
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucio Nobile | + 3647 word(s) | 3647 | 2020-07-03 10:53:52 | | | |
| 2 | Catherine Yang | Meta information modification | 3647 | 2020-07-09 03:30:32 | | |
Video Upload Options
The application of nanotechnology, molecular biotechnologies, and nano-sciences for medical purposes has been termed nanomedicine, a promising growing area of medical research. The aim of this paper is to provide an overview of and discuss nanotechnology applications in the early epochs of life, from transplacental transfer to neonatal/pediatric conditions. Diagnostic and therapeutic applications, mainly related to the respiratory tract, the neurosensory system, and infections, are explored and discussed. Preclinical studies show promising results for a variety of conditions, including for the treatment of pregnancy complications and fetal, neonatal, and pediatric diseases. However, given the complexity of the functions and interactions between the placenta and the fetus, and the complex and incompletely understood determinants of tissue growth and differentiation during early life, there is a need for much more data to confirm the safety and efficacy of nanotechnology in this field.
1. Introduction
Research activities aimed at exploring the human applications of nanotechnology, nanomaterials, and nanomechanics are becoming increasingly performed.
The application of nanotechnology, molecular biotechnologies, and nano-sciences for medical purposes has been termed nanomedicine, a multi-disciplinary branch of medicine for diagnosis (nano-diagnostics), controlled drug delivery (nano-therapeutics), monitoring biological parameters and biomarkers, and, more recently, theranostics, a novel approach which contains in the same system both the diagnosis/imaging agent and therapy. Nanomedicine is a growing area of medical research. To confirm this trend, 6696 articles were extracted from PubMed/MEDLINE that were published in the period from 2003 to 2019 [1].
In a previous paper, we discussed novel applications of nanotechnology in medicine and bone tissue engineering, exploring typical applications of these emerging technologies [2].
The diagnostic and therapeutic applications of nanotechnology in the early epochs of life are becoming increasingly reported. Diagnostic and therapeutic applications have been proposed, and conditions mainly related to the respiratory tract, the neurosensory system (including the eye), and infections have been studied.
The aim of this narrative review is to provide an overview of and discuss typical applications in the early epochs of life in order to explore the potential of nanoparticles (NPs), starting from transplacental transfer and extending to the diagnosis or treatment of pediatric diseases [3].
2. Transplacental Drug Delivery System
The placenta is a fetomaternal organ consisting of two components: the fetal placenta, which develops from the same blastocyst that forms the fetus, and the maternal placenta, which develops from the maternal uterus. The placenta is an important metabolic organ that acts as a lung, liver, and kidney for the fetus; it regulates the passage of several metabolic products between the maternal and fetal bloodstreams (Figure 1). Minimizing the exposure of the fetus to drugs and reducing adverse effects in the mother are the main challenges faced by researchers in this field.
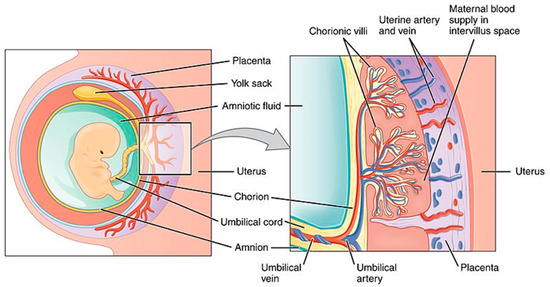
Figure 1. Schematic view of the placenta. Source: Anatomy & Physiology;Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013.
Nanoparticles cross the placental barrier by paracellular passage (particles under 25 nm) and transcellular passage, which is an active process requiring multiple steps [4]. The transportation of nanoparticles through the placenta depends on the physico-chemical characteristics of these substances (e.g., size, shape, charge, and degree of hydrophilicity) as well as the conjugation with other particles to maintain drugs in the maternal bloodstream (i.e., polyethylene glycol and placental chondroitin sulfate A binding protein) or allow transplacental passage [4][3]. The ultimate goals of nanoparticle administration during pregnancy are of paramount importance for clinicians and include the treatment of pregnancy complications, the prevention of preterm birth and related complications, and the promotion of fetal growth and wellbeing. These factors have an impact even on adult health.
Surface-functionalized nanoparticles are candidates for the therapy of pregnancy complications as they could help prevent fetal transfer of specific substances and may enhance placental-specific drug delivery [5]. For example, prevention of premature birth, ectopic pregnancies, and fetal growth restriction have been studied in animal and in vitro models. However, human applications are still unexplored due to reasons including the risk of acute immune reactions (i.e., anaphylaxis); moreover, placenta is a species-specific mammalian organ and therefore observations from animal models cannot be automatically translated to humans.
Another tool for the treatment of pregnancy complications and fetal conditions are short interfering ribonucleic acids (siRNAs). These compounds may induce the silencing of a target gene through a protein complex and have high specificity for pathogenic targets. Some authors found that siRNAs loaded into liposome complexes were internalized by the placenta without being transferred to the fetus, enabling the possibility to selectively treat placental disorders [5]. Other authors developed and tested, in an animal model, a novel siRNA delivery system consisting of a lipid nanoparticle that protects the mRNA from clearance, allowing its distribution to the liver for the treatment of a severe inherited metabolic disorder: ornithine transcarbamylase deficiency [6].
