
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nipith Charoenngam | + 2222 word(s) | 2222 | 2021-10-13 09:00:39 | | | |
| 2 | Lindsay Dong | + 248 word(s) | 2470 | 2021-11-22 04:27:30 | | | | |
| 3 | Lindsay Dong | + 248 word(s) | 2470 | 2021-11-22 04:27:50 | | |
Video Upload Options
Vitamin D plays an important role in maintaining a healthy mineralized skeleton. It is also considered an immunomodulatory agent that regulates innate and adaptive immune systems. The aim of this narrative review is to provide general concepts of vitamin D for the skeletal and immune health, and to summarize the mechanistic, epidemiological, and clinical evidence on the relationship between vitamin D and rheumatic diseases. Multiple observational studies have demonstrated the association between a low level of serum 25-hydroxyvitamin D [25(OH)D] and the presence and severity of several rheumatic diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), spondyloarthropathies, and osteoarthritis (OA). Nevertheless, the specific benefits of vitamin D supplements for the treatment and prevention of rheumatic diseases are less accepted as the results from randomized clinical trials are inconsistent, although some conceivable benefits of vitamin D for the improvement of disease activity of RA, SLE, and OA have been demonstrated in meta-analyses. It is also possible that some individuals might benefit from vitamin D differently than others, as inter-individual difference in responsiveness to vitamin D supplementation has been observed in genomic studies. Although the optimal level of serum 25(OH)D is still debatable, it is advisable it is advisable that patients with rheumatic diseases should maintain a serum 25(OH)D level of at least 30 ng/mL (75 nmol/L) to prevent osteomalacia, secondary osteoporosis, and fracture, and possibly 40–60 ng/mL (100–150 nmol/L) to achieve maximal benefit from vitamin D for immune health and overall health.
1. Introduction
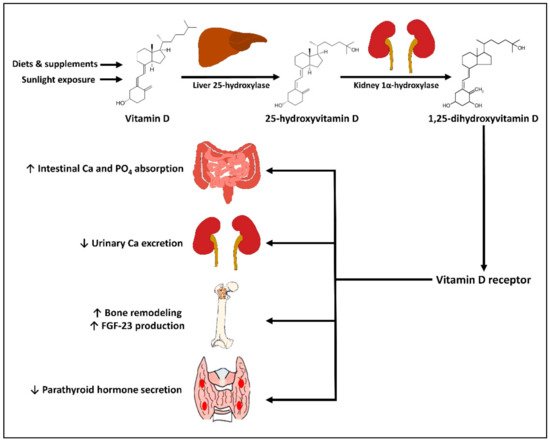
2. Physiology of Vitamin D
3. Vitamin D in Prevention and Treatment of Rheumatic Diseases
3.1. Rheumatoid Arthritis
Vitamin D is believed to play a role in modulating the pathogenesis and disease activity of RA, based on the actions of 1,25(OH)2D on the adaptive immune response that suppresses the proliferation and activity of TH1 and TH17 and enhances the Treg activity [12]. Furthermore, genomic studies have shown that certain polymorphisms of the gene encoding VDR and DBP are associated with susceptibility to RA, suggesting that the vitamin D signaling pathway may be involved in the pathogenesis of RA [13][14].
Multiple observational studies have shown the association of vitamin D status or intake with incidence and severity of RA [15]. For example, in a prospective cohort study by Merlino et al., women in the highest tertile of vitamin D intake had a lower risk for RA by 33% compared with those in the lowest tertile [16]. Moreover, a higher amount of ultraviolet B exposure was shown to be associated with a decreased risk of incident RA in the Nurse Health Study cohort of 106,368 women aged 30–55 years old [17]. This finding is in line with the evidence that the risks of some immune-mediated diseases (e.g., type 1 diabetes, multiple sclerosis, and RA) are higher in high-latitude regions where there is a relatively low amount of ultraviolet radiation and a high prevalence of vitamin D deficiency [18][19]. These observations, therefore, support that vitamin D obtained from either oral intake or sunlight exposure could possibly be protective against RA. In the COMOrbidities in Rheumatoid Arthritis (COMORA) study consisting of 1413 patients with RA from 15 countries, the serum level of 25(OH)D was inversely correlated with disease activity, as assessed by the Disease Activity Score-28 (DAS28) after adjusting for potential confounders [20].
