Major Depressive Disorder (MDD), colloquially known as depression, is a debilitating condition affecting an estimated 3.8% of the population globally, of which 5.0% are adults and 5.7% are above the age of 60. MDD is differentiated from common mood changes and short-lived emotional responses due to subtle alterations in gray and white matter, including the frontal lobe, hippocampus, temporal lobe, thalamus, striatum, and amygdala. It can be detrimental to a person’s overall health if it occurs with moderate or severe intensity. It can render a person suffering terribly to perform inadequately in their personal, professional, and social lives. Depression, at its peak, can lead to suicidal thoughts and ideation. Antidepressants manage clinical depression and function by modulating the serotonin, norepinephrine, and dopamine neurotransmitter levels in the brain.
1. Introduction
Major Depressive Disorder (MDD) is considered to be the most frequent psychiatric disorder
[1] and, according to the World Health Organization (WHO), the leading cause of disability
[2]. Low mood, diminished interest in daily activities, guilt, loss of pleasure, difficulty concentrating, low self-esteem, trouble sleeping, and altered appetite are some of the MDD symptoms. These issues can become chronic or recurrent, with severe consequences on a person’s ability to carry out daily activities. At its worst, depression can lead to suicidal thoughts
[3]. Depression has been related to an increased chance of suffering from other severe illnesses, such as cardiovascular disease
[4], stroke
[3], Alzheimer’s disease
[5], epilepsy
[6], diabetes
[7], and cancer
[8]. Depressive symptoms are more commonly seen in older people, but this is due to factors linked with ageing, including physical disability
[9], cognitive deficits, socioeconomic drawbacks, and other factors
[10]. Treatment-resistant depression (TRD) can be caused by continuous exposure to environmental stressors during development
[11].
Almost all antidepressants work the same way and effectively treat severe MDD across the lifespan
[12][13]. However, antidepressant therapy has a number of adverse side effects, including sedation, headaches, decreased blood pressure, insomnia, weight gain, indigestion, feeling agitated, dry mouth, diarrhea, and sexual dysfunction
[14]. This frequently leads to poor patient compliance, resulting in a recurrence of depressive symptoms and a higher risk of suicide
[14].
2. Neurochemistry of Depression: The Monoamine Hypothesis
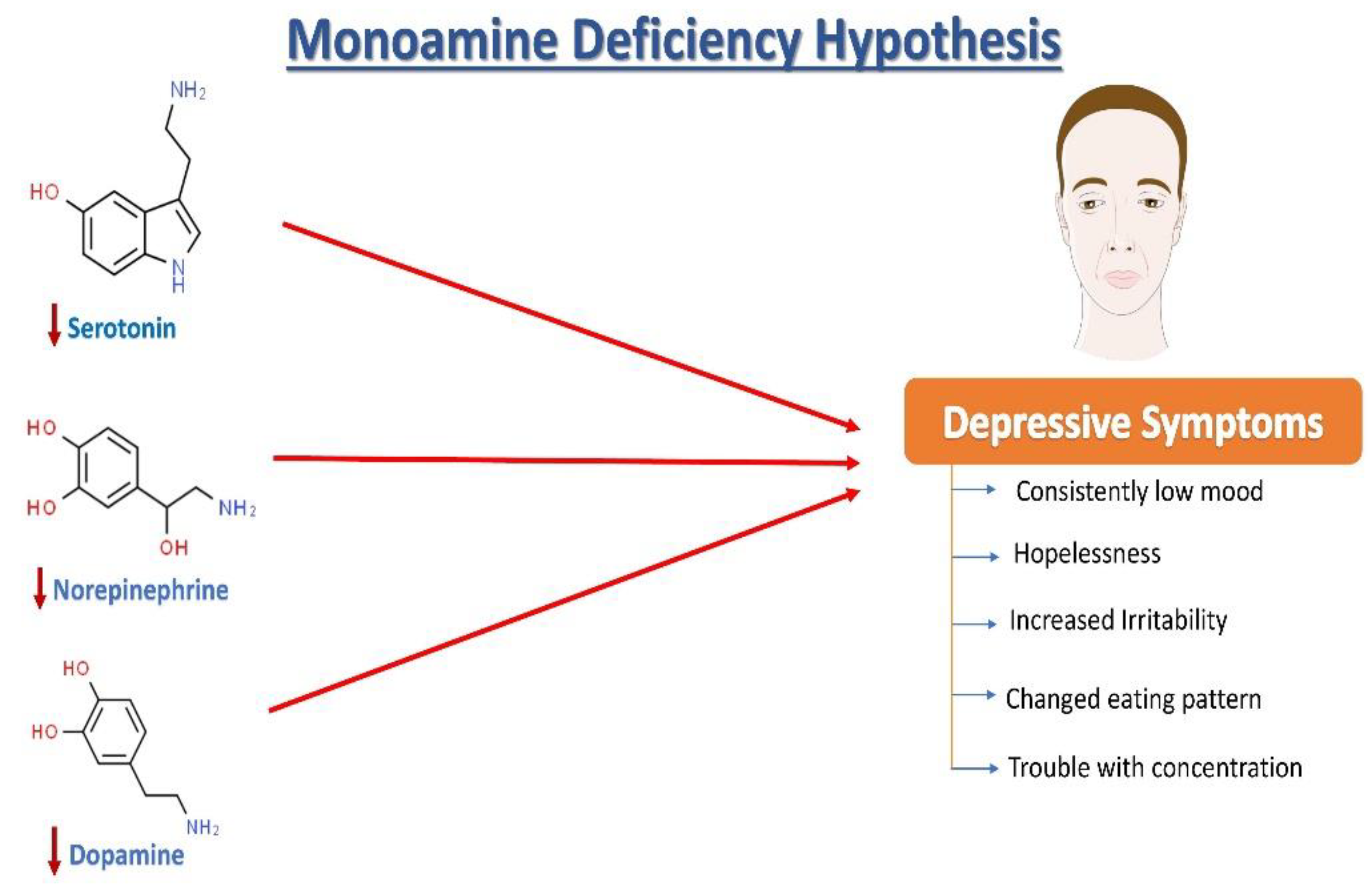
Norepinephrine (NE), serotonin (5-hydroxytryptamine, 5HT), and dopamine (DA) dysregulation are linked to the pathological changes seen in depression
[15] (
Figure 1). According to the monoamine hypothesis of depression, NE, 5HT, and DA work in synchrony to regulate emotions and mood
[15]. In the depressed mood, the dysregulation of these three monoamines is observed, along with extracellular 5HT levels being lower than average. It has been reported that monoamines and metabolites are found to be lower in the urine, blood, and cerebrospinal fluid (CSF) of patients with depression as compared to age-matched controls
