
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Masamichi Hirose | + 1522 word(s) | 1522 | 2021-11-04 03:43:52 | | | |
| 2 | Peter Tang | Meta information modification | 1522 | 2021-11-16 02:57:26 | | |
Video Upload Options
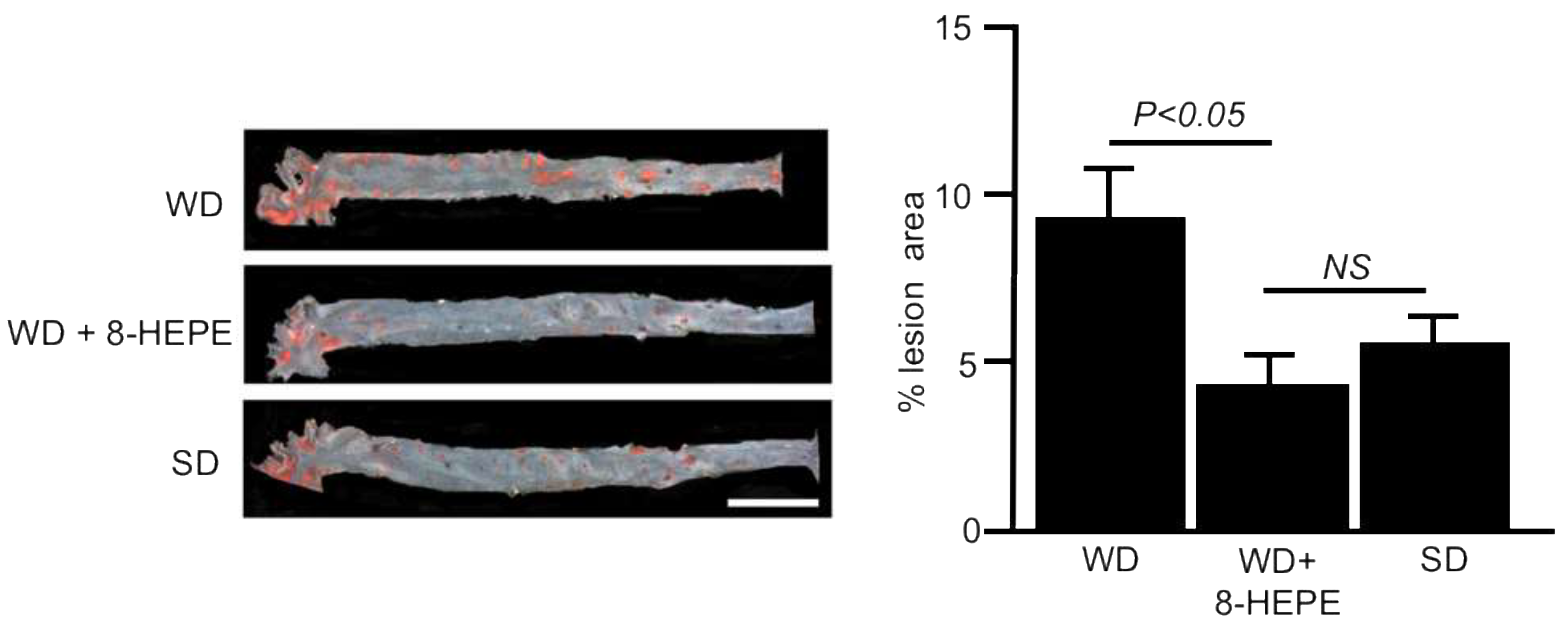
Euphausia pacifica (E. pacifica), also called North Pacific krill, is a small, red crustacean similar to shrimp that flourishes in the North Pacific Ocean. E. pacifica oil contains 8-hydroxyeicosapentaenoic acid (8-HEPE) at a level more than 10 times higher than Euphausia superba oil. 8-HEPE can activate the transcription of peroxisome proliferator-activated receptor alpha (PPARα), PPARγ, and PPARδ to levels 10, 5, and 3 times greater than eicosapentaenoic acid, respectively. 8-HEPE has beneficial effects against metabolic syndrome (reduction in body weight gain, visceral fat area, amount of gonadal white adipose tissue, and gonadal adipocyte cell size), dyslipidemia (reduction in serum triacylglycerol and low-density lipoprotein cholesterol and induction of serum high-density lipoprotein cholesterol), atherosclerosis, and nonalcoholic fatty liver disease (reduction in triglyceride accumulation and hepatic steatosis in the liver) in mice.
