
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Śliżewska | + 1709 word(s) | 1709 | 2021-01-07 08:03:50 | | | |
| 2 | Vicky Zhou | Meta information modification | 1709 | 2021-01-12 09:53:04 | | | | |
| 3 | Katarzyna Śliżewska | Meta information modification | 1709 | 2021-01-15 15:33:11 | | |
Video Upload Options
The gut microbiome can play important role in maintaining homeostasis in the human body. An imbalance in the gut microbiome can lead to pro-inflammatory immune responses and the initiation of disease processes, including cancer. The research results prove some strains of probiotics by modulating intestinal microbiota and immune response can be used for cancer prevention or/and as adjuvant treatment during anticancer chemotherapy.
1. Introduction
The human gastrointestinal tract is a reservoir of a complex and dynamic population of microorganisms (the gut microbiota) mainly containing bacteria (in number over 1014), which exerts a significant influence on the host during homeostasis and disease [1]. The presence of such a large count of intestinal bacteria means that the human body has about 10 times more prokaryotic cells than eukaryotic cells [2]. In the human intestines are found bacterial phyla: Firmicutes, Bacteroides, Actinobacteria, Fusobacteria, Proteobacteria, Verrucomicrobia, Sinicobacteria and Spirochaetes [3]. Two bacterial phyla, gram-positive Firmicutes (Bacillus spp., Lactobacillus spp. and Clostridium spp.) and gram-negative Bacteroidetes, predominate in human gut and represent about 90% of the bacterial population [4][5]. The gut microbiota develops and matures during the first 3 years of human life [2]. Enterotype (the type and proportion of microorganisms found in the intestines) may indirectly affect the host’s energy balance. The appropriate balance between bacterial populations ensures homeostasis of the gastrointestinal tract. However, the composition of the intestinal microbiome is susceptible to change. Thus, many factors such as improper diet, stress, gastrointestinal diseases, obesity or taking medications can lead to intestinal homeostasis disorders. As a result of imbalance of the digestive system may be proinflammatory immune responses and initiate disease processes, including cancer. Intestinal dysbiosis may be the reason for the tumorigenesis of both local gastro-intestinal cancers and tumors localized in distant sites of the body [6].
The use of probiotics has a beneficial effect on the human gut microbiome. Their main advantage is the effect on the development of the microbiota inhabiting the organism in the way ensuring proper balance between the bacteria that are necessary for a normal function of the organism and pathogens. Beneficial functions of probiotics lead to the restoration (in case of disturbance) and maintenance of intestinal homeostasis. Probiotics are live microorganisms which, when administered in adequate amounts, confer a health benefit on the host [7][8]. The sources of probiotics in the human diet are mainly silage (e.g., cabbage and cucumbers) and fermented milk products (e.g., yogurt, kefir). Probiotic microorganisms commonly used in human nutrition belong mainly to the genera: Lactobacillus, Bifidobacterium, Lactococcus, Streptococcus and Enterococcus. Moreover, some strains of Bacillus and Saccharomyces are used [9].
2. Mechanism of Probiotics Action in Cancer Prevention and Therapy
The anticarcinogenic activity of probiotics is based on: (1) modification of the intestinal microbiota composition, (2) metabolic activity of the intestinal microbiota, (3) production of compounds with anticarcinogenic activity, such as short-chain fatty acids and conjugated linoleic acid, (4) inhibition of cell proliferation and induction apoptosis in cancer cells, (5) influence on other mutagenic and carcinogenic factors, (6) binding and degradation of carcinogenic compounds present in the intestinal lumen, (7) immunomodulation and (8) improvement of the intestinal barrier. Figure 1 shows potential mechanisms of action of probiotics in the prevention of colorectal cancer development.
