed extract (11.5 µg/mL) |
Human breast cancer cell line (MCF-7) | Potent cytotoxic effect with IC50 11.5 µg/mL; ↑caspase-3,8 and 9, and Bax | [56] |
| NSO nanoemulsion (10–100 µL/mL) |
Human breast cancer cell line (MCF-7) | ↓Cell proliferation; ↑apoptosis and necrosis | [57] |
| TQ (25 µmol/L) |
Human breast cancer cell line (MCF-7) | Inhibit tumor cell growth; ↑p53; induce apoptosis | [58] |
| Seeds incorporated platinum nanoparticles (NS-PtNP) (25, 50, 100 and 150 µg/mL) |
HeLa cervical cancer and MDA-MB-231 breast cancer cell lines | Dose-dependent cytotoxic effect with IC50 value 36.86 µg/mL (MDA-MB-231) and 19.83 µg/mL (HeLa), respectively | [59] |
| TQ (0.78 µM) |
HeLa cervical cancer cell line | Dose-dependent antiproliferative effect | [60] |
| TQ (2, 4, 6 and 8 µM) |
Human colon cancer cell line (LoVo) | Inhibit metastasis; ↑JNK, p38; ↓P13K, ERK1/2, IKKα/β and NF-κB | [61] |
| TQ (20 µmol/L) |
Human colon cancer cell line (LoVo) | Reduce cell proliferation; ↓p-P13K, p-Akt, p-GSK3β, β-catenin and COX-2; ↓PGE2, LEF-1 and TCF-4 | [62] |
| TQ (10–120 µmol/L) |
Human bladder cancer cell lines (253J and T24) | Inhibit proliferation and metastasis; ↓MYC, Axin-2, MMP-7, MET and cyclin-D1; ↓Wnt/β-catenin signaling cascade | [63] |
| TQ (40, 60 and 80 µM) |
Human bladder cancer cell lines (253J and T24) | Significant cytotoxicity and reduction in cell proliferation; ↑caspase-3, cleaved PARP, Bax, cyt c and AIF; ↑ER-stress marker GRP78, IRE1, ATF6, ATF4 and CHOP; ↓Bcl-2 and Bcl-xl; induce apoptosis | [64] |
| TQ (10–50 µM) |
Pancreatic ductal adenocarcinoma cell lines (AsPC1 and MiaPaCa-2) | Inhibit cell viability; reduce tumor size; ↑p53, p21; ↓Bcl-2 and HDAC; induce apoptosis and G2 cell cycle arrest | [65] |
| TQ (0.5–20 µM) |
Human renal tubular epithelial cell line (HK2) and human renal cancer cell lines (769-P and 786-O) | Inhibit metastatic phenotype and epithelial-mesenchymal transition; ↑E-cadherin; ↓Snail, ZEB1 and vimentin; ↑LKB1/AMPK signaling | [66] |
| TQ (0–100 µmol/L) |
Human renal cancer cell lines (ACHN and 786-O) | Inhibition of metastasis; ↑LC3; ↑AMPK/mTOR signaling; induce autophagy | [67] |
| TQ (40 and 50 µM) |
Human kidney cancer cell lines (A498 and Caki-1) | Anti-proliferative effects with GI50 value 40.07 µM (A498) and 51.04 µM (Caki-1), respectively; ↑Bax; ↓Bcl-2 and p-Akt; induce apoptosis | [68] |
| Hexanic seed extract (0–150 µg/mL) |
Human ovary cancer cell line (A2780) | Strong cytotoxic activity of SF2 with IC50 10.89 µg/mL; ↑caspase-3 and 9; ↓MMP; induce apoptosis | [69] |
| Seed extract and NSO with OM-90(0.5 and 2.4 mg/mL) | AGS human gastric adenocarcinoma cell line | Activates mitochondrial pathways; induce apoptosis | [70] |