1. Introduction
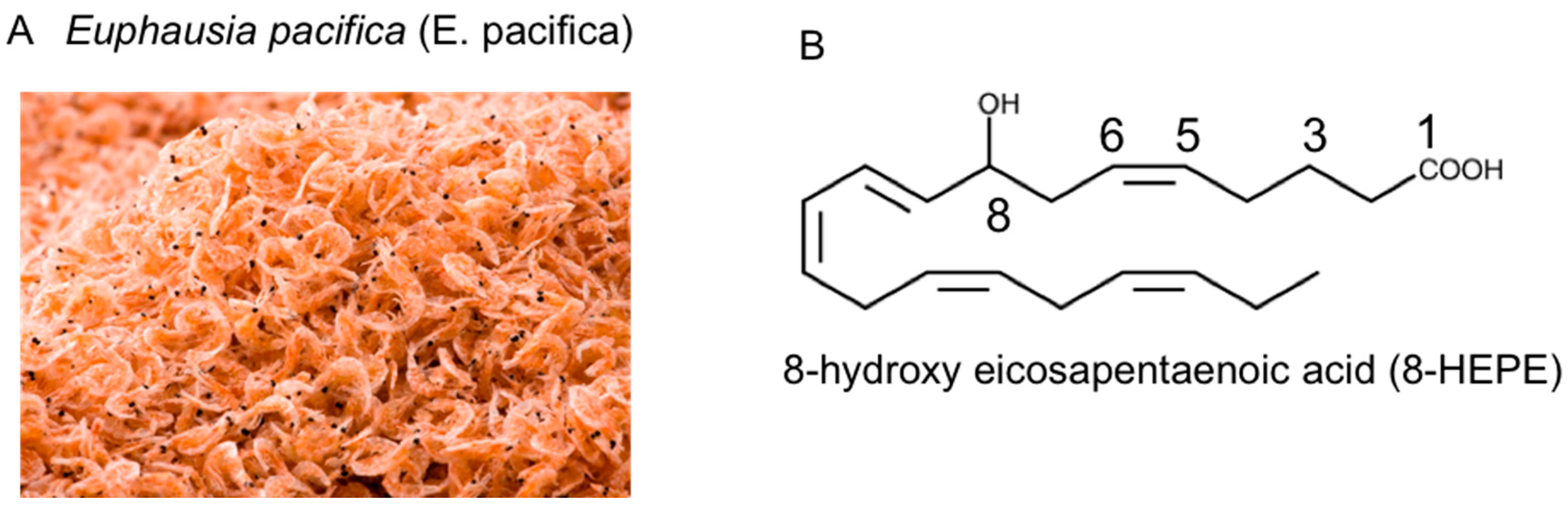
2. Chemical Features of E. pacifica
2.1. Lipids of E. pacifica
2.2. 8-HEPE Extracted from E. pacifica and PPAR Activation
3. Potential Benefits of 8-HEPE Extracted from E. pacifica against NAFLD and Its Associated Diseases
3.1. Effects of 8-HEPE Extracted from E. pacifica on Metabolic Syndrome
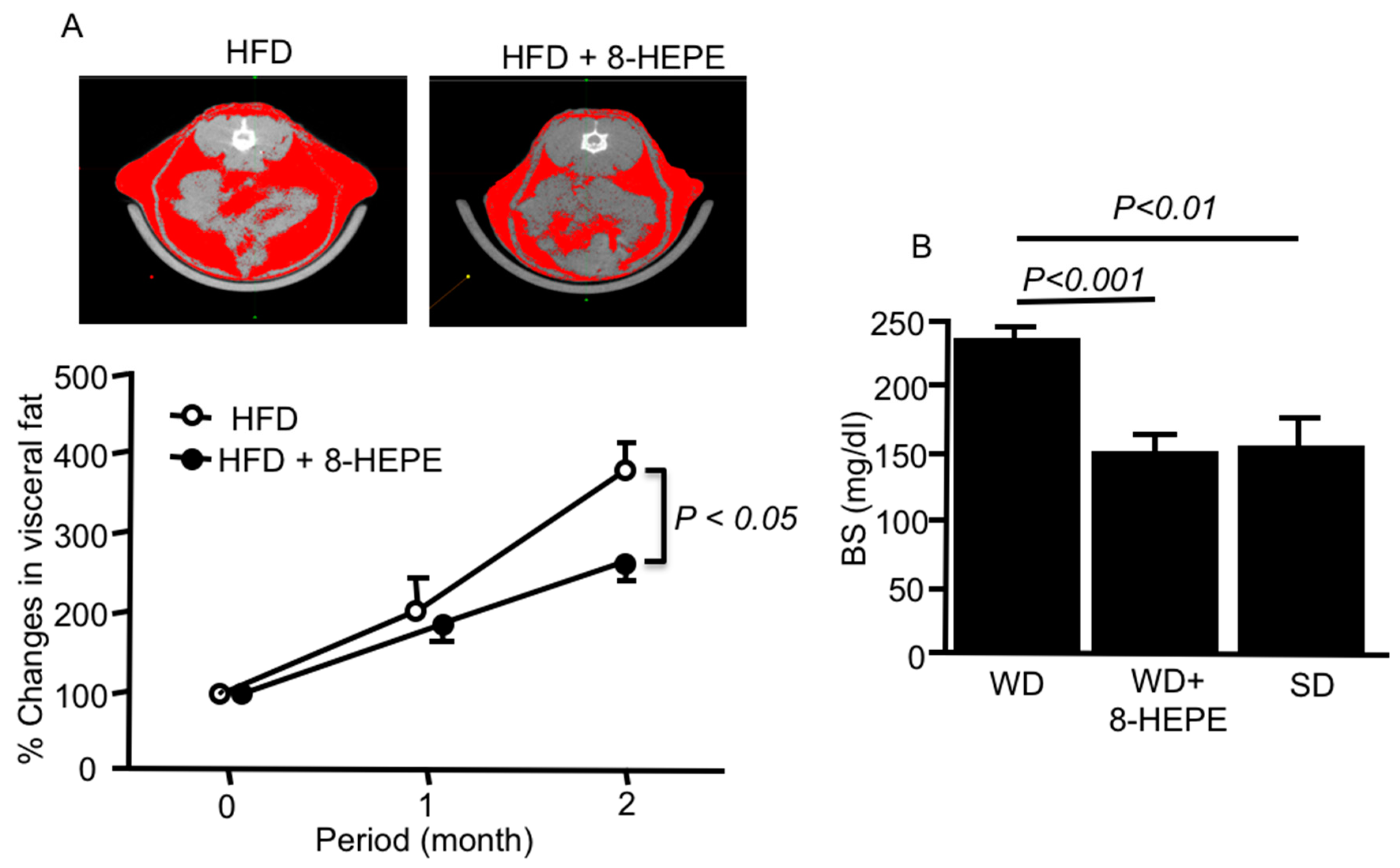
3.2. Effects of 8-HEPE Extracted from E. pacifica on Dyslipidemia
3.3. Effects of 8-HEPE Extracted from E. pacifica on NAFLD
3.4. Effects of 8-HEPE Extracted from E. Pacifica on Atherosclerosis
References
- Yamada, H.; Yamazaki, Y.; Koike, S.; Hakozaki, M.; Nagahora, N.; Yuki, S.; Yano, A.; Tsurumi, K.; Okumura, T. Lipids, fatty acids and hydroxy-fatty acids of Euphausia pacifica. Sci. Rep. 2017, 7, 9944.
- Yamada, H.; Oshiro, E.; Kikuchi, S.; Hakozaki, M.; Takahashi, H.; Kimura, K.I. Hydroxyeicosapentaenoic acids from the Pacific krill show high ligand activities for PPARs. J. Lipid Res. 2014, 55, 895–904.