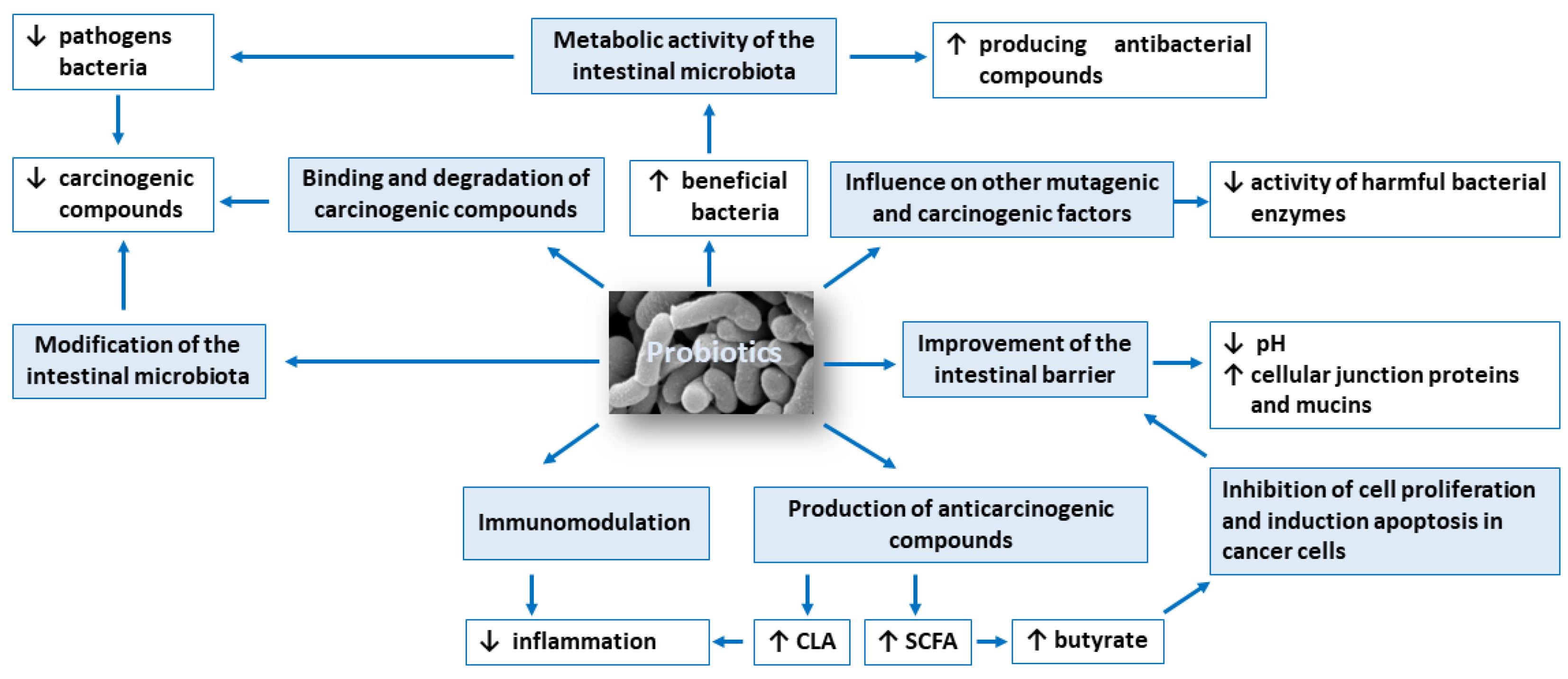
Symbols: ↓ decrease, ↑ increase.
2.1. Modification of the Intestinal Microbiota Composition
The healthy intestinal microbiota must be properly balanced and diversified to ensure homeostasis (eubiosis). Disturbance of the intestinal microbiota balance may result in a shortage of beneficial bacteria and an excess of pathogens (dysbiosis). Moreover, dysbiosis can cause a chronic inflammation and raise the production of carcinogenic compounds which increases the risk of developing colorectal cancer [10][11].