| TQ (0.1–30 µM) |
Human prostate cancer cell lines (PC3 and DU145) | Inhibit metastatic phenotype and epithelial-mesenchymal transition; ↓TGF-β, Smad2 and Smad3 | [71] |
| TQ (0–80 µM) |
Head and neck squamous cells carcinoma cell lines (SCC25 and CAL27) | Dose-dependent cytotoxicity with IC50 value 12.12 µM (CAL27) and 24.62 µM (SCC25), respectively; induce apoptosis | [72] |
| TQ + Resveratrol (46 µM) |
Hepatocellular carcinoma cell line (HepG2) | Significant cell inhibition; ↑caspase-3; ↓GSH and MDA; induce apoptosis | [73] |
| NSO (50–250 µg/mL) |
Human liver cancer (HepG2), human breast cancer (MCF-7), human lung cancer (A-549) and normal human embryonic kidney (HEK293) cell lines | High cytotoxic effect in HepG2 cells with IC50 48µg/mL; ↑ROS and LPO; ↓GSH and MMP; ↑p53, caspase-3 and 9, Bax; ↓Bcl-2; induce apoptosis | [74] |
| TQ (In vitro: 1–50 µMIn vivo: 20 and 100 mg/kg for 3 days; i.v.) |
TNBC cells and orthotopic TNBC xenograft mice model | Inhibit cell proliferation, migration and invasion; ↓tumor growth; ↓eEF-2K, Src/FAK and Akt | [75] |
| TQ + Paclitaxel (In vitro: 0–100 µM In vivo: 2.4 mg/kg/day for 12 days; i.p) |
Mouse breast cancer cell line (4T1) and EAC cells-induced female Balb/c mice model | Dose-dependent cytotoxicity; ↑caspase-3,7 and 12, PARP; ↓p65, p53 and Akt1; ↓JAK-STAT signaling | [76] |
| Ethanolic seed extract (250 mg/kg/day for 5 days, p.o.) |
Diethyl nitrosamine-induced hepatocarcinogenesis in Wistar rat model | Antiangiogenic effect; ↓serum VEGF and AFP levels, and liver HGFβ level | [54] |
| Ethanolic seed extract and TQ (150, 250 and 300 mg/kg (extract) 6 days/week and 20 mg/kg (TQ) for 3 days/week, p.o.) |
Diethyl nitrosamine-induced hepatocellular carcinoma in albino-Wistar rat model | Reduction in cell proliferation; ↑Antioxidant activity; ↓PCNA, c-fos, Bcl-2; ↓EGFR/ERK1/2 signaling | [77] |
| TQ + 5-fluorouracil (35 mg/kg/day for 3 days/week for 9 weeks; p.o.) |
Azoxymethane-induced colon cancer in Wistar rat model | Subdues tumor growth; ↑TGF-β1, TGF-β/RII, Smad4, DKK-1, CDNK-1A and GPx; ↓Wnt, β-catenin, NF-κB, VEGF, COX2, iNOS and TBRAS | [78] |
| TQ + Piperine (10 mg/kg/day for 14 days; i.p) |
EMT6/P cells- inoculated Balb/c mice | Inhibit angiogenesis; ↓Tumor size; ↑serum INF-ᵧ level; ↓VEGF; induce apoptosis | [79] |
| TQ + Resveratrol (50 mg/kg/day for 14 days; i.p) |
EMT6/P cells- inoculated Balb/c mice | Inhibit angiogenesis; ↓Tumor size; ↑serum INF-ᵧ level; ↓VEGF; induce apoptosis | [80] |
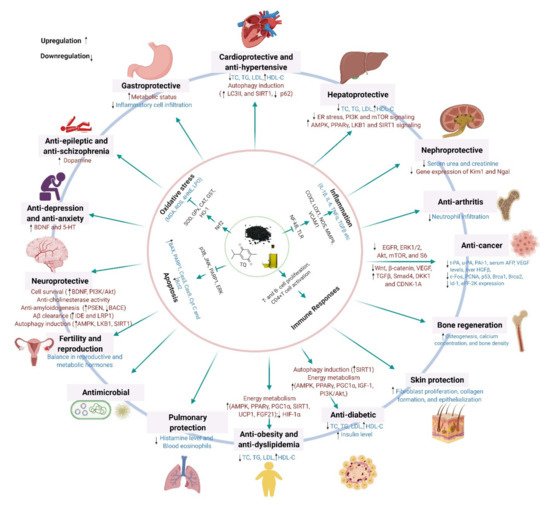
4. Molecular Mechanisms Underlying the Pharmacological Effects across Health and Disease Conditions
References
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed. Pharmacother. 2017, 95, 780–788.