[16].
Figure 1. The monoamine hypothesis of depression. The reduced levels of serotonin, norepinephrine, and dopamine have been observed and are understood as one of the main factors responsible for the generation of depressive symptoms.
The sympathetic nervous system’s primary neurotransmitter, NE, is produced in the locus coeruleus (LC) and is capable of sending projections all over the CNS. Research on depression began with a focus on the noradrenergic system, starting with introducing the “motor activation deficit” hypothesis
[16]. Patients with depression and victims of suicidal behavior have been seen to show deficits in the noradrenergic system in the LC compared with healthy individuals
[17]. Furthermore, some genetic changes in norepinephrine transporters (NETs) are highly plausible to be associated with psychiatric diseases
[18][19]. It has been discovered that heterogeneously projecting neurons from the LC can separately lead to modulation of fear and learning, emphasizing that an imbalance in the activation of these neuronal groups may be linked with post-traumatic stress disorder (PTSD) and depression in rats
[15]. Another monoamine neurotransmitter, DA, is well known to play an essential role in motivation, concentration, reward, psychomotor speed, and emotional response
[15]. Notably, changes in DA functioning have also been linked to depression-related fatigue symptoms
[20]. Depressed patients have lower DA metabolites in their CSF, which supports this hypothesis
[21]. Pharmacologically, increased dopamine binding and availability of the dopamine transporter (DAT) in the striatum have been observed to improve depression symptoms in humans
[22]. Furthermore, ropinirole, a dopaminergic targeting drug, has improved antidepressant treatment responsiveness in refractory patients
[23].
Serotonin is synthesized in the dorsal raphe nucleus from tryptophan (via a 5-OH-tryptophan intermediate). The dorsal raphe nucleus sends projections to the whole CNS including, remarkably, the brain areas that are vulnerable to stress, such as the hippocampus. Serotonin is a widely distributed neurotransmitter that also acts as a neuromodulator
[24][25][26]. Ever since its discovery, 5HT in the brain has been linked to circadian rhythms, sleep, cognitive abilities, appetite, motor activities, and many more biological functions
[27]. Furthermore, 5HT involvement in response to stress
[28] and psychiatric disease
[29] has received a lot of attention. A decrease in serum 5HT
[30] and plasma tryptophan
[31] levels and alterations in metabolite levels of 5HIAA (5-hydroxyindoleacetic acid) in CSF have been observed in patients with a depressive illness
[31]. Serotonin and its metabolites however have not been found to be a persistent biomarker of depression
[26]. The function of 5HT receptors (particularly autoreceptors) and 5HT transporters is also being studied in patients with depression
[26].