Sobhani et al. [12] compared samples of feces from healthy people and colorectal cancer patients. Their research shows that the number of Bacteroides and Prevotella genus were significantly higher in the colorectal cancer group. In the intestinal ecosystem, several species of the Lactobacillus type were present in lower amounts than bacteria of the genera Bacteroides, Eubacterium, Fusobacterium, Prevotella and Proteobacteria. It was also found that several species of the genus Salmonella and Clostridium were present in greater numbers in patients with colorectal cancer [13]. Some strains of Bacteroides spp. and Clostridium spp. are classified as bacteria which are involved in the pathogenesis of colorectal cancer. Bacteroides fragilis produces enterotoxigenic toxin (fragilysin), which affects the induction of inflammatory mediators, which leads to the progression of cancer [14]. The pathogenic strain of Escherichia coli can synthesize several toxins, for example, cytotoxic necrotizing agent (CNF), cytolethal distending toxins (CDT), and other various virulence factors. Streptococcus gallolyticus and Enterococcus faecalis can also be connected to colorectal cancer [15][16].
The intestinal microbiota has been linked to development of the gastrointestinal cancers also by production of toxic and genotoxic bacterial metabolites that can lead to mutations by binding specific cell surface receptors and affecting intracellular signal transduction [17].
Competitive exclusion of pathogenic microorganisms by probiotics can be related to competition for nutrients and adhesion to the intestinal mucosa. There are limited nutrients available at the distal part of the colon. Probiotics compete for nutrients and grow at the expense of different intestinal microbiota [18].
Probiotic strains compete with pathogenic microorganisms for adhesion and colonization to biological membranes, forming stable, thin layers called a biofilm [19]. The microbiological biofilm develops as a result of the adhesion of microorganisms to the surface and the production of extracellular polymers that facilitate adhesion and form a structural matrix. Biofilm is characterized by structural heterogeneity, genetic diversity, complexity of interaction and the presence of extracellular substances, i.e., polysaccharides, proteins, nucleic acids, phospholipids. The secretion of these compounds is the result of adaptation to the environment. Polysaccharide polymer substances play a major role in adhesion entirely, bridging the gaps created between microorganisms in biofilm. In the result, microorganisms become focused through polymerization or association. In the first stages of biofilm formation, polysaccharides are secreted the most intensively and help first cells attach to the surface. On the other hand, proteins are first accumulated on the cell surface and then, when released, they associate on the target surface. This is usually a mixture of collagen, elastin and other proteins. They form an extracellular matrix to which microorganisms adhere [20][21].
The results of few clinical trial studies showed the beneficial effect of probiotics on the composition of gut microbiota, thus on the host by improving intestinal barrier integrity, inhibiting growth of pathogens, and reducing metabolism of pro-carcinogenic substances. Ohara et al. investigated the differences between the intestinal flora of colorectal cancer patients and healthy subjects and assessed the possibility of using probiotics to prevent colorectal carcinogenesis [22]. After ingestion of the probiotic (Lactobacillus gasseri OLL2716: LG21), the Lactobacillus detection rate increased, a decrease in the total amount of Clostridium perfringens was found. Moreover, fecal pH indicated acidosis, synthesis of fecal putrefaction products was inhibited, while increase in the short-chain fatty acids and isobutyric acid concentration was observed.
A deterioration of the intestinal environment was observed in the colorectal cancer patients in comparison to the healthy controls, and the intestinal environment improved when probiotics was taken, suggest the possibility of preventing colorectal carcinoma with probiotics. Kotzampassi et al. demonstrated beneficial effects of probiotics (Lactobacillus: acidophilus, plantarum; Bifidobacterium lactis and Saccharomyces boulardii) in patients undergoing colorectal surgery for cancer [23]. The probiotics formulation significantly decreased the risk of postoperative complications, namely mechanical ventilation, infections and anastomotic leakage.
2.2. Inhibition of Cell Proliferation and Induction Apoptosis in Cancer Cells
Proliferation and apoptosis of cancer cells determine the rate of cancer development. During the cancer development process, these cells proliferate more than undergo apoptosis. Probiotics that are able to modulate the cellular proliferation and apoptosis are of great interest because cancer cells would be eliminated less aggressively, and apoptosis brings no damage to the neighbor cells and does not cause inflammation [24]. The apoptosis signaling pathways can be activated by probiotic bacteria (e.g., lactic acid bacteria; LAB) through a mitochondria-dependent (intrinsic) and a death receptor–dependent, mitochondria-independent (extrinsic) pathway (Figure 2) [25].