3. Nervous System
Nanoparticles are currently being studied as drug carriers to cross the blood-brain barrier (BBB) for the therapy of several central nervous system diseases, as nanoscaffolds for axonal regeneration, tools for neurological interventions, and brain diagnostics. The main obstacle drugs have to face to reach their molecular targets in the central nervous system is the blood-brain barrier, as shown in Figure 2.
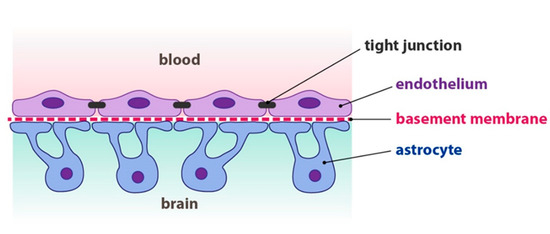
Figure 2. Structure of the blood-brain barrier. Source: Fontana 2013. Reproduced with permission of Josef Fontana.
The BBB is a highly selective barrier that separates circulating blood from the brain and prevents free diffusion of circulating substances from the blood into the brain. Intrauterine inflammation has been associated with preterm birth and also with a variety of neurobehavioral disorders, ranging from cognitive and learning disabilities to motor dysfunction disorders such as cerebral palsy. In a mouse model of intrauterine inflammation, Burd et al. [7] demonstrated that intra-amniotic delivery of hydroxyl polyamidoamine dendrimer (G4-OH)-based nanodrugs may be effective in preventing complications such as preterm birth and its consequences (i.e., neurodevelopmental delay, chronic lung disease, and/or poor growth)
Nanoparticles can also exert adverse effects on the central nervous system via several possible mechanisms, including oxidative stress, autophagy, lysosome dysfunction, and the activation of specific pathways which result in cell damage, dysfunction, or death. Other studies suggested that nanoparticles could induce considerable neurotoxicity in animals (i.e., blood-brain barrier destruction and/or neuronal degeneration). Since nervous tissue regeneration is limited, most of the nerve damage is irreversible. Furthermore, animal models have shown that nanoparticles administered to the mother during gestation may harm fetal development through direct or indirect mechanisms. Even traces of particles in maternal blood can migrate to the fetal compartment [8]. However, it is difficult to predict the toxicological behavior of nanoparticles in humans, given the lack of standards in previous studies and the complexity of the biological responses to these compounds.
Regeneration in the brain, which is a very complex process, involves cellular replacement, but also synaptic and functional repair. Nanotechnology has been proposed as a promising tool to facilitate nervous system regeneration, with multiple mechanisms being comprehensively reviewed by Liaw et al. [9]. Briefly, nanoparticles may be used as carriers of drugs, growth factors, or genetic material and may exert actions on cells involved in neuroregeneration (glial cells), immune function (leukocytes), and/or circulation (endothelial cells), or may form scaffolds to support neuronal growth and modify the extracellular environment. Carbon nanotubes can be used as scaffolds for neuronal growth and neural stem cell growth and differentiation. A nanoscaffold is a three-dimensional structure composed of polymer fibers that enhances damaged cell adherence and regeneration of tissues. As the tissue grows, the scaffold is metabolized and completely cleared [8].
SiRNA nanomedicines can overcome obstacles such as the BBB, particle clearance in the bloodstream, and cell entry, allowing the treatment of several brain diseases [10]. Clinical studies in childhood are needed to confirm this possibility.
4. Respiratory System
The development of the respiratory system during fetal life and early childhood includes dramatic changes. Lung development can be divided into five stages—embryonic, pseudoglandular, canalicular, saccular, and alveolar—that start in fetal life (the first four stages) and take several years to complete (the alveolar phase), as shown in Figure 3.
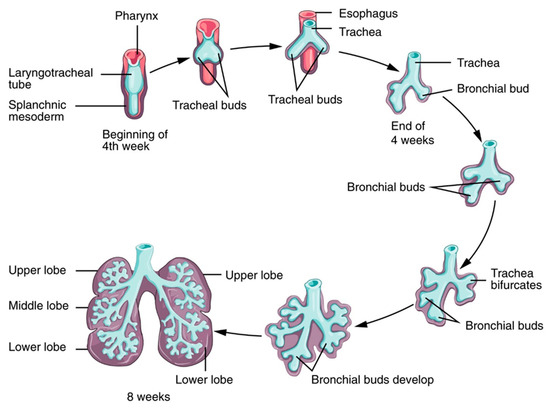
Figure 3. Lung development. Source: Anatomy and Physiology by Rice University, licensed under a Creative Commons Attribution 4.0 International License.
The phases are characterized by morphogenesis of the conducting airways, formation of alveoli (the terminal regions of the lungs where gas exchange occurs), differentiation of cells with region-specific functions of the lung (such as protection from foreign substances), efficient movement of air into and out of the lung, and efficient gas exchange at the alveolar–capillary interface. These processes are finely regulated by a complex network of cell signals, cell-derived molecules, hormones, and genetic expression.
One of the key components of the respiratory system is pulmonary surfactant. Pulmonary surfactant covers and stabilizes the alveoli and has peculiar biophysical properties, including the reduction of alveolar superficial tension and defense against microorganisms and toxic substances.
