
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anabel Torrente | + 1446 word(s) | 1446 | 2021-06-02 05:59:14 | | | |
| 2 | Peter Tang | Meta information modification | 1446 | 2021-06-09 11:35:10 | | | | |
| 3 | Peter Tang | Meta information modification | 1446 | 2021-06-10 07:59:27 | | |
Video Upload Options
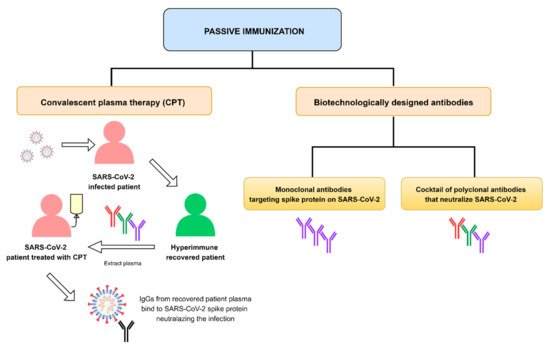
Therapeutic monoclonal antibodies (mAbs) have been the subject of widespread investigation focusing on two target-based groups, i.e., non-SARS-CoV-2 specific mAbs, that target immune system responses, and SARS-CoV-2 specific mAbs, designed to neutralize the virus protein structure.
1. Introduction
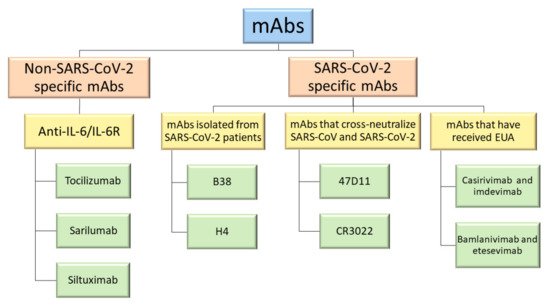
2. Non-SARS-CoV-2 Specific Monoclonal Antibodies
3. SARS-CoV-2 Specific Monoclonal Antibodies
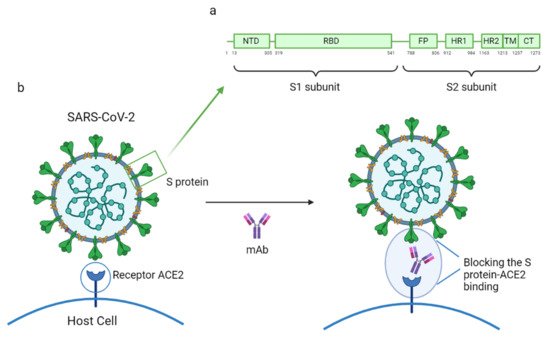
|
Groups of Specific mAbs |
Name |
Binding Site and Mechanism of Action |
|---|---|---|
|
MAbs isolated from SARS-CoV-2 patients |
B5 |
SARS-CoV-2 RBD; partial competition with ACE2 |
|
B38 |
SARS-CoV-2 RBD; complete competition with ACE2 |
|
|
H2 |
SARS-CoV-2 RBD; no competition with ACE2 |
|
|
H4 |
SARS-CoV-2 RBD; complete competition with ACE2 |
|
|
EY6A |
SARS-CoV-2 RBD and SARS-CoV RBD with lower affinity; site spatially separate from that of ACE2 |
|
|
MAbs that cross-neutralize SARS-CoV and SARS-CoV-2 |
47D11 |
SARS-CoV-2 and SARS-CoV RBD; conserved epitope in the RBD |
|
CR3022 |
SARS-CoV RBD and SARS-CoV-2 RBD with lower affinity; conserved epitope in the RBD. Do not neutralize SARS-CoV-2 |
|
|
MAbs that have received Emergency Use Authorization (EUA) |
Bamlanivimab (LY-CoV555) |
SARS-CoV-2 RBD; EUA revoked |
|
Casirivimab (REGN10933) and imdevimab (REGN10987) in a combined therapy |
Non-overlapping epitopes of the SARS-CoV-2 RBD |
|
|
Bamlanivimab (LY-CoV555) and etesevimab (LY-CoV016) in a combined therapy |
Different, but overlapping, epitopes of the SARS-CoV-2 RBD |
References
- Renn, A.; Fu, Y.; Hu, X.; Hall, M.D.; Simeonov, A. Fruitful Neutralizing Antibody Pipeline Brings Hope To Defeat SARS-Cov-2. Trends Pharmacol. Sci. 2020, 41, 815–829.
- Cantini, F.; Goletti, D.; Petrone, L.; Najafi Fard, S.; Niccoli, L.; Foti, R. Immune Therapy, or Antiviral Therapy, or Both for COVID-19: A Systematic Review. Drugs 2020, 80, 1929–1946.
- Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Korompoki, E.; Fotiou, D.; Migkou, M.; Tzanninis, I.-G.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Emerging treatment strategies for COVID-19 infection. Clin. Exp. Med. 2021, 21, 167–179.
- RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040.
- WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19—Interim WHO Solidarity Trial Results. N. Engl. J. Med. 2021, 384, 497–511.
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2020, 396, 1345–1352.
- Owji, H.; Negahdaripour, M.; Hajighahramani, N. Immunotherapeutic approaches to curtail COVID-19. Int. Immunopharmacol. 2020, 88, 106924.
- Jahanshahlu, L.; Rezaei, N. Monoclonal antibody as a potential anti-COVID-19. Biomed. Pharmacother. 2020, 129, 110337.
- An, Z. Therapeutic Monoclonal Antibodies; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; ISBN 9780470485408.
- Zarkali, A.; Karageorgopoulos, D.E.; Rafailidis, P.I.; Falagas, M.E. Frequency of the off-label use of monoclonal antibodies in clinical practice: A systematic review of the literature. Curr. Med. Res. Opin. 2014, 30, 471–480.
- Brechner, R.J.; Rosenfeld, P.J.; Babish, J.D.; Caplan, S. Pharmacotherapy for Neovascular Age-Related Macular Degeneration: An Analysis of the 100% 2008 Medicare Fee-For-Service Part B Claims File. Am. J. Ophthalmol. 2011, 151, 887–895.
- DeFrancesco, L. COVID-19 antibodies on trial. Nat. Biotechnol. 2020, 38, 1242–1252.
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273.
- European Medicines Agency. Treatments and Vaccines for COVID-19—European Medicines Agency. Available online: (accessed on 16 March 2021).
- Khan, F.A.; Stewart, I.; Fabbri, L.; Moss, S.; Robinson, K.; Smyth, A.R.; Jenkins, G. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021, 1–13.
- Huang, Y.; Yang, C.; Xu, X.; Xu, W.; Liu, S. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149.
- Valdez-Cruz, N.A.; García-Hernández, E.; Espitia, C.; Cobos-Marín, L.; Altamirano, C.; Bando-Campos, C.G.; Cofas-Vargas, L.F.; Coronado-Aceves, E.W.; González-Hernández, R.A.; Hernández-Peralta, P.; et al. Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb. Cell Fact. 2021, 20, 88.
- Marovich, M.; Mascola, J.R.; Cohen, M.S. Monoclonal Antibodies for Prevention and Treatment of COVID-19. JAMA 2020, 324, 131.
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020, 368, 1274–1278.