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40.
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411.
- Chaudhry, Z.; Khera, R.A.; Hanif, M.A.; Ayub, M.A.; Sumrra, S.H. Chapter 13—Cumin. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–178.
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Altern. Med. 2019, 2019.
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745.
- Kabir, Y.; Shirakawa, H.; Komai, M. Nutritional composition of the indigenous cultivar of black cumin seeds from Bangladesh. Prog. Nutr. 2019, 21, 428–434.
- Kazemi, M. Phytochemical composition, antioxidant, anti-inflammatory and antimicrobial activity of Nigella sativa L. essential oil. J. Essent. Oil Bear. Plants 2014, 17, 1002–1011.
- Singh, S.; Das, S.S.; Singh, G.; Schuff, C.; De Lampasona, M.P.; Catalán, C.A.N. Composition, in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.). Biomed. Res. Int. 2014, 2014.
- El-Gindy, Y.; Zeweil, H.; Zahran, S.; El-Rahman, M.A.; Eisa, F. Hematologic, lipid profile, immunity, and antioxidant status of growing rabbits fed black seed as natural antioxidants. Trop. Anim. Health Prod. 2020, 52, 999–1004.
- Imam, A.; Sulaiman, N.A.; Oyewole, A.L.; Amin, A.; Shittu, S.T.T.; Ajao, M.S. Pro-neurogenic and antioxidant efficacy of Nigella sativa oil reduced vulnerability cholinesterase dysfunction and disruption in amygdala-dependent behaviours in chlorpyrifos exposure. J. Krishna Inst. Med. Sci. Univ. 2018, 7, 1–12.
- Ismail, N.; Ismail, M.; Azmi, N.H.; Abu Bakar, M.F.; Basri, H.; Abdullah, M.A. Modulation of hydrogen peroxide-induced oxidative stress in human neuronal cells by thymoquinone-rich fraction and thymoquinone via transcriptomic regulation of antioxidant and apoptotic signaling genes. Oxid. Med. Cell. Longev. 2016, 2016.
- Mabrouk, A. Protective effect of thymoquinone against lead-induced antioxidant defense system alteration in rat liver. Acta Biol. Hung. 2017, 68, 248–254.
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Bauer, D.; Soliman, K.F.A. The Antioxidant Effects of Thymoquinone in Activated BV-2 Murine Microglial Cells. Neurochem. Res. 2016, 41, 3227–3238.
- Ardiana, M.; Pikir, B.S.; Santoso, A.; Hermawan, H.O.; Al-Farabi, M.J. Effect of Nigella sativa Supplementation on Oxidative Stress and Antioxidant Parameters: A Meta-Analysis of Randomized Controlled Trials. Sci. World J. 2020, 2020.
- Dwita, L.P.; Yati, K.; Gantini, S.N. The anti-inflammatory activity of nigella sativa balm sticks. Sci. Pharm. 2019, 87, 3.
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51.
- Attia, H.N.; Ibrahim, F.M.; Maklad, Y.A.; Ahmed, K.A.; Ramadan, M.F. Characterization of antiradical and anti-inflammatory activities of some cold pressed oils in carrageenan-induced rat model of acute inflammation. Der Pharma Chem. 2016, 8, 148–158.
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995.
- Aziz, N.; Son, Y.J.; Cho, J.Y. Thymoquinone suppresses irf-3-mediated expression of type i interferons via suppression of tbk1. Int. J. Mol. Sci. 2018, 19, 1355.