Surface tension is the force that develops at the interface between two or more fluids or between fluids and a solid wall. The forces of cohesion that act between the molecules of a fluid mean that those on the outer surface are subjected to a resulting force directed inwards; there is therefore a tendency of molecular stray from the limit surface of the liquid, which tends to contract and assume the minimum possible extent.
In the lungs, there is an interface between the liquid that covers the alveolar walls and the gas at their internal. Again, the surface liquid layer tends to contract and expel the air outwards, resulting in the collapse of the alveoli. The pressure generated within the alveoli due to surface tension is called collapse pressure and, according to Laplace’s law, is directly proportional to twice the surface tension and inversely proportional to the radius of the alveoli. Thus, the increase in surface tension determines an increment in the collapse pressure of the alveoli. In the physiological lung, surface tension is greatly reduced by the presence of surfactant, a mixture of different phospholipids, proteins, and ions produced by type II alveolar epithelial cells that cover about 10% of the area of the alveolar walls. Surfactant production begins at around the 20th week of gestation and reaches significant amounts in the amniotic fluid only after the 28th week [11].
The main components of surfactant are phospholipid dipalmitoylphosphatidylcholine, apoproteins, and calcium ions (see below). The tensioactive capacity lies in the amphibious dipalmitoylphosphatidylcholine at the liquid–gas interface. The absence of surfactant can lead to atelectasis (collapse of the alveoli) and respiratory insufficiency. Quantitatively, the surface tension of normal liquids covering the alveoli without surfactants is 50 dine/cm; this decreases to 5–30 dine/cm with surfactant. Consequently, the collapse pressure for medium-sized alveoli (r = 100 µm) falls from about 18 to 4 cm H2O.
To summarize, lung surfactant has important functions:
- increases lung compliance and therefore reduces the work required to expand the lung;
- contributes to the stability of the alveoli by preventing their collapse;
- limits the risk of pulmonary edema by decreasing the recall force of fluids inside the alveoli;
- allows the expansion of the lung at birth during the first respiratory efforts.
Moreover, surfactant can be used as a shuttle for the delivery of drugs and nanocarriers. Pulmonary surfactant is composed of approximately 90% lipids and 10% proteins. Four proteins—SP-A, -B, -C, and -D—are important for surfactant functions. SP-A and SP-D are large hydrophilic glycoproteins involved in pulmonary host defense through binding to inhaled particles and pathogens. SP-B and SP-C are smaller hydrophobic proteins that regulate the very low surface tension observed in the lung. The expression of surfactant proteins is regulated by genetic and environmental factors, including inflammation [12][13].
Interactions between surfactant and nanoparticles depend on the physico-chemical properties of the nanoparticles and also on the molecular composition, dynamic surface phase behavior, and monolayer biomechanics of the pulmonary surfactant [14][15][16]. The interaction between nanoparticles and surfactant has been studied with different methods. Hu et al. [14] showed that many factors, including surface charge, degree of hydrophobicity, and acquisition of a lipoprotein corona from endogenous surfactant, influence nanoprotein adsorption and blood stream translocation. Hydrophobic nanoparticles, such as carbon-based nanomaterials, can be retained at the surfactant lining layer and can lead to increased inflammation. Hydrophilic nanoparticles can be used for systemic drug delivery due to their rapid absorption and translocation to other organs and tissues. Anionic nanoparticles may inhibit the biophysical function of pulmonary surfactant by binding to surfactant proteins and interfering with their functions. Cationic nanoparticles may be taken up by cells and may cause acute toxicity. Therefore, neutral nanoparticles might be the safest option for pulmonary drug delivery.
In experimental models, some authors reported that hydrophilic nanomaterials (halloysite and bentonite) induced concentration-dependent damage on surfactant phospholipid function [17]. The shape and porous structure of nanoparticles are important factors to consider.
Other authors found that nanoparticles have different effects on lung inflammation and function depending on their metal properties: compared to non-metal nanoparticles (which elicit a dose-dependent inflammatory effect), silver nanoparticles do not induce significant inflammation, but only change in lung elastance [18][19].
On the other hand, the interaction between nanoparticles and surfactant may offer important therapeutic advantages: delivering drugs or nanoparticles in combination with surfactant can improve drug stability and prevent degradation and clearance in vivo, thereby allowing the delivery of macromolecular drugs. For example, nanovesicles carrying antibiotics have been successfully tested in animal models and clinical trials are ongoing [20][21]. Other potential applications include the diagnosis and therapy of different pulmonary diseases, vaccines, and cancer therapy.
Surfactant itself (animal-derived or synthetic) is an established therapy for respiratory distress syndrome, particularly among newborns: the administration of this drug is invasive, as it requires endotracheal instillation through a tube or a catheter. However, this maneuver can be associated with lung function deterioration and long-term complications among infants, and studies aimed at finding the most convenient method of administration are ongoing [22][23]. Other potential applications include the diagnosis and therapy of different pulmonary diseases, vaccines, and cancer therapy. Some authors designed inhalable nanoparticles mimicking some of the lipid components of surfactant [24]. Hopefully, technological advancement in this field will eventually lead to the production of inhalable surfactant preparations, and invasive surfactant administration will eventually be overcome.