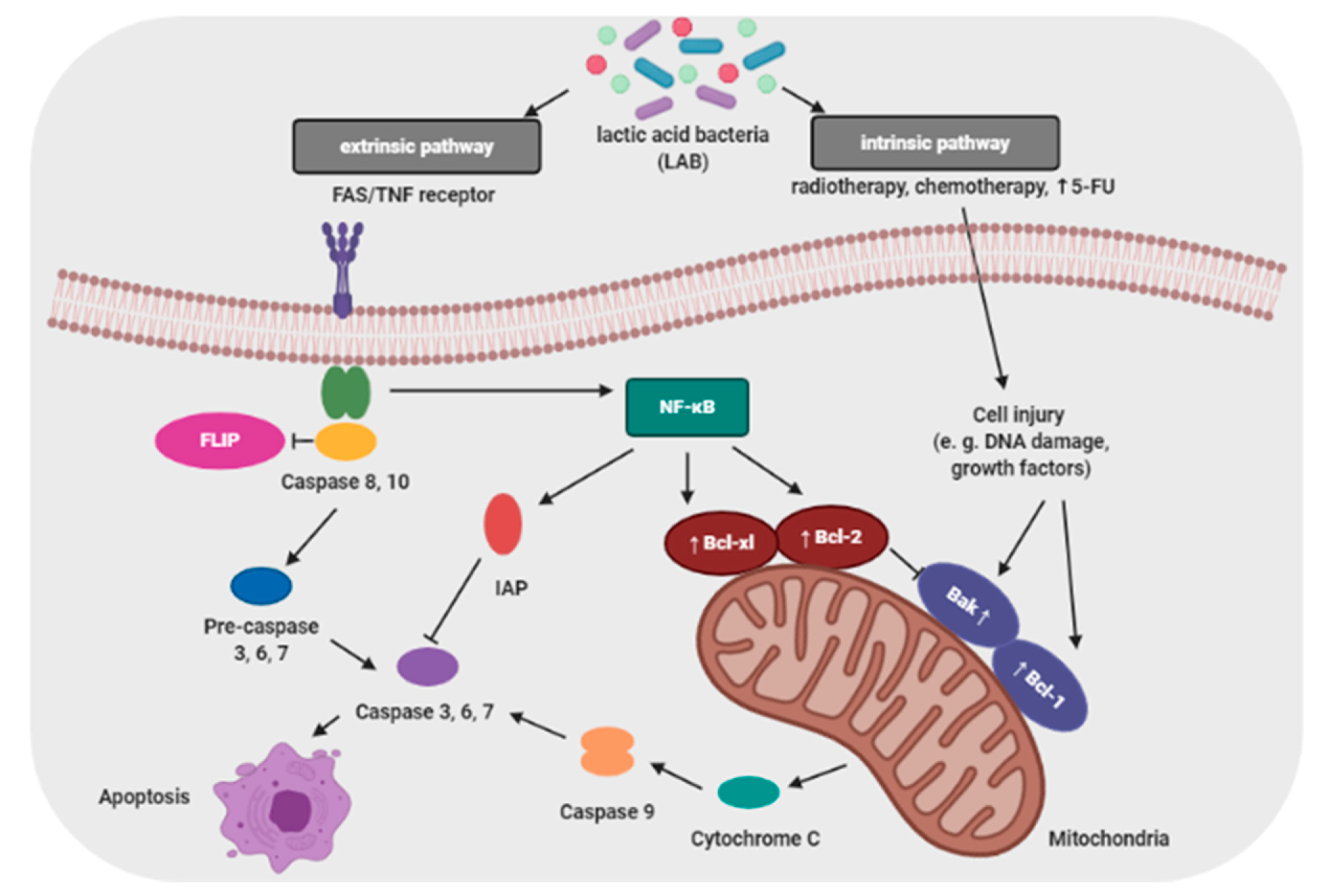
Baldwin et al. evaluated the antiproliferative activity of Lactobacillus acidophilus and Lactobacillus casei against LS513 gastric cell lines through cellular apoptosis [27]. Hwang et al. demonstrated that probiotic induced apoptosis in gastric cancer cells (KATO3) by inhibiting NF-κB and mTOR-mediated signaling [28]. Cousin et al. demonstrated that probiotic bacteria induce chromatin condensation, apoptotic bodies, DNA fragmentation, caspase activation, inactivation of mitochondrial trans-membrane potential and cell cycle arrest [29]. Probiotic strains such as Lactobacillus reuteri have been reported to influence hematological cancers, which enhanced TNF-induced apoptosis in human chronic myeloid leukemia derived cells [30].