3.2. Systemic Lupus Erythematosus
3.3. Spondyloarthropathies
3.4. Gout and Hyperuricemia
3.5. Osteoarthritis
3.6. Other Rheumatic Diseases
References
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma 2019, 10, 1082–1093.
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281.
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012, 3, 118–126.
- Pike, J.W.; Meyer, M.B.; Lee, S.-M.; Onal, M.; Benkusky, N.A. The vitamin D receptor: Contemporary genomic approaches reveal new basic and translational insights. J. Clin. Investig. 2017, 127, 1146–1154.
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537.
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930.
- Booth, D.R.; Ding, N.; Parnell, G.P.; Shahijanian, F.; Coulter, S.; Schibeci, S.D.; Atkins, A.R.; Stewart, G.J.; Evans, R.M.; Downes, M.; et al. Cistromic and genetic evidence that the vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun. 2016, 17, 213–219.
- Hossein-nezhad, A.; Spira, A.; Holick, M.F. Influence of Vitamin D Status and Vitamin D3 Supplementation on Genome Wide Expression of White Blood Cells: A Randomized Double-Blind Clinical Trial. PLoS ONE 2013, 8, e58725.
- Blau, J.E.; Collins, M.T. The PTH-Vitamin D-FGF23 axis. Rev. Endocr. Metab. Disord. 2015, 16, 165–174.
- Adams, J.S.; Rafison, B.; Witzel, S.; Reyes, R.E.; Shieh, A.; Chun, R.; Zavala, K.; Hewison, M.; Liu, P.T. Regulation of the extrarenal CYP27B1-hydroxylase. J. Steroid Biochem. Mol. Biol. 2014, 144 (Pt A), 22–27.
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18.
- Aslam, M.M.; John, P.; Bhatti, A.; Jahangir, S.; Kamboh, M.I. Vitamin D as a Principal Factor in Mediating Rheumatoid Arthritis-Derived Immune Response. Biomed. Res. Int. 2019, 2019, 3494937.
- Bagheri-Hosseinabadi, Z.; Imani, D.; Yousefi, H.; Abbasifard, M. Vitamin D receptor (VDR) gene polymorphism and risk of rheumatoid arthritis (RA): Systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 3555–3569.
- Rozmus, D.; Ciesielska, A.; Plominski, J.; Grzybowski, R.; Fiedorowicz, E.; Kordulewska, N.; Savelkoul, H.; Kostyra, E.; Cieslinska, A. Vitamin D Binding Protein (VDBP) and Its Gene Polymorphisms-The Risk of Malignant Tumors and Other Diseases. Int. J. Mol. Sci. 2020, 21, 7822.
- Lee, Y.H.; Bae, S.C. Vitamin D level in rheumatoid arthritis and its correlation with the disease activity: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 827–833.
- Merlino, L.A.; Curtis, J.; Mikuls, T.R.; Cerhan, J.R.; Criswell, L.A.; Saag, K.G.; Iowa Women’s Health, S. Vitamin D intake is inversely associated with rheumatoid arthritis: Results from the Iowa Women’s Health Study. Arthritis Rheum. 2004, 50, 72–77.
- Arkema, E.V.; Hart, J.E.; Bertrand, K.A.; Laden, F.; Grodstein, F.; Rosner, B.A.; Karlson, E.W.; Costenbader, K.H. Exposure to ultraviolet-B and risk of developing rheumatoid arthritis among women in the Nurses’ Health Study. Ann. Rheum. Dis. 2013, 72, 506–511.
- Staples, J.A.; Ponsonby, A.-L.; Lim, L.L.Y.; McMichael, A.J. Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: Latitude, regional ultraviolet radiation, and disease prevalence. Environ. Health Perspect. 2003, 111, 518–523.