Respiratory tract infections are among the most frequent worldwide and represent one of the most important causes of death in childhood. Antimicrobial resistance is a major challenge, as it results in increased morbidity and mortality worldwide. Since the development of new antimicrobials is expensive and has scarce success, one strategy to overcome this issue could be the application of synthetic products with antimicrobial properties. Moreover, newborns and young children have still immature metabolic pathways that can result in increased drug toxicity and poorly known drug interactions, potentially resulting in transient or persistent adverse events. Polymers with intrinsic antimicrobial effects or that can be conjugated with antimicrobials might replace antimicrobials. A comprehensive review of these compounds has been recently published [25]. Furthermore, nanomodified endotracheal tubes have been shown to reduce bacterial adhesion on the inner surface of the tube itself and potentially reduce the likelihood of ventilator-acquired infections [26].
Lower airway inflammation can be the result of different diseases and is often characterized by neutrophil leukocytes recruitment. Current therapies locally administered have low efficacy as multiple systems reduce drug availability, i.e., hydrophobicity and clearance by local defense systems. Some authors developed a delivery platform that takes advantage of the extracellular proteolysis of a microgel to deliver nanoparticle-embedded hydrophobic drugs to neutrophils and then to the lower airways [27]. In another study, Vij et al. successfully tested, in animal models, the efficacy of a PEGylated immuno-conjugated PLGA-nanoparticle to selectively deliver a drug to neutrophil cells [28].
Furthermore, the delivery of siRNAs to endothelial cells has been investigated in animal models for the treatment of pulmonary inflammatory conditions and other diseases [29].
Asthma is a common chronic inflammatory disease of the lungs starting in childhood and is characterized by intermittent airway obstruction, bronchial hyper-reactivity, and chronic airway inflammation. Diagnostic tests include the measurement of lung exhaled air flow and characteristics by means of specific instruments, i.e., spirometers. The electronic nose (E-nose) is a novel device based on nanosensors capable of detecting specific volatile organic compounds in exhaled gas, thus confirming an asthma diagnosis and allowing stratification and subtype characterization [30]. Asthma therapy relies mainly on the inhalation of certain drugs; however, poor deposition of the inhaled drug in the lung presents a challenge for the effectiveness of therapy. Drug nanoformulations are currently being evaluated following promising preclinical studies [31][32][33].
Concerning respiratory function monitoring and diagnostics, Bhattacharjee et al. [34] developed a point-of-care testing device consisting of a mouthpiece, paper-sensor, micro-heater assemblage, and monitoring unit which could facilitate the diagnosis of chronic obstructive lung diseases. The sensor was developed by depositing gold and cadmium sulfide nanoparticles on a paper surface in which the former enhanced the electrical and thermal conductivities while the latter allowed high precision humidity sensing.
Clinical studies are needed in order to assess the safety and efficacy of nanoparticles as drug carriers for respiratory and systemic conditions.
5. Ocular System
The regeneration of damaged ocular parts resulting in vision loss is a complex and current issue, and engineering approaches are becoming increasingly tested.
Potential targets are congenital and acquired diseases which may occur during childhood, including genetic retinal defects (i.e., retinitis pigmentosa) and retinopathy of prematurity (ROP), characterized by excessive vascular proliferation leading to retinal detachment [35]. Given that vascular endothelial growth factor (VEGF) plays a crucial role in the pathogeneses of ROP and other retinal diseases, injection of anti-VEGF agents (i.e., bevacizumab) is effective in reverting retinal neovascularization while avoiding detrimental effects of other procedures (such as laser therapy).
Some authors developed a nano-sized siRNA therapeutic delivery system loaded with VEGF receptor 2 and found that, in an animal model of ocular neovascularization, it was a safe and effective method of reducing vessel proliferation [36].
According to the International Classification of ROP, the instrumental fundus examination of the eye of premature infants allows the identification of five different stages of evolution of the disease. From stage 1 to stage 3, the retina is still adherent, while in stage 4, partial retinal detachment begins. In the presence of a partial detachment and increased vascular activity, the retina may further detach from the epithelial layer of the choroid to which it adheres physiologically. If vascular activity diminishes after medical treatment (ROP regression), it is advisable to employ a geometric–mechanical criterion to determine the onset of its further propagation, which also depends on many other pathogenic factors, for example myopia. It is necessary to adopt a fracture mechanics approach to this delamination problem.
Many models regarding the mechanics of the eye have been proposed in the literature. To our knowledge, only one paper describes a mathematical model to study the mechanics of retinal detachment [37]. Based on an energy criterion, the problem of quasi-static and axisymmetric propagation of a detaching retina has been solved. The propagating boundary value problem has been reformulated in a variational form allowing variations of the boundary and deformation of the detaching retina. In our opinion, the model should be reformulated by using a more rigorous adhesion–delamination theory.
In a later paper [38], the authors extended the model, taking into account the geometrical variations induced by the evolution of myopia. According to the clinical evidence, detachment propagation is initiated when the energy release rate exceeds the critical energy release rate reached at severe levels of myopia without other stress factors acting on the retina.