The increased incidence of apoptosis of cancer cells induced by probiotics has been attributed to SCFAs, particularly butyrate, which are able to induce epigenetic changes, paralyze the cell cycle, and stimulate the expression of proapoptotic genes. Relationship exist between the amount of SCFAs in the feces and cell proliferation in the colonic crypts [31].
Probiotic Lactobacillus spp. induced selective genotoxic, cytotoxic, pro-apoptotic effects on leukemia and colon cancer cell lines, and as anti-inflammatory effects on macrophage cells at the molecular level.
In vitro experimental evidence suggests that probiotics used for their anti-cancer activity operate via a process of genotoxicity and cytotoxicity against tumor cells. Liu et al. explored the effects of Lactobacillus casei 01 on 4-nitroquinoline Noxide (4-NQO) induced genotoxicity and colon cancer cell line (HT29) [32]. Nami et al. reported that the metabolites from Lactobacillus acidophilus 36 YL exhibited the most potent cytotoxic effect against human cervical cancer cell lines (HeLa) and colorectal cancer cell lines (HT-29) [33].
Since in vitro studies using cell lines indicated that probiotics had proapoptotic effects on carcinoma cells probiotics-based regimens might be used as an adjuvant treatment during anticancer chemotherapy [27][30][32][34].
References
- Liśkiewicz, P.; Pełka-Wysiecka, J.; Wroński, M.; Bąba-Kubiś, A.; Samochowiec, J. Intestinal flora and the pathophysiology of depression and anxiety disorders—Current state of the art and future perspectives. Psychiatry 2018, 15, 70–76.
- Gonzalez, A.; Stombaugh, J.; Lozupone, C.; Turnbaugh, P.J.; Gordon, J.I.; Knight, R. The mind-body-microbial continuum. Dialogues. Clin. Neurosci. 2011, 13, 55–62.
- Snyder, L.; Peter, J.E.; Henkin, T.M.; Champress, W. Molecular Genetics of Bacteria, 4th ed.; ASM Press: Washington, DC, USA, 1997; pp. 53–66.
- Joseph, N.; Vasodavan, K.; Saipudin, N.A.; Yusof, B.N.M.; Kumar, S.; Nordin, S.A. Gut microbiota and short-chain fatty acids (SCFAs) profiles of normal and overweight school children in Selangor after probiotics administration. J. Funct. Foods 2009, 57, 103–111.
- Lach, G.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics 2018, 15, 36–59.
- Goodman, B.; Gardner, H. The microbiome and cancer. J. Pathol. 2018, 244, 667–676.
- Food and Agriculture Organization (FAO). Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; FAO: London, ON, Canada, 2002.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021.
- Dos Reis, S.A.; Da Conceição, L.L.; Siqueira, N.P.; Rosa, D.D.; Da Silva, L.L.; Peluzio, M.C.G. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19.
- Koboziev, I.; Webb, C.R.; Furr, K.L.; Grisham, M.B. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free. Radic. Biol. Med. 2013, 68, 122–133.
- Sobhani, I.; Tap, J.; Roudot-Thoraval, F.; Roperch, J.P.; Letulle, S.; Langella, P.; Corthier, G.; Tran Van Nhieu, J.; Furet, J.P. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 2011, 6, e16393.
- Kahouli, I.; Tomaro-Duchesneau, C.; Prakash, S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of action and current perspectives. J. Med. Microbiol. 2013, 62, 1107–1123.
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C. The bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 2015, 60, 208–215.
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131.