3. Growth Factors Involved in Depression
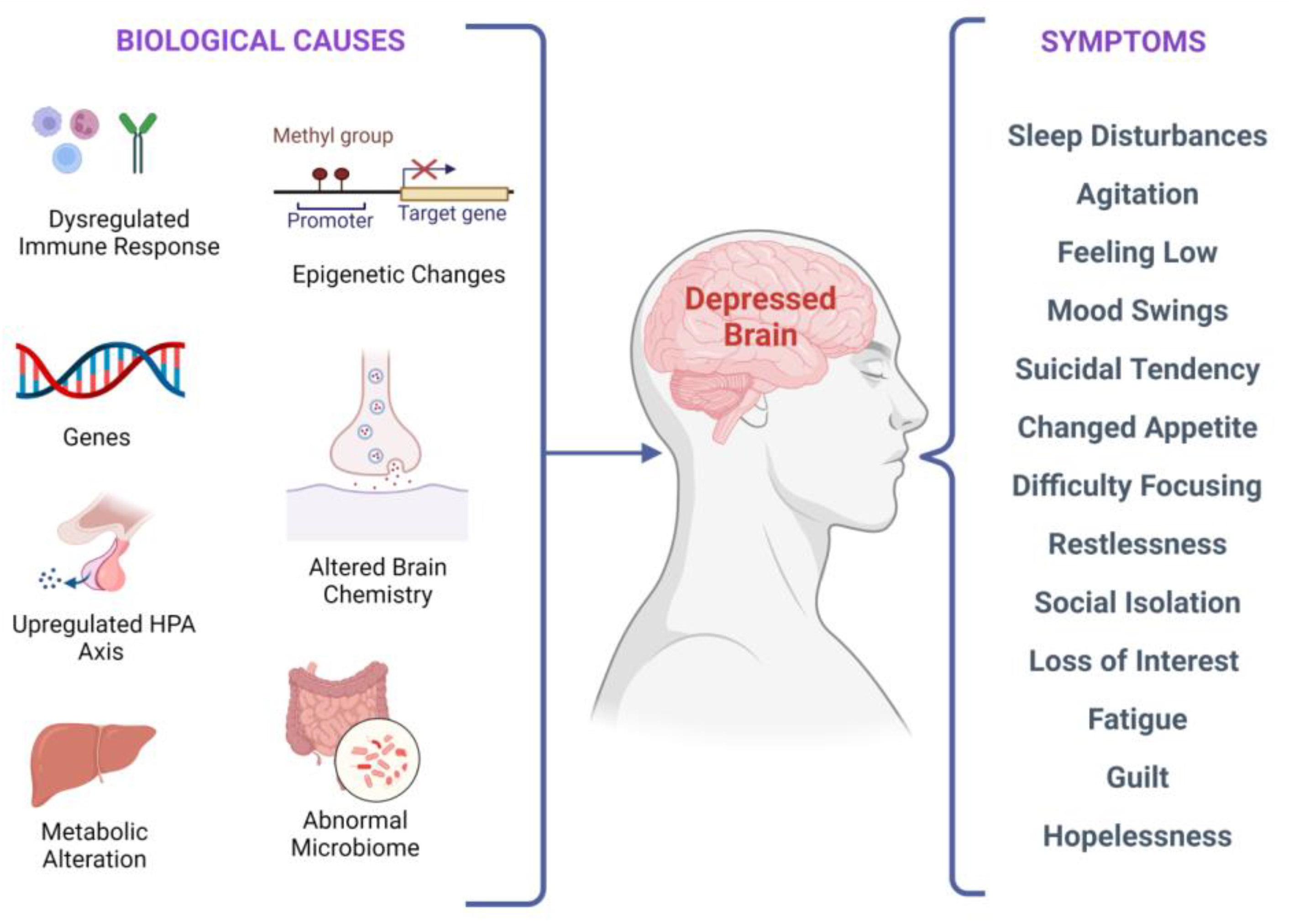
There are several biological factors associated with depressive symptoms, as shown in
Figure 2. It has been investigated that stress-induced epigenetic changes can lead to depression
[32][33]. Two meta-analyses of studies examining temporal lobe structures in MDD indicated that patients with recurrent depression have a smaller hippocampus
[34][35]. Synaptic plasticity in neural circuits associated with depressive behaviors is regulated by the brain-derived neurotrophic factor (BDNF)
[36][37][38]. Interestingly, stress-induced impairments in the brain structure and synaptic plasticity may be reversed by BDNF upregulation, leading to flexibility in cognition and an elevated capacity to acclimatize to environmental changes that might stimulate depressive episodes. According to current research, in depressed subjects, BDNF levels in the blood are lower, and they increase with antidepressant treatment
[39]. Additionally, elevated BDNF plasma levels have been linked to better treatment outcomes regardless of the medication used
[40].