- Vieira, V.M.; Hart, J.E.; Webster, T.F.; Weinberg, J.; Puett, R.; Laden, F.; Costenbader, K.H.; Karlson, E.W. Association between residences in U.S. northern latitudes and rheumatoid arthritis: A spatial analysis of the Nurses’ Health Study. Environ. Health Perspect. 2010, 118, 957–961.
- Hajjaj-Hassouni, N.; Mawani, N.; Allali, F.; Rkain, H.; Hassouni, K.; Hmamouchi, I.; Dougados, M. Evaluation of Vitamin D Status in Rheumatoid Arthritis and Its Association with Disease Activity across 15 Countries: “The COMORA Study”. Int. J. Rheumatol. 2017, 2017, 5491676.
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392.
- Sahebari, M.; Nabavi, N.; Salehi, M. Correlation between serum 25(OH)D values and lupus disease activity: An original article and a systematic review with meta-analysis focusing on serum VitD confounders. Lupus 2014, 23, 1164–1177.
- Monticielo, O.A.; Teixeira, T.d.M.; Chies, J.A.B.; Brenol, J.C.T.; Xavier, R.M. Vitamin D and polymorphisms of VDR gene in patients with systemic lupus erythematosus. Clin. Rheumatol. 2012, 31, 1411–1421.
- Sun, J.; Zhang, S.; Liu, J.S.; Gui, M.; Zhang, H. Expression of vitamin D receptor in renal tissue of lupus nephritis and its association with renal injury activity. Lupus 2019, 28, 290–294.
- Costenbader, K.H.; Feskanich, D.; Holmes, M.; Karlson, E.W.; Benito-Garcia, E. Vitamin D intake and risks of systemic lupus erythematosus and rheumatoid arthritis in women. Ann. Rheum. Dis. 2008, 67, 530–535.
- Zheng, R.; Gonzalez, A.; Yue, J.; Wu, X.; Qiu, M.; Gui, L.; Zhu, S.; Huang, L. Efficacy and Safety of Vitamin D Supplementation in Patients with Systemic Lupus Erythematosus: A Meta-analysis of Randomized Controlled Trials. Am. J. Med. Sci. 2019, 358, 104–114.
- De Martinis, M.; Ginaldi, L.; Sirufo, M.M.; Bassino, E.M.; De Pietro, F.; Pioggia, G.; Gangemi, S. IL-33/Vitamin D Crosstalk in Psoriasis-Associated Osteoporosis. Front. Immunol. 2021, 11, 3416.
- Cubillos, S.; Krieg, N.; Norgauer, J. Effect of Vitamin D on Peripheral Blood Mononuclear Cells from Patients with Psoriasis Vulgaris and Psoriatic Arthritis. PLoS ONE 2016, 11, e0153094.
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173.
- Petho, Z.; Kulcsar-Jakab, E.; Kalina, E.; Balogh, A.; Pusztai, A.; Gulyas, K.; Horvath, A.; Szekanecz, Z.; Bhattoa, H.P. Vitamin D status in men with psoriatic arthritis: A case-control study. Osteoporos. Int. 2015, 26, 1965–1970.
- Sağ, M.S.; Sağ, S.; Tekeoğlu, İ.; Solak, B.; Kamanlı, A.; Nas, K.; Harman, H.; Kantar, M. Comparison of 25-hidroksi Vitamin D serum concentrations in patients with psoriasis and psoriatic arthritis. J. Back Musculoskelet. Rehabil. 2018, 31, 37–43.
- McCullough, P.J.; Lehrer, D.S.; Amend, J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J. Steroid Biochem. Mol. Biol. 2019, 189, 228–239.
- McCullough, P.J.; McCullough, W.P.; Lehrer, D.; Travers, J.B.; Repas, S.J. Oral and Topical Vitamin D, Sunshine, and UVB Phototherapy Safely Control Psoriasis in Patients with Normal Pretreatment Serum 25-Hydroxyvitamin D Concentrations: A Literature Review and Discussion of Health Implications. Nutrients 2021, 13, 1511.