- Molska, M.; Reguła, J. Potential mechanisms of probiotics action in the prevention and treatment of colorectal cancer. Nutrients 2019, 11, 2453.
- Javanmard, A.; Ashtari, S.; Sabet, B.; Davoodi, S.H.; Rostami-Nejad, M.; Akbari, M.E.; Niaz, A.; Mortazavian, A.M. Probiotics and their role in gastrointestinal cancers prevention and treatment; an overview. Gastroenterol. Hepatol. 2018, 11, 284–295.
- Fooks, L.; Gibson, G. Probiotics as modulators of the gut flora. Br. J. Nutr. 2002, 88, S39–S49.
- Ohland, C.L.; MacNaughton, W.K. Probiotic bacteria and intestinal barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, 807–819.
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867.
- Dieltjens, L.; Appermans, K.; Lissens, M.; Lories, B.; Kim, W.; Van der Eycken, E.V.; Foster, K.R.; Steenackers, H.P. Inhibiting bacterial cooperation is an evolutionarily robust anti-biofilm strategy. Nat. Commun. 2020, 107, 1–11.
- Ohara, T.; Yoshino, K.; Kitajima, M. Possibility of preventing colorectal carcinogenesis with probiotics. Hepatogastroenterol 2010, 57, 1411–1415.
- Kotzampassi, K.; Stavrou, G.; Damoraki, G.; Georgitsi, M.; Basdanis, G.; Tsaousi, G.; Giamarellos-Bourboulis, E.J. A four-probiotics regimen reduces postoperative complications after colorectal surgery: A randomized, double-blind, placebo-controlled study. World J. Surg. 2015, 39, 2776–2783.
- Fearon, E.R. Molecular genetics of colorectal cancer. Annu. Rev. Pathol. Mech. 2011, 6, 479–507.
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014, 20, 7878–7886.
- De Vries, E.G.; Gietema, J.A.; de Jong, S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin. Cancer Res. 2006, 12, 2390–2393.
- Baldwin, C.; Millette, M.; Oth, D.; Ruiz, M.T.; Luquet, F.M.; Lacroix, M. Probiotic Lactobacillus acidophilus and L. casei mix sensitize colorectal tumoral cells to 5-fluorouracilinduced apoptosis. Nutr. Cancer. 2010, 62, 371–378.
- Hwang, J.W.; Baek, Y.M.; Yang, K.E.; Yoo, H.S.; Cho, C.K.; Lee, Y.W. Lactobacillus casei extract induces apoptosis in gastric cancer by inhibiting NF-κB and mTOR-mediated signalling. Integr. Cancer Ther. 2013, 12, 165–173.
- Cousin, F.J.; Jouan-Lanhouet, S.; Dimanche-Boitrel, M.T.; Corcos, L.; Jan, G. Milk fermented by Propionibacterium freudenreichii induces apoptosis of HGT-1 human gastric cancer cells. PLoS ONE 2012, 7, 31892.
- Iyer, C.; Kosters, A.; Sethi, G.; Kunnumakkara, A.B.; Aggarwal, B.B.; Versalovic, J. Probiotic Lactobacillus reuteri promotes TNFinduced apoptosis in human myeloid leukemia-derived cells by modulation of NF-kappaB and MAPK signalling. Cell. Microbiol. 2008, 10, 1442–1452.
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052.
- Liu, C.T.; Chu, F.J.; Chou, C.C.; Yu, R.C. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat. Res. 2011, 721, 157–162.
- Nami, Y.; Abdullah, N.; Haghshenas, B.; Radiah, D.; Rosli, R.; Khosroushahi, A.Y. Probiotic potential and biotherapeutic effects of newly isolated vaginal Lactobacillus acidophilus 36YL strain on cancer cells. Anaerobe 2014, 28, 29–36.
- Shinnoh, M.; Horinaka, M.; Yasuda, T.; Yoshikawa, S.; Morita, M. Clostridium butyricum MIYAIRI 588 shows antitumor effects by enhancing the release of TRAIL from neutrophils through MMP-8. Int. J. Oncol. 2013, 42, 903–911.




