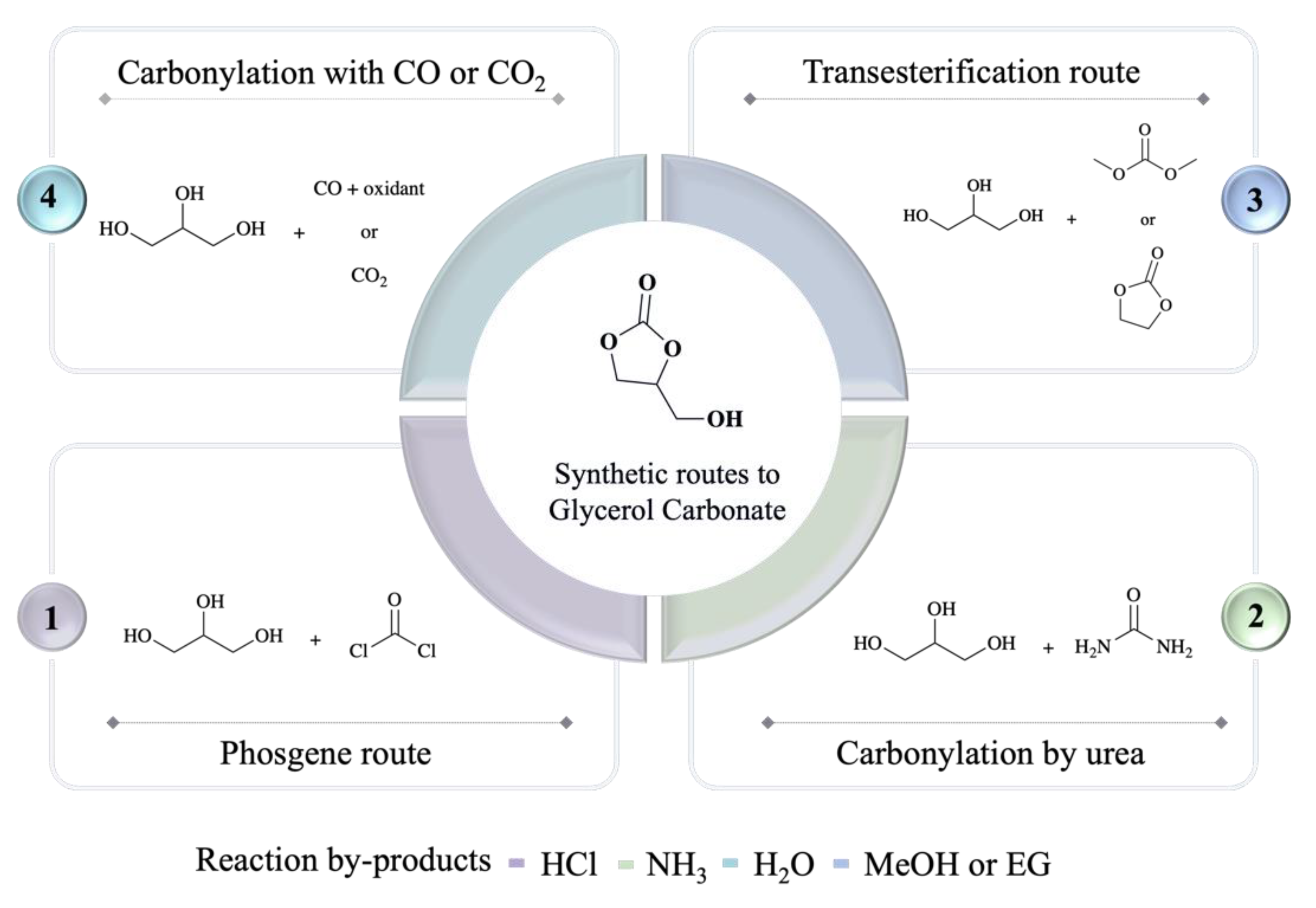
The reaction system is generally carried out under the conditions of 0.5–5 MPa and 70–170 °C. The reaction raw materials are cheap and easy to obtain, the reaction by-product is only water, and the atom utilization rate is high. It can also realize the synthesis of the target product in one step without intermediate reaction steps. Under certain reaction conditions, both CO
2 and CO can be used as carbonylation reagents to react with glycerol to generate GC, but compared with CO
2, CO has higher chemical activity. In the reaction of glycerol and CO to produce GC, the most common oxidant is oxygen, which can afford GC in high yield under relatively mild conditions
[17].
Therefore, such a route realizes the sustainable conversion of cheap and easily available raw materials into high value-added chemicals.
4.2. CO2 Direct Carbonylation Route
Also known as the CO2 conversion method, it refers to the direct reaction of CO2 and glycerol under certain conditions to produce GC.
Carbon dioxide is the main greenhouse gas, which is cheap and easy to obtain and non-toxic, while glycerol is a large by-product of the biodiesel industry. If the two are combined to synthesize high value-added chemicals, it will turn double waste into “treasure”, has extremely high environmental value. In addition, the atomic utilization rate of the reaction can reach 87%
[20], and the only by-product of the reaction is water, which shows that the reaction system is highly compatible with the concept of atomic economy and green chemistry.