- McCullough, P.; Amend, J. Results of daily oral dosing with up to 60,000 international units (iu) of vitamin D3 for 2 to 6 years in 3 adult males. J. Steroid Biochem. Mol. Biol. 2017, 173, 308–312.
- Theodoridis, X.; Grammatikopoulou, M.G.; Stamouli, E.-M.; Talimtzi, P.; Pagkalidou, E.; Zafiriou, E.; Haidich, A.-B.; Bogdanos, D.P. Effectiveness of oral vitamin D supplementation in lessening disease severity among patients with psoriasis: A systematic review and meta-analysis of randomized controlled trials. Nutrition 2021, 82, 111024.
- Cai, G.; Wang, L.; Fan, D.; Xin, L.; Liu, L.; Hu, Y.; Ding, N.; Xu, S.; Xia, G.; Jin, X.; et al. Vitamin D in ankylosing spondylitis: Review and meta-analysis. Clin. Chim. Acta 2015, 438, 316–322.
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717.
- Ben-Shabat, N.; Watad, A.; Shabat, A.; Bragazzi, N.L.; Comaneshter, D.; Cohen, A.D.; Amital, H. Low Vitamin D Levels Predict Mortality in Ankylosing Spondylitis Patients: A Nationwide Population-Based Cohort Study. Nutrients 2020, 12, 1400.
- Guzman-Prado, Y.; Samson, O.; Segal, J.P.; Limdi, J.K.; Hayee, B.H. Vitamin D Therapy in Adults With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, 1819–1830.
- Charoenngam, N.; Shirvani, A.; Kalajian, T.A.; Song, A.; Holick, M.F. The Effect of Various Doses of Oral Vitamin D3 Supplementation on Gut Microbiota in Healthy Adults: A Randomized, Double-blinded, Dose-response Study. Anticancer Res. 2020, 40, 551–556.
- Jung, K.H.; Kim, T.H.; Sheen, D.H.; Lim, M.K.; Lee, S.K.; Kim, J.Y.; Park, H.; Chae, S.C.; Shim, S.C. Associations of vitamin d binding protein gene polymorphisms with the development of peripheral arthritis and uveitis in ankylosing spondylitis. J. Rheumatol. 2011, 38, 2224–2229.
- Henderson, C.M.; Fink, S.L.; Bassyouni, H.; Argiropoulos, B.; Brown, L.; Laha, T.J.; Jackson, K.J.; Lewkonia, R.; Ferreira, P.; Hoofnagle, A.N.; et al. Vitamin D–Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N. Engl. J. Med. 2019, 380, 1150–1157.
- Kew, R.R. The Vitamin D Binding Protein and Inflammatory Injury: A Mediator or Sentinel of Tissue Damage? Front. Endocrinol. 2019, 10, 470.
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511.
- Charoenngam, N.; Ponvilawan, B.; Ungprasert, P. Vitamin D insufficiency and deficiency are associated with a higher level of serum uric acid: A systematic review and meta-analysis. Mod. Rheumatol. 2020, 30, 385–390.
- Nimitphong, H.; Saetung, S.; Chailurkit, L.O.; Chanprasertyothin, S.; Ongphiphadhanakul, B. Vitamin D supplementation is associated with serum uric acid concentration in patients with prediabetes and hyperuricemia. J. Clin. Transl. Endocrinol. 2021, 24, 100255.
- Ponvilawan, B.; Charoenngam, N. Vitamin D and uric acid: Is parathyroid hormone the missing link? J. Clin. Transl. Endocrinol. 2021, 25, 100263.
- Sugimoto, R.; Watanabe, H.; Ikegami, K.; Enoki, Y.; Imafuku, T.; Sakaguchi, Y.; Murata, M.; Nishida, K.; Miyamura, S.; Ishima, Y.; et al. Down-regulation of ABCG2, a urate exporter, by parathyroid hormone enhances urate accumulation in secondary hyperparathyroidism. Kidney Int. 2017, 91, 658–670.
- Ponvilawan, B.; Charoenngam, N.; Ungprasert, P. Primary hyperparathyroidism is associated with a higher level of serum uric acid: A systematic review and meta-analysis. Int. J. Rheum. Dis. 2020, 23, 174–180.