Nanotechnology may be used to increase drug bioavailability in the intraocular tissues, thus reducing the need for repeated injections and complications [39]. The use of nanoparticulate systems—liposomes, nanoparticles, and nanoemulsions, with a size less than 1000 nm—has been explored as an appropriate alternative to conventional options. In particular, liposomes are composed of phospholipids and cholesterol, forming a layer capable of surrounding an aqueous compartment, thus allowing the encapsulation of drugs and protection from degradation; polymeric nanoparticles, either natural or synthetic, are polymeric colloidal particles, which adsorb, absorb, attach, or encapsulate drug molecules. Solid lipid nanoparticles consist of a nanosized lipidic core stabilized by a layer of surfactants. The nanostructured lipid carrier represents a novel system allowing greater drug loading and availability compared to older systems. Polymeric micelles are a nanosized (10 to 100 nm) self-assembly of amphiphilic block copolymers (hydrophobic core and hydrophilic shell). Nanoemulsions typically include droplets of a specific liquid stabilized by surfactants and dispersed in another liquid.
Carbon dots (C-dots) constitute a functional nanomaterial with unique properties (including the inhibition of VEGF-stimulated angiogenesis in choroidal blood vessels), which have been evaluated as effective anti-VEGF carriers across the cornea, reaching therapeutic levels even when topically administered [40].
Nanoparticles can be used to enhance the bioavailability of topic drug formulations and potentially also to treat disorders of the posterior ocular segment.
Nanomaterials have also been employed as scaffolds to promote the adhesion of stem cells, proliferation, and differentiation, or as vectors for genes, growth factors, cytokines, and drugs to enhance ocular tissue regeneration and regulate a variety of host responses [41].
Finally, novel formulations and drug delivery systems have been explored. Some authors designed and validated, in a preclinical model, an intraocularly implantable device called the nanofluidic Vitreal System for Therapeutic Administration (nViSTA), based on a nanochannel membrane for continuous and controlled drug release in an attempt to avoid repeated drug injections [42].
Clinical studies are needed to confirm the encouraging results of the experimental evidence.
6. Future Directions
Nanomaterials are being tested for multiple diagnostic and therapeutic applications in medicine. Transplacental transfer of nanoparticles from the mother to the fetus and applications of nanotechnology in the early epochs of life are important emerging fields of study. Preclinical studies show promising results for a variety of conditions, including the treatment of pregnancy complications and fetal, neonatal, and pediatric conditions. However, given the complexity of the functions and interactions between the placenta and the fetus, and the complex and incompletely understood determinants of tissue growth and differentiation during early life, there is a need for much more data to confirm the safety and efficacy of nanotechnology in this field.
References
- Nicola Luigi Luigi; Nanomedicine: Insights from a Bibliometrics-Based Analysis of Emerging Publishing and Research Trends. Medicina 2019, 55, 785, 10.3390/medicina55120785.
- Stefano Nobile; Lucio Nobile; Nanotechnology for biomedical applications: Recent advances in neurosciences and bone tissue engineering. Polymer Engineering & Science 2017, 57, 644-650, 10.1002/pen.24595.
- Thádia Evelyn De Araújo; Iliana Claudia Balga Milián; Guilherme De Souza; Rafaela José Da Silva; Alessandra Monteiro Rosini; Pâmela Mendonça Guirelli; Priscila Silva Franco; Bellisa Freitas Barbosa; Eloisa Amália Vieira Ferro; Idessania Nazareth Costa; et al. Experimental models of maternal–fetal interface and their potential use for nanotechnology applications. Cell Biology International 2019, 44, 36-50, 10.1002/cbin.11222.
- Baozhen Zhang; Ruijing Liang; Mingbin Zheng; Lintao Cai; Xiujun Fan; Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications.. International Journal of Molecular Sciences 2019, 20, 3642, 10.3390/ijms20153642.
- Lucie Valero; Khair Alhareth; Jenifer Espinoza Romero; Warren Viricel; Jeanne Leblond; Audrey Chissey; Hélène Dhotel; Caroline Roques; Danielle Campiol Arruda; Virginie Escriou; et al.Nathalie MignetThierry FournierKarine Andrieux Liposomes as Gene Delivery Vectors for Human Placental Cells. Molecules 2018, 23, 1085, 10.3390/molecules23051085.
- Mary G. Prieve; Pierrot Harvie; Sean D. Monahan; Debashish Roy; Allen G. Li; Teri L. Blevins; Amber E. Paschal; Matt Waldheim; Eric C. Bell; Anna Galperin; et al.Jean-Rene Ella-MenyeMichael E. Houston Targeted mRNA Therapy for Ornithine Transcarbamylase Deficiency. Molecular Therapy 2018, 26, 801-813, 10.1016/j.ymthe.2017.12.024.
- Irina Burd; Fan Zhang; Tahani Dada; Manoj K. Mishra; Talaibek Borbiev; Wojciech G. Lesniak; Haitham Baghlaf; Sujatha Kannan; Rangaramanujam M. Kannan; Fetal uptake of intra-amniotically delivered dendrimers in a mouse model of intrauterine inflammation and preterm birth. Nanomedicine: Nanotechnology, Biology and Medicine 2014, 10, 1343-1351, 10.1016/j.nano.2014.03.008.