- Samarghandian, S.; Farkhondeh, T.; Samini, F. A review on possible therapeutic effect of nigella sativa and thymoquinone in neurodegenerative diseases. CNS Neurol. Disord. Drug Targets 2018, 17, 412–420.
- Cobourne-Duval, M.K.; Taka, E.; Mendonca, P.; Soliman, K.F.A. Thymoquinone increases the expression of neuroprotective proteins while decreasing the expression of pro-inflammatory cytokines and the gene expression NFκB pathway signaling targets in LPS/IFNγ -activated BV-2 microglia cells. J. Neuroimmunol. 2018, 320, 87–97.
- Velagapudi, R.; El-Bakoush, A.; Lepiarz, I.; Ogunrinade, F.; Olajide, O.A. AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol. Cell Biochem. 2017, 435, 149–162.
- Alhibshi, A.H.; Odawara, A.; Suzuki, I. Neuroprotective efficacy of thymoquinone against amyloid beta-induced neurotoxicity in human induced pluripotent stem cell-derived cholinergic neurons. Biochem. Biophys. Rep. 2019, 17, 122–126.
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Abdullah, M.A.; Basri, H. Thymoquinone-rich fraction nanoemulsion (TQRFNE) decreases Aβ40 and Aβ42 levels by modulating APP processing, up-regulating IDE and LRP1, and down-regulating BACE1 and RAGE in response to high fat/cholesterol diet-induced rats. Biomed. Pharmacother. 2017, 95, 780–788.
- Ismail, N.; Ismail, M.; Azmi, N.H.; Bakar, M.F.A.; Yida, Z.; Stanslas, J.; Sani, D.; Basri, H.; Abdullah, M.A. Beneficial effects of TQRF and TQ nano- and conventional emulsions on memory deficit, lipid peroxidation, total antioxidant status, antioxidants genes expression and soluble Aβ levels in high fat-cholesterol diet-induced rats. Chem. Biol. Interact. 2017, 275, 61–73.
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Abdel-Mottaleb, Y.; Badary, O.A. Thymoquinone alleviates the experimentally induced Alzheimer’s disease inflammation by modulation of TLRs signaling. Hum. Exp. Toxicol. 2018, 37, 1092–1104.
- Abulfadl, Y.S.; El-Maraghy, N.N.; Ahmed, A.A.E.; Nofal, S.; Badary, O.A. Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: Biochemical, histological and behavioral changes. Neurol. Res. 2018, 40, 324–333.
- Elibol, B.; Terzioglu-Usak, S.; Beker, M.; Sahbaz, C. Thymoquinone (TQ) demonstrates its neuroprotective effect via an anti-inflammatory action on the Aβ(1–42)-infused rat model of Alzheimer’s disease. Psychiatry Clin. Psychopharmacol. 2019, 29, 379–386.
- Alhebshi, A.H.; Odawara, A.; Gotoh, M.; Suzuki, I. Thymoquinone protects cultured hippocampal and human induced pluripotent stem cells-derived neurons against α-synuclein-induced synapse damage. Neurosci. Lett. 2014, 570, 126–131.
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126.
- Ebrahimi, S.S.; Oryan, S.; Izadpanah, E.; Hassanzadeh, K. Thymoquinone exerts neuroprotective effect in animal model of Parkinson’s disease. Toxicol. Lett. 2017, 276, 108–114.
- Soleimannejad, K.; Rahmani, A.; Hatefi, M.; Khataminia, M.; Hafezi Ahmadi, M.R.; Asadollahi, K. Effects of Nigella sativa Extract on Markers of Cerebral Angiogenesis after Global Ischemia of Brain in Rats. J. Stroke Cerebrovasc. Dis. 2017, 26, 1514–1520.
- Guan, D.; Li, Y.; Peng, X.; Zhao, H.; Mao, Y.; Cui, Y. Thymoquinone protects against cerebral small vessel disease: Role of antioxidant and anti-inflammatory activities. J. Biol. Regul. Homeost. Agents 2018, 32, 225–231.