- Ishay, A.; Herer, P.; Luboshitzky, R. Effects of Successful Parathyroidectomy on Metabolic Cardiovascular Risk Factors In Patients With Severe Primary Hyperparathyroidism. Endocr. Pract. 2011, 17, 584–590.
- Broulik, P.D.; Brouliková, A.; Adámek, S.; Libanský, P.; Tvrdoň, J.; Broulikova, K.; Kubinyi, J. Improvement of hypertension after parathyroidectomy of patients suffering from primary hyperparathyroidism. Int. J. Endocrinol. 2011, 2011, 309068.
- Al-Naqeeb, J.; Saeed, M.; Dye, B.; Jeranko, M. Association of Gout with Vitamin D: A Population-Based Study. Arthritis Rheumatol. 2019, 71. Available online: https://acrabstracts.org/abstract/association-of-gout-with-vitamin-d-a-population-based-study/ (accessed on 1 September 2021).
- Bergink, A.P.; Zillikens, M.C.; Van Leeuwen, J.P.T.M.; Hofman, A.; Uitterlinden, A.G.; van Meurs, J.B.J. 25-Hydroxyvitamin D and osteoarthritis: A meta-analysis including new data. Semin. Arthritis Rheum. 2016, 45, 539–546.
- Tripathy, S.K.; Gantaguru, A.; Nanda, S.N.; Velagada, S.; Srinivasan, A.; Mangaraj, M. Association of vitamin D and knee osteoarthritis in younger individuals. World J. Orthop. 2020, 11, 418–425.
- Rejnmark, L. Effects of vitamin d on muscle function and performance: A review of evidence from randomized controlled trials. Ther. Adv. Chronic Dis. 2011, 2, 25–37.
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839.
- Robinson, A.B.; Thierry-Palmer, M.; Gibson, K.L.; Rabinovich, C.E. Disease activity, proteinuria, and vitamin D status in children with systemic lupus erythematosus and juvenile dermatomyositis. J. Pediatr. 2012, 160, 297–302.
- Azali, P.; Barbasso Helmers, S.; Kockum, I.; Olsson, T.; Alfredsson, L.; Charles, P.J.; Piehl Aulin, K.; Lundberg, I.E. Low serum levels of vitamin D in idiopathic inflammatory myopathies. Ann. Rheum. Dis. 2013, 72, 512.
- An, L.; Sun, M.-H.; Chen, F.; Li, J.-R. Vitamin D levels in systemic sclerosis patients: A meta-analysis. Drug Des. Devel. Ther. 2017, 11, 3119–3125.
- Alibaz-Oner, F.; Asmaz-Haliloglu, Ö.; Gogas-Yavuz, D.; Can, M.; Haklar, G.; Direskeneli, H. Vitamin D Levels in Takayasu’s Arteritis and a Review of the Literature on Vasculitides. J. Clin. Lab. Anal. 2016, 30, 529–533.
- Kriegel, M.A.; Manson, J.E.; Costenbader, K.H. Does vitamin D affect risk of developing autoimmune disease?: A systematic review. Semin. Arthritis Rheum. 2011, 40, 512–531.e518.
- Hulshof, M.M.; Bavinck, J.N.B.; Bergman, W.; Masclee, A.A.M.; Heickendorff, L.; Breedveld, F.C.; Dijkmans, B.A.C. Double-blind, placebo-controlled study of oral calcitriol for the treatment of localized and systemic scleroderma. J. Am. Acad. Dermatol. 2000, 43, 1017–1023.
- Häuser, W.; Perrot, S.; Sommer, C.; Shir, Y.; Fitzcharles, M.-A. Diagnostic confounders of chronic widespread pain: Not always fibromyalgia. Pain Rep. 2017, 2, e598.
- Yong, W.C.; Sanguankeo, A.; Upala, S. Effect of vitamin D supplementation in chronic widespread pain: A systematic review and meta-analysis. Clin. Rheumatol. 2017, 36, 2825–2833.