- Limin Wei; Longquan Shao; Xiaoli Feng; Aijie Chen; Yanli Zhang; Jianfeng Wang; Central nervous system toxicity of metallic nanoparticles. International Journal of Nanomedicine 2015, 10, 4321-4340, 10.2147/IJN.S78308.
- Kevin Liaw; Ozgul Gok; Louis DeRidder; Sujatha Kannan; Rangaramanujam M. Kannan; Quantitative assessment of surface functionality effects on microglial uptake and retention of PAMAM dendrimers. Journal of Nanoparticle Research 2018, 20, 111, 10.1007/s11051-018-4219-1.
- Meng Zheng; Wei Tao; Yan Zou; Omid C. Farokhzad; Bingyang Shi; Nanotechnology-Based Strategies for siRNA Brain Delivery for Disease Therapy. Trends in Biotechnology 2018, 36, 562-575, 10.1016/j.tibtech.2018.01.006.
- Moore, K.L.; Persaud, T.V.N.; Torchia, M.G. . The Developing Human: Clinically Oriented Embryology; Elsevier—Health Sciences Division: Amsterdam, the Netherlands, 2019; pp. 181-192.
- Sara D’Aronco; Manuela Simonato; Luca Vedovelli; Aldo Baritussio; Giuseppe Verlato; Stefano Nobile; Chiara Giorgetti; Matteo Nespeca; Virgilio P. Carnielli; Paola Cogo; et al. Surfactant protein B and A concentrations are increased in neonatal pneumonia. Pediatric Research 2015, 78, 401-406, 10.1038/pr.2015.123.
- 13. Nespeca, M.; Giorgetti, C.; Nobile, S.; Ferrini, I.; Simonato, M.; Verlato, G.; Cogo, P.; Carnielli, V.P.; Does Whole-Body Hypothermia in Neonates with Hypoxic–Ischemic Encephalopathy Affect Surfactant Disaturated-Phosphatidylcholine Kinetics?. PLoS ONE 2016, 11, e0153328, 10.1371/journal.
- Guoqing Hu; Bao Jiao; Xinghua Shi; Russell P. Valle; Qihui Fan; Yi Y. Zuo; Physicochemical Properties of Nanoparticles Regulate Translocation across Pulmonary Surfactant Monolayer and Formation of Lipoprotein Corona. ACS Nano 2013, 7, 10525-10533, 10.1021/nn4054683.
- Mridula V. Dwivedi; Rakesh Kumar Harishchandra; Olga Koshkina; Michael Maskos; Hans-Joachim Galla; Size Influences the Effect of Hydrophobic Nanoparticles on Lung Surfactant Model Systems. Biophysical Journal 2014, 106, 289-298, 10.1016/j.bpj.2013.10.036.
- Moritz Beck-Broichsitter; Clemens Ruppert; Thomas Schmehl; Andreas Günther; Werner Seeger; Biophysical inhibition of synthetic vs. naturally-derived pulmonary surfactant preparations by polymeric nanoparticles. Biochimica et Biophysica Acta (BBA) - Biomembranes 2014, 1838, 474-481, 10.1016/j.bbamem.2013.10.016.
- Dorota Kondej; Tomasz R. Sosnowski; Effect of clay nanoparticles on model lung surfactant: a potential marker of hazard from nanoaerosol inhalation.. Environmental Science and Pollution Research 2015, 23, 4660-9, 10.1007/s11356-015-5610-4.
- Danielle Botelho; Bey F. Leo; Christopher Massa; Srijata Sarkar; Terry Tetley; Kian F. Chung; Shu Chen; Mary P. Ryan; Alexandra Porter; Elena N. Atochina-Vasserman; et al.Junfeng ZhangStephan SchwanderAndrew Gow Exposure to Silver Nanospheres Leads to Altered Respiratory Mechanics and Delayed Immune Response in an in Vivo Murine Model. Frontiers in Pharmacology 2018, 9, 213, 10.3389/fphar.2018.00213.
- Sinbad Sweeney; Bey Fen Leo; Shu Chen; Nisha Abraham-Thomas; Andrew J. Thorley; Andrew Gow; Stephan Schwander; Junfeng Jim Zhang; M.S.P. Shaffer; Kian Fan Chung; et al.Mary P. RyanAlexandra E. PorterTeresa D. Tetley Pulmonary surfactant mitigates silver nanoparticle toxicity in human alveolar type-I-like epithelial cells. Colloids and Surfaces B: Biointerfaces 2016, 145, 167-175, 10.1016/j.colsurfb.2016.04.040.
- Ching-Yun Hsu; Calvin T. Sung; Ibrahim A Aljuffali; Chun-Han Chen; Kai-Yin Hu; Jia-You Fang; Intravenous anti-MRSA phosphatiosomes mediate enhanced affinity to pulmonary surfactants for effective treatment of infectious pneumonia. Nanomedicine: Nanotechnology, Biology and Medicine 2018, 14, 215-225, 10.1016/j.nano.2017.10.006.
- Mohammad Doroudian; Ronan MacLoughlin; Fergus Poynton; Adriele Prina-Mello; Seamas Donnelly; Nanotechnology based therapeutics for lung disease. Thorax 2019, 74, 965-976, 10.1136/thoraxjnl-2019-213037.