- Gülşen, İ.; Ak, H.; Çölçimen, N.; Alp, H.H.; Akyol, M.E.; Demir, İ.; Atalay, T.; Balahroğlu, R.; Rağbetli, M.Ç. Neuroprotective Effects of Thymoquinone on the Hippocampus in a Rat Model of Traumatic Brain Injury. World Neurosurg. 2016, 86, 243–249.
- Ahirwar, D.; Ahirwar, B. Antidepressant effect of nigella sativa in stress-induced depression. Res. J. Pharm. Technol. 2020, 13, 1611–1614.
- Alam, M.; Zameer, S.; Najmi, A.K.; Ahmad, F.J.; Imam, S.S.; Akhtar, M. Thymoquinone Loaded Solid Lipid Nanoparticles Demonstrated Antidepressant-Like Activity in Rats via Indoleamine 2,3- Dioxygenase Pathway. Drug Res. 2020, 70, 206–213.
- Farh, M.; Kadil, Y.; Tahri, E.H.; Abounasr, M.; Riad, F.; El Khasmi, M.; Tazi, A. Evaluation of anxiolytic, antidepressant, and memory effects of Nigella sativa seeds oil in rat. Phytotherapie 2017, 1–9.
- Beheshti, F.; Norouzi, F.; Abareshi, A.; Anaeigoudari, A.; Hosseini, M. Acute administration of Nigella sativa showed anxiolytic and anti-depression effects in rats. Curr. Nutr. Food Sci. 2018, 14, 422–431.
- Tahmasebi, S.; Oryan, S.; Mohajerani, H.R.; Akbari, N.; Palizvan, M.R. Probiotics and Nigella sativa extract supplementation improved behavioral and electrophysiological effects of PTZ-induced chemical kindling in rats. Epilepsy Behav. 2020, 104.
- Bepari, A.; Parashivamurthy, B.M.; Niazi, S.K. Evaluation of the effect of volatile oil extract of Nigella sativa seeds on maximal electroshock-induced seizures in albino rats. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 273–284.
- Shao, Y.Y.; Li, B.; Huang, Y.M.; Luo, Q.; Xie, Y.M.; Chen, Y.H. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl. Neurosci. 2017, 8, 9–14.
- Shao, Y.; Feng, Y.; Xie, Y.; Luo, Q.; Chen, L.; Li, B.; Chen, Y. Protective Effects of Thymoquinone Against Convulsant Activity Induced by Lithium-Pilocarpine in a model of Status Epilepticus. Neurochem. Res. 2016, 41, 3399–3406.
- Vafaee, F.; Hosseini, M.; Hassanzadeh, Z.; Edalatmanesh, M.A.; Sadeghnia, H.R.; Seghatoleslam, M.; Mousavi, S.M.; Amani, A.; Shafei, M.N. The effects of Nigella sativa hydro-alcoholic extract on memory and brain tissues oxidative damage after repeated seizures in rats. Iran. J. Pharm. Res. 2015, 14, 547–557.
- Khan, R.A.; Najmi, A.K.; Khuroo, A.H.; Goswami, D.; Akhtar, M. Ameliorating effects of thymoquinone in rodent models of schizophrenia. Afr. J. Pharm. Pharmacol. 2014, 8, 413–421.
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Role of Oxidative Stress, MAPKinase and Apoptosis Pathways in the Protective Effects of Thymoquinone Against Acrylamide-Induced Central Nervous System Toxicity in Rat. Neurochem. Res. 2020, 45, 254–267.
- Tabeshpour, J.; Mehri, S.; Abnous, K.; Hosseinzadeh, H. Neuroprotective Effects of Thymoquinone in Acrylamide-Induced Peripheral Nervous System Toxicity Through MAPKinase and Apoptosis Pathways in Rat. Neurochem. Res. 2019, 44, 1101–1112.