- Lee, D.; Shin, Y.; Roh, J.S.; Ahn, J.; Jeoong, S.; Shin, S.S.; Yoon, M. Lemon Balm Extract ALS-L1023 regulates obesity and improves insulin sensitivity via activation of hepatic PPARα in high-fat diet-fed obese C57BL/6J mice. Int. J. Mol. Sci. 2020, 21, 4256.
- Yu, Y.H.; Ginsberg, H.N. Adipocyte signaling and lipid homeostasis: Sequelae of insulin-resistant adipose tissue. Circ. Res. 2005, 96, 1042–1052.
- Hirose, M.; Yamada, H.; Kamata, Y.; Yoshida, K.; Sasaki, H.; Tanji, M.; Ibi, M. Preventive Effect of New Functional Material Derived from E. pacifica on Visceral Fat. Society of Cerebral-Cardio-Vascular Anti-Aging 2015 Abstract. Available online: http://www.plus-s-ac.com/ccvaa/english.html (accessed on 28 November 2015).
- Yamada, H.; Kikuchi, S.; Hakozaki, M.; Motodate, K.; Nagahora, N.; Hirose, M. 8-Hydroxyeicosapentaenoic acid Ddcreases plasma and hepatic triglycerides via activation of peroxisome proliferator-activated receptor alpha in high-fat diet-induced obese mice. J. Lipids. 2016, 7498508.