- Giovanni Vento; Roberta Pastorino; Luca Boni; Francesco Cota; Virgilio Paolo Carnielli; Filip Cools; Carlo Dani; Fabio Mosca; J. Jane Pillow; Graeme Polglase; et al.Paolo TagliabueAnton H. L. C. Van KaamMaria Luisa VenturaMilena TanaChiara TironeClaudia AuriliaAlessandra LioCinzia RicciAlessandro GambacortaChiara ConsigliDanila D’OnofrioCamilla GizziLuca MassenziViviana CardilliAlessandra CasatiRoberto BottinoFederica PontiggiaElena CiarmoliStefano MartinelliLaura IlardiMariaRosa ColnaghiPiero Giuseppe MatassaValentina VendettuoliPaolo VillaniFrancesca FuscoDiego GazzoloAlberto RicottiFederica FerreroIlaria StasiRosario MagaldiGianfranco MaffeiGiuseppe PrestaRoberto PerniolaFrancesco MessinaGiovanna MontesanoChiara PoggiLucio GiordanoEnza RomaCarolina GrassiaGaetano AusanioFabrizio SandriGiovanna MescoliFrancesco GiuraGiampaolo GaraniAgostina SolinasMaria LucenteGabriella NigroAntonello Del VecchioFlavia PetrilloLuigi OrfeoLidia GrapponeLorenzo QuartulliAntonio ScorranoHubert MessnerAlex StafflerGiancarlo GarganoEleonora BalestriStefano NobileCaterina CacaceValerio MeliSara DallaglioBetta PasquaLoretta MattiaEloisa GittoMarcello VitalitiMaria Paola ReStefania VedovatoAlessandra GrisonAlberto BerardiFrancesco TorcettaIsotta GuidottiSandra Di FabioEugenia MaranellaIsabella MondelloStefano VisentinFrancesca Tormena Efficacy of a new technique - INtubate-RECruit-SURfactant-Extubate - "IN-REC-SUR-E" - in preterm neonates with respiratory distress syndrome: study protocol for a randomized controlled trial.. Trials 2016, 17, 414, 10.1186/s13063-016-1498-7.
- Stefano Nobile; Paolo Marchionni; Giovanni Vento; Valentina Vendettuoli; Claudio Marabini; Alessandra Lio; Cinzia Ricci; Domenica Mercadante; MariaRosa Colnaghi; Fabio Mosca; et al.Costantino RomagnoliVirgilio Carnielli New Insights on Early Patterns of Respiratory Disease among Extremely Low Gestational Age Newborns. Neonatology 2017, 112, 53-59, 10.1159/000456706.
- Heidi M Mansour; Meenach; Vogt; Ronald C McGarry; Kimberly W Anderson; J. Zach Hilt; Samantha A Meenach; Frederick G Vogt; Design, physicochemical characterization, and optimization of organic solution advanced spray-dried inhalable dipalmitoylphosphatidylcholine (DPPC) and dipalmitoylphosphatidylethanolamine poly(ethylene glycol) (DPPE-PEG) microparticles and nanoparticles for targeted respiratory nanomedicine delivery as dry powder inhalation aerosols. International Journal of Nanomedicine 2013, 8, 275-293, 10.2147/IJN.S30724.
- Nor Fadhilah Kamaruzzaman; Li Peng Tan; Ruhil Hayati Hamdan; Siew Shean Choong; Weng Kin Wong; Amanda Gibson; Alexandru Chivu; Maria De Fatima Pina; Antimicrobial Polymers: The Potential Replacement of Existing Antibiotics?. International Journal of Molecular Sciences 2019, 20, 2747, 10.3390/ijms20112747.
- Mary C Machado; Daniel Cheng; Keiko M Tarquinio; Thomas J Thomas Webster; Nanotechnology: Pediatric Applications. Pediatric Research 2010, 67, 500-504, 10.1203/pdr.0b013e3181d68e78.
- Joscelyn C. Mejías; Osric A. Forrest; Camilla Margaroli; David A. Frey Rubio; Liliana Viera; Jindong Li; Xin Xu; Amit Gaggar; Rabindra Tirouvanziam; Krishnendu Roy; et al. Neutrophil-targeted, protease-activated pulmonary drug delivery blocks airway and systemic inflammation. JCI Insight 2019, 4, 131468, 10.1172/jci.insight.131468.
- Neeraj Vij; Taehong Min; Manish Bodas; Aakruti Gorde; Indrajit Roy; Neutrophil targeted nano-drug delivery system for chronic obstructive lung diseases. Nanomedicine: Nanotechnology, Biology and Medicine 2016, 12, 2415-2427, 10.1016/j.nano.2016.06.008.
- James E. Dahlman; Carmen Barnes; Omar F. Khan; Aude Thiriot; Siddharth Jhunjhunwala; Taylor E. Shaw; Yiping Xing; Hendrik B. Sager; Gaurav Sahay; Lauren Speciner; et al.Andrew BaderRoman L. BogoradHao YinTim RacieYizhou DongShan JiangDanielle SeedorfApeksha DaveKamaljeet Singh SandhuMatthew J. WebberTatiana NovobrantsevaVera M. RudaAbigail K.R. Lytton-JeanChristopher G. LevinsBrian KalishDayna K. MudgeMario PérezLudmila AbezgauzPartha DuttaLynelle SmithKlaus CharisseMark W. KieranKevin FitzgeraldMatthias NahrendorfDganit DaninoRubin M. TuderUlrich H. Von AndrianAkin AkincDipak PanigrahyAvi SchroederVictor KotelianskyRobert LangerDaniel G. AndersonKamaljeet S. SanduVictor Kotelianski In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nature Nanotechnology 2014, 9, 648-655, 10.1038/nnano.2014.84.