- Firdaus, F.; Zafeer, M.F.; Anis, E.; Ahmad, F.; Hossain, M.M.; Ali, A.; Afzal, M. Evaluation of phyto-medicinal efficacy of thymoquinone against Arsenic induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. Phytomedicine 2019, 54, 224–230.
- Firdaus, F.; Zafeer, M.F.; Ahmad, M.; Afzal, M. Anxiolytic and anti-inflammatory role of thymoquinone in arsenic-induced hippocampal toxicity in Wistar rats. Heliyon 2018, 4, e00650.
- Firdaus, F.; Zafeer, M.F.; Waseem, M.; Ullah, R.; Ahmad, M.; Afzal, M. Thymoquinone alleviates arsenic induced hippocampal toxicity and mitochondrial dysfunction by modulating mPTP in Wistar rats. Biomed. Pharmacother. 2018, 102, 1152–1160.
- Imam, A.; Ogunniyi, A.; Ibrahim, A.; Abdulmajeed, W.I.; Oyewole, L.A.; Lawan, A.H.; Sulaimon, F.A.; Adana, M.Y.; Ajao, M.S. Dichlorvos induced oxidative and neuronal responses in rats: Mitigative efficacy of Nigella sativa (Black Cumin). Niger. J. Physiol. Sci. 2018, 33, 83–88.
- Demir, E.; Taysi, S.; Ulusal, H.; Kaplan, D.S.; Cinar, K.; Tarakcioglu, M. Nigella sativa oil and thymoquinone reduce oxidative stress in the brain tissue of rats exposed to total head irradiation. Int. J. Radiat. Biol. 2020, 96, 228–235.
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347.
- Fathy, M.; Nikaido, T. In vivo attenuation of angiogenesis in hepatocellular carcinoma by Nigella sativa. Turk. J. Med. Sci. 2018, 48, 178–186.
- Almatroudi, A.; Khadri, H.; Azam, M.; Rahmani, A.H.; Khaleefah, A.; Khaleefah, F.; Khateef, R.; Ansari, M.A.; Allemailem, K.S. Antibacterial, Antibiofilm and Anticancer Activity of Biologically Synthesized Silver Nanoparticles Using Seed Extract of Nigella sativa. Processes 2020, 8, 388.
- Bumidin, M.S.; Johari, F.A.; Risan, N.F.; Nasir, M.H.M. The effect of aqueous extracts of nigella sativa on breast cancer cell line Mcf-7: An in vitro study. Sci. Herit. J. 2018, 2, 13–17.
- Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Anticancer activity of an ultrasonic nanoemulsion formulation of Nigella sativa L. essential oil on human breast cancer cells. Ultrason. Sonochem. 2016, 31, 449–455.
- Dastjerdi, M.N.; Mehdiabady, E.M.; Iranpour, F.G.; Bahramian, H. Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. Int. J. Prev. Med. 2016, 7, 66.
- Aygun, A.; Gülbagca, F.; Ozer, L.Y.; Ustaoglu, B.; Altunoglu, Y.C.; Baloglu, M.C.; Atalar, M.N.; Alma, M.H.; Sen, F. Biogenic platinum nanoparticles using black cumin seed and their potential usage as antimicrobial and anticancer agent. J. Pharm. Biomed. Anal. 2020, 179, 112961.
- Butt, A.S.; Nisar, N.; Ghani, N.; Altaf, I.; Mughal, T.A. Isolation of thymoquinone from Nigella sativa L. and Thymus vulgaris L., and its anti-proliferative effect on HeLa cancer cell lines. Trop. J. Pharm. Res. 2019, 18, 37–42.
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678.
- Hsu, H.-H.; Chen, M.-C.; Day, C.H.; Lin, Y.-M.; Li, S.-Y.; Tu, C.-C.; Padma, V.V.; Shih, H.-N.; Kuo, W.-W.; Huang, C.-Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017, 23, 1171.
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022.