The biggest problem with this route is that it is difficult to break the carbon-oxygen double bond in the CO2 molecule, which makes the chemical properties of CO2 highly stable and difficult to activate.
The choice of suitable combinations of catalyst and dehydration agent is crucial to achieving feasible yields in the synthesis of GC from glycerol and carbon dioxide. Usually, quiet harsh conditions are necessary with temperatures ranging from 120 °C to 180 °C and pressures varying from 30 bar to 150 bar
[21]. Nevertheless, the current research shows that the glycerol conversion rate and GC yield of this route are not high, and the production cost is relatively high.
In order to solve the dehydration problem of the reaction, it was attempted the direct synthesis of GC with glycerol and carbon dioxide under the combined action of 1,8-diazabicycloundec-7-ene/CH
2Br
2 [22]. This method used mild reaction conditions (70 °C, 1 MPa) and high yield of GC was obtained (86%). However, CH
2Br
2 is toxic and expensive, which hinders the industrial application of this method.
It was reported that the yield of GC could be enhanced when acetonitrile was used as solvent and dehydration reagent in the carbonylation of glycerol and CO
2. Such as, Podila et al.
[23] showed that dibutyltin oxides also catalyze the synthesis of GC, resulting in yields of 7% in presence of acetonitrile. Additionally, in the presence of acetonitrile, La
2O
3 in combination with ZnO or Cu resulted in yields of up to 15% as reported from other research groups
[24][25][26].
Although the improved yield was obtained using acetonitrile as the dehydration agent
[27], acetonitrile is easily hydrolyzed to acetic acid, which could etherify with glycerol to decrease the yield of GC. The dehydration agent is important for the carbonylation of alcohols with CO
2. 2-Cyanopyridine was also reported to be an effective dehydration agent
[28][29][30]. For example, Liu et al.
[31] found it to be effective for the carbonylation of glycerol with CO
2 directly catalyzed by the CeO
2 catalyst. Additionally, as reported by Su et al.
[32], it was shown that if 2-cyanopyridine is used as dehydration agent and promoter, a GC yield of 18.7% was obtained without any further additives. Recently, Qiao Zhang et al.
[33] described the application of CaC
2 as a dehydrating agent for the direct synthesis of carbamates from amines, CO
2, and MeOH. Based on the use of CaC
2, GC was successfully and directly synthesized from glycerol and CO
2 under zinc catalysis and N-donor ligand, in 92% yield. CaC
2 itself is also a sustainable chemical and promotes the transformation of CO
2 into valuable products.
For developing a green methodology, McGregor et al., for the first time, demonstrated the catalytic activities of untreated biochar and biochar ash, catalytic material that can readily be produced from low-value biomass residues, in the synthesis of GC from the reaction of glycerol, acetonitrile, and CO
2 [34].
It was provided novel insights into the behavior of dehydrating agents during the conversion of glycerol in the presence of carbon dioxide and a catalyst
[35]. Particularly, they observed that conducting the reaction in the presence of adiponitrile resulted in a five-fold increase in GC yield when compared to acetonitrile, which is currently the most applied dehydrating agent.
In a very recent paper, Hu et al.
[36] prepared a cobalt-based zeolitic imidazolate framework-67 (ZIF-67) as a catalyst for the carboxylation of glycerol in the presence of acetonitrile. The conversion, yield, and selectivity achieved with ZIF-67 were 32, 29, and 92%, respectively, conducting the reaction at 210 °C for 12 h. ZIF- 67 exhibited high crystallinity, a high specific surface area, good thermal stability as well as numerous Lewis basic sites, indicating its high catalytic activity.
In recent years, photocatalysis is becoming a hot field as introducing light into the thermally driven reaction system can improve the catalytic performance. He et al.
[37] prepared a series of xLa
2O
2CO
3–ZnO catalysts, which were used for the photo-thermal transformation of glycerol and CO
2 into GC. The photo-thermal synergism as well as the cooperation between ZnO and La
2O
2CO
3 contributed to its catalytic performance, thus achieving a glycerol conversion of 6.9% under the reaction conditions of 150 °C, 5.5 MPa CO
2, and 20 mmol g
−1 with a reaction time of 6 h when 20% La
2O
2CO
3–ZnO was used as the catalyst.
4.3. The Future of CO2 Direct Carbonylation Route
Although many researchers have worked on this route, experiments have proved that the direct reaction between glycerol and CO2 is extremely difficult. This reaction is thermodynamically limited from the production of water during the process; therefore, in order to remove water and to shift the equilibrium towards GC, the use of suitable dehydrating agents has been proposed over the years. Nevertheless, most of the reagents employed to remove water also result in formation of other by-products.
In many experimental examples, the following similar difficulties and problems are encountered: the conversion of glycerol is too low, the carbon dioxide reaction activity is reduced, the reaction needs to be carried out under high pressure conditions, the dehydrating agents are not effective.
Therefore, even if the reaction itself is extremely attractive and important, its application is still very limited. Due to the limitation of thermodynamics, it is impossible to promote the reaction between glycerol and CO2 simply by developing a new high-efficiency catalyst. Moreover, the method of adding dehydrating agents to move the reaction to the right has very limited effect. In short, for the current research on this reaction system, the development of a more effective reaction system will be the main way to solve the problem.