- Silvano Dragonieri; Giorgio Pennazza; Pierluigi Carratu; Onofrio Resta; Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157-165, 10.1007/s00408-017-9987-3.
- Bhavna; Farhan J. Ahmad; Gaurav Mittal; Gaurav K. Jain; Geena Malhotra; Roop K. Khar; Aseem Bhatnagar; Nano-salbutamol dry powder inhalation: A new approach for treating broncho-constrictive conditions. European Journal of Pharmaceutics and Biopharmaceutics 2009, 71, 282-291, 10.1016/j.ejpb.2008.09.018.
- Nashwa El-Gendy; Eric M. Gorman; Eric J. Munson; Cory J. Berkland; Budesonide nanoparticle agglomerates as dry powder aerosols with rapid dissolution.. Journal of Pharmaceutical Sciences 2009, 98, 2731-46, 10.1002/jps.21630.
- Joscelyn C. Mejías; Krishnendu Roy; In-vitro and in-vivo characterization of a multi-stage enzyme-responsive nanoparticle-in-microgel pulmonary drug delivery system.. Journal of Controlled Release 2019, 316, 393-403, 10.1016/j.jconrel.2019.09.012.
- Samarjit Dey; Anirban Bhattacharjee; Debasis Pradhan; Prithwis Bhattacharyya; Daniala Chhunthang; Akash Handique; Angkita Barman; Mohd Yunus; How useful is extravascular lung water measurement in managing lung injury in intensive care unit?. Indian Journal of Critical Care Medicine 2017, 21, 494-499, 10.4103/ijccm.ijccm_40_17.
- Nobile, S.; Gnocchini, F.; Pantanetti, M.; Battistini, P.; Carnielli, V.P.; The importance of oxygen control reaffirmed: Experience of ROP reduction at a single tertiary-care NICU. . J. Pediatr. Ophtalmol. Strab. 2014, 51, 112-115.
- Farkhondeh Chaharband; Narsis Daftarian; Mozhgan Rezaei Kanavi; Reyhaneh Varshochian; Maliheh Hajiramezanali; Parisa Norouzi; Ehsan Arefian; Fatemeh Atyabi; Rassoul Dinarvand; Trimethyl chitosan-hyaluronic acid nano-polyplexes for intravitreal VEGFR-2 siRNA delivery: Formulation and in vivo efficacy evaluation. Nanomedicine: Nanotechnology, Biology and Medicine 2020, 26, 102181, 10.1016/j.nano.2020.102181.
- J. M. Lakawicz; W. J. Bottega; J. L. Prenner; H. F. Fine; An analysis of the mechanical behaviour of a detaching retina. Mathematical Medicine and Biology: A Journal of the IMA 2014, 32, 137-161, 10.1093/imammb/dqt023.
- Joseph M. Lakawicz; William J. Bottega; Howard F. Fine; Jonathan L. Prenner; On the mechanics of myopia and its influence on retinal detachment. Biomechanics and Modeling in Mechanobiology 2019, 19, 603-620, 10.1007/s10237-019-01234-1.
- Fernando J. Cabrera; Daniel C. Wang; Kartik Reddy; Ghanashyam Acharya; Crystal S. Shin; Challenges and opportunities for drug delivery to the posterior of the eye.. Drug Discovery Today 2019, 24, 1679-1684, 10.1016/j.drudis.2019.05.035.
- Asaf Shoval; Amos Markus; Zhixin Zhou; Xia Liu; Rémi Cazelles; Itamar Willner; Yossi Mandel; Anti-VEGF-Aptamer Modified C-Dots-A Hybrid Nanocomposite for Topical Treatment of Ocular Vascular Disorders.. Small 2019, 15, e1902776, 10.1002/smll.201902776.
- Fitsum Feleke Sahle; Sangyoon Kim; Kumar Kulldeep Niloy; Faiza Tahia; Cameron V. Fili; Emily Cooper; David J. Hamilton; Tao L. Lowe; Nanotechnology in regenerative ophthalmology. Advanced Drug Delivery Reviews 2019, 148, 290-307, 10.1016/j.addr.2019.10.006.
- Nicola Di Trani; Priya Jain; Corrine Ying Xuan Chua; Jeremy S. Ho; Giacomo Bruno; Antonia Susnjar; Fernanda P. Pons-Faudoa; Antons Sizovs; R. Lyle Hood; Zachary W. Smith; et al.Andrea BalleriniCarly Sue FilgueiraAlessandro Grattoni Nanofluidic microsystem for sustained intraocular delivery of therapeutics. Nanomedicine: Nanotechnology, Biology and Medicine 2019, 16, 1-9, 10.1016/j.nano.2018.11.002.