- Zhang, M.; Du, H.; Huang, Z.; Zhang, P.; Yue, Y.; Wang, W.; Liu, W.; Zeng, J.; Ma, J.; Chen, G.; et al. Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact. 2018, 292, 65–75.
- Relles, D.; Chipitsyna, G.I.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Thymoquinone promotes pancreatic cancer cell death and reduction of tumor size through combined inhibition of histone deacetylation and induction of histone acetylation. Adv. Prev. Med. 2016, 2016, 1407840.
- Kou, B.; Kou, Q.; Ma, B.; Zhang, J.; Sun, B.; Yang, Y.; Li, J.; Zhou, J.; Liu, W. Thymoquinone inhibits metastatic phenotype and epithelial-mesenchymal transition in renal cell carcinoma by regulating the LKB1/AMPK signaling pathway. Oncol. Rep. 2018, 40, 1443–1450.
- Zhang, Y.; Fan, Y.; Huang, S.; Wang, G.; Han, R.; Lei, F.; Luo, A.; Jing, X.; Zhao, L.; Gu, S. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018, 109, 3865–3873.
- Dera, A.; Rajagopalan, P. Thymoquinone attenuates phosphorylation of AKT to inhibit kidney cancer cell proliferation. J. Food Biochem. 2019, 43, e12793.
- Shokoohinia, Y.; Bahrami, G.; Taherabadi, F.; Jaffari, F.; Hosseinzadeh, L. Apoptosis cell death effect of linoleic acid from nigella sativa on human ovary cancer cells through mitochondrial intrinsic pathway. J. Rep. Pharm. Sci. 2018, 7, 20–26.
- Czajkowska, A.; Gornowicz, A.; Pawłowska, N.; Czarnomysy, R.; Nazaruk, J.; Szymanowski, W.; Bielawska, A.; Bielawski, K. Anticancer Effect of a Novel Octahydropyrazino[2,1-a:5,4-a’]diisoquinoline Derivative and Its Synergistic Action with Nigella sativa in Human Gastric Cancer Cells. Biomed. Res. Int. 2017, 2017, 9153403.
- Kou, B.; Liu, W.; Zhao, W.; Duan, P.; Yang, Y.; Yi, Q.; Guo, F.; Li, J.; Zhou, J.; Kou, Q. Thymoquinone inhibits epithelial-mesenchymal transition in prostate cancer cells by negatively regulating the TGF-β/Smad2/3 signaling pathway. Oncol. Rep. 2017, 38, 3592–3598.
- Kotowski, U.; Heiduschka, G.; Kadletz, L.; Fahim, T.; Seemann, R.; Schmid, R.; Schneider, S.; Mitterbauer, A.; Thurnher, D. Effect of thymoquinone on head and neck squamous cell carcinoma cells in vitro: Synergism with radiation. Oncol. Lett. 2017, 14, 1147–1151.
- Ismail, N.; Abdel–Mottaleb, Y.; Ahmed, A.A.E.; El-Maraghy, N.N. Novel combination of thymoquinone and resveratrol enhances anticancer effect on hepatocellular carcinoma cell line. Future J. Pharm. Sci. 2018, 4, 41–46.
- Al-Oqail, M.M.; Al-Sheddi, E.S.; Al-Massarani, S.M.; Siddiqui, M.A.; Ahmad, J.; Musarrat, J.; Al-Khedhairy, A.A.; Farshori, N.N. Nigella sativa seed oil suppresses cell proliferation and induces ROS dependent mitochondrial apoptosis through p53 pathway in hepatocellular carcinoma cells. S. Afr. J. Bot. 2017, 112, 70–78.
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605.
- Şakalar, Ç.; İzgi, K.; İskender, B.; Sezen, S.; Aksu, H.; Çakır, M.; Kurt, B.; Turan, A.; Canatan, H. The combination of thymoquinone and paclitaxel shows anti-tumor activity through the interplay with apoptosis network in triple-negative breast cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 4467–4477.