- Hansen, M.K.; McVey, M.J.; White, R.F.; Legos, J.J.; Brusq, J.M.; Grillot, D.A.; Issandou, M.; Barone, F.C. Selective CETP inhibition and PPARalpha agonism increase HDL cholesterol and reduce LDL cholesterol in human ApoB100/human CETP transgenic mice. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 196–202.
- Saito, M.; Ishida, N.; Yamada, H.; Ibi, M.; Hirose, M. 8-HEPE- concentrated materials from Pacific krill improve plasma cholesterol levels and hepatic steatosis in high cholesterol diet-fed low-density lipoprotein (LDL) receptor-deficient mice. Biol. Pharm. Bull. 2020, 43, 919–924.
- Wen, Y.; Dai, J.H.; Gao, Q. Effects of Omega-3 fatty acid on major cardiovascular events and mortality in patients with coronary heart disease: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 470–475.
- Lopez-Huertas, E. The effect of EPA and DHA on metabolic syndrome patients: A systematic review of randomised controlled trials. Br. J. Nutr. 2012, 107, 185–194.
- Lee, J.; Park, Y.; Koo, S. ATP-binding cassette transporter A1 and HDL metabolism: Effects of fatty acids. J. Nutr. Biochem. 2012, 23, 1–7.
- Landry, Y.; Denis, M.; Nandi, S.; Bell, S.; Vaughan, A.; Zha, X. ATP-binding cassette transporter A1 expression disrupts raft membrane microdomains through its ATPase-related functions. J. Biol. Chem. 2006, 281, 36091–36101.
- Phillips, M. Molecular mechanisms of cellular cholesterol efflux. J. Biol. Chem. 2014, 289, 24020–24029.
- Vedhachalam, C.; Duong, P.; Nickel, M.; Nguyen, D.; Dhanasekaran, P.; Saito, H.; Rothblat, G.H.; Lund-Katz, S.; Phillips, M.C. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high-density lipoprotein particles. J. Biol. Chem. 2007, 282, 25123–25130.
- Wilson, C.G.; Tran, J.L.; Erion, D.M.; Vera, N.B.; Febbraio, M.; Weiss, E.J. Hepatocyte-specific disruption of CD36 attenuates fatty liver and improves insulin sensitivity in HFD-fed mice. Endocrinology 2016, 157, 570–585.
- Hajri, T.; Han, X.X.; Bonen, A.; Abumrad, N.A. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J. Clin. Invest. 2002, 109, 1381–1389.
- Bhatia, L.S.; Curzen, N.P.; Calder, P.C.; Byrne, C.D. Non-alcoholic fatty liver disease: A new and important cardiovascular risk factor? Eur. Heart J. 2012, 33, 1190–1200.
- Gastaldelli, A.; Kozakova, M.; Hojlund, K.; Flyvbjerg, A.; Favuzzi, A.; Mitrakou, A.; Balkau, B. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009, 49, 1537–1544.
- Sung, K.C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty liver, insulin resistance, and features of metabolic syndrome: Relationships with coronary artery calcium in 10,153 people. Diabetes Care. 2012, 35, 2359–2364.
- Parolini, C.; Bjorndal, B.; Busnelli, M.; Manzini, S.; Ganzetti, G.S.; Dellera, F.; Ramsvik, M.; Bruheim, I.; Berge, R.K.; Chiesa, G. Effect of Dietary Components from Antarctic Krill on Atherosclerosis in apoE-Deficient Mice. Mol. Nutr. Food Res. 2017, 61, 1700098.
- Ibi, M.; Saito, M.; Ishida, N.; Yamada, Y.; Hirose, M. 8-HEPE concentrated materials from pacific krill suppresses atherosclerosis in apoE-deficient mice. Circulation 2019, 140 (Suppl. 1), A11153.
- Park, Y.M. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014, 46, e99.
- Kato, R.; Mori, C.; Kitazato, K.; Arata, S.; Obama, T.; Mori, M.; Takahashi, K.; Aiuchi, T.; Takano, T.; Itabe, H. Transient increase in plasma oxidized LDL during the progression of atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 33–39.
- Moore, K.J.; Kunjathoor, V.V.; Koehn, S.L.; Manning, J.J.; Tseng, A.A.; Silver, J.M.; McKee, M.; Freeman, M.W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 2005, 115, 2192–2201.
- Zhu, X.; Ng, H.P.; Lai, Y.C.; Craigo, J.K.; Nagilla, P.S.; Raghani, P.; Nagarajan, S. Scavenger receptor function of mouse Fcγ receptor III contributes to progression of atherosclerosis in apolipoprotein E hyperlipidemic mice. J. Immunol. 2014, 193, 2483–2495.