- Shahin, Y.R.; Elguindy, N.M.; Abdel Bary, A.; Balbaa, M. The protective mechanism of Nigella sativa against diethylnitrosamine-induced hepatocellular carcinoma through its antioxidant effect and EGFR/ERK1/2 signaling. Environ. Toxicol. 2018, 33, 885–898.
- Kensara, O.A.; El-Shemi, A.G.; Mohamed, A.M.; Refaat, B.; Idris, S.; Ahmad, J. Thymoquinone subdues tumor growth and potentiates the chemopreventive effect of 5-fluorouracil on the early stages of colorectal carcinogenesis in rats. Drug Des. Dev. Ther. 2016, 10, 2239–2253.
- Talib, W.H. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci. Pharm. 2017, 85, 27.
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408.
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116.
- Alkhalaf, M.I.; Hussein, R.H.; Hamza, A. Green synthesis of silver nanoparticles by Nigella sativa extract alleviates diabetic neuropathy through anti-inflammatory and antioxidant effects. Saudi J. Biol. Sci. 2020, 27, 2410–2419.
- Abdelrazek, H.M.A.; Kilany, O.E.; Muhammad, M.A.A.; Tag, H.M.; Abdelazim, A.M. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male Wistar rats. Oxid. Med. Cell. Longev. 2018, 2018.
- Feng, Y.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687.
- Iqbal, M.J.; Butt, M.S.; Sohail, M.; Suleria, H.A.R. The antioxidant potential of black cumin (Nigella sativa l.) extracts through different extraction methods. Curr. Bioact. Compd. 2019, 15, 623–630.
- Mohammed, N.K.; Abd Manap, M.Y.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Hussin, A.S.M. The Effects of Different Extraction Methods on Antioxidant Properties, Chemical Composition, and Thermal Behavior of Black Seed (Nigella sativa L.) Oil. Evid. Based Complement. Altern. Med. 2016, 2016.
- Staniek, K.; Gille, L. Is thymoquinone an antioxidant? BMC Pharm. 2010, 10, A9.
- Gao, W.; Xiong, Y.; Li, Q.; Yang, H. Inhibition of Toll-Like Receptor Signaling as a Promising Therapy for Inflammatory Diseases: A Journey from Molecular to Nano Therapeutics. Front. Physiol. 2017, 8, 508.
- Hannan, M.A.; Rahman, M.A.; Rahman, M.S.; Sohag, A.A.M.; Dash, R.; Hossain, K.S.; Farjana, M.; Uddin, M.J. Intermittent fasting, a possible priming tool for host defense against SARS-CoV-2 infection: Crosstalk among calorie restriction, autophagy and immune response. Immunol. Lett. 2020, 226, 38–45.
- Mahmoud, Y.K.; Abdelrazek, H.M.A. Cancer: Thymoquinone antioxidant/pro-oxidant effect as potential anticancer remedy. Biomed. Pharmacother. 2019, 115, 108783.
- Jrah-Harzallah, H.; Ben-Hadj-Khalifa, S.; Almawi, W.Y.; Maaloul, A.; Houas, Z.; Mahjoub, T. Effect of thymoquinone on 1,2-dimethyl-hydrazine-induced oxidative stress during initiation and promotion of colon carcinogenesis. Eur. J. Cancer 2013, 49, 1127–1135.
- Linjawi, S.A.; Khalil, W.K.; Hassanane, M.M.; Ahmed, E.S. Evaluation of the protective effect of Nigella sativa extract and its primary active component thymoquinone against DMBA-induced breast cancer in female rats. Arch. Med. Sci. 2015, 11, 220–229.
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone Pretreatment Overcomes the Insensitivity and Potentiates the Antitumor Effect of Gemcitabine Through Abrogation of Notch1, PI3K/Akt/mTOR Regulated Signaling Pathways in Pancreatic Cancer. Dig. Dis. Sci. 2015, 60, 1067–1080.
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK pathway: Metabolism and growth control in tumour suppression. Nat. Rev. Cancer 2009, 9, 563–575.