Figure 2. Biological factors and clinical manifestations associated with depression. There are multiple biological causes at molecular, genetic, epigenetic, cellular, and systems levels. These causes result in clinical depression and can have a plethora of symptoms that may vary in different individuals.
Furthermore, anxiety, depression risk, neuroticism, and serotonergic neurotransmission have all been linked to altered serum BDNF levels and BDNF gene polymorphism
[2]. Lithium augmentation refers to the addition of lithium to an antidepressant in the acute treatment phase of depression
[41][42]. Antidepressant augmentation with lithium is a well-studied augmentation therapy for patients with depression who have not been responding well to antidepressant therapy
[41]. Moreover, lithium augmentation has the ability to increase BDNF concentrations in the serum
[40].
The reduced levels of nerve growth factor (NGF) are involved in the pathophysiology of depression. Fluoxetine and lithium are known to treat depression by upregulating NGF protein levels in the hippocampus
[43]. Neurotrophin-3 (NT-3) is responsible for neuron proliferation, differentiation, and survival. NT-3 also promotes the growth of axons and dendrites. Clinical data from post-mortem research showed that people with depressive disorders have lower NT-3 levels in their parietal brain
[44][45]. The decreased glial cell line-derived neurotrophic factor (GDNF) in the serum is correlated with the development of depressive symptoms. GDNF has a crucial role in the survival and maintenance of monoaminergic neurons. It has been reported that reduced GDNF levels abnormally regulate serotonergic neurons and reduce post and pre-synaptic serotonin receptors in patients with depression
[46]. Fibroblast growth factor-2 (FGF-2) is another growth factor associated with depression
[47]. Recent research has suggested that BDNF may signal via the Akt and GSK3 pathways
[48].
Further, it has been seen that erythropoietin impacts neuroplasticity and could be used to treat depression in the future. A study by Miskowiak et al. recruited 40 patients with a bipolar mood disorder and 40 patients with TRD. The patients received either a weekly intravenous infusion of erythropoietin or saline. It was observed that a single dose of erythropoietin (Eprex; 40,000IU) could improve cognitive functioning and reduce the neurocognitive processing involved in negative emotional information in normal versus people with depression in a way that is similar to antidepressants’ effects
[49]. In the limited research conducted on unipolar depression, inositol, a component of the intracellular phosphatidyl-inositol second-messenger system, has also been shown to be effective
[50]. Finally, peripheral VGF (nonacronymic) showed a reduced expression in MDD patients, and recombinant VGF administration causes antidepressant effects in rats
[50].
4. Neural Stem Cells and Depression
NSCs have garnered interest in recent years with the extensive published literature elucidating that the adult brain maintains multipotent NSCs in contrast to the old dogma of the brain being a generally invariable and quiescent organ that lacks the flexibility to regenerate. With their most generally accepted distinguishing traits, NSCs are also ascribed to the so-called tissue stem cells
[51], featuring the power to stay undifferentiated without an outlined phenotype under specific conditions, the power of dividing and proliferating (self-renewal), and also the ability to be differentiated into a progeny like neurons, oligodendroglia, and astroglia upon neurogenesis initiation. They are the unique types of competent cells found within the adult mammalian brain’s “neurogenic” regions, such as the hippocampus
[52], subventricular zone
[53], and neural structures
[54], and might create neurons both spontaneously and in response to local signals induction. Neurogenesis (NG) is assumed to need an explicit set of signaling cues to be delivered to cells that are neurogenic in a very spatially and temporally coordinated manner by their surroundings so as to activate stem cells or progenitors to develop new neurons and, in addition to the well-known modulators
[55], injury is considered to be sufficient to activate neurogenesis. Neurogenesis is also stimulated by the expression of BDNF
[56]. NSCs are often extracted from adult brain tissues, including post-mortem brain tissue
[57], and become significant candidates for increasing or restoring the quality and function of brain tissue affected with CNS-related illnesses. The NSCs are clonally expanded in vitro, genetically manipulated, or stimulated to transform CNS cell lineages
[57]. Understanding how adult neurogenesis is regulated has required significant work.
Growth factors, transmitters, enzymes, tissue hormones, neuromodulators, and antibodies are predicted to be secreted into the local tissue environment by activated cells, eliciting desirable tissue responses. In damaged neuronal and glial networks, the newly empowered cells and their progeny can operate as functional enhancers and scaffold “healing agents.” These properties have led to substantial advancements in the invention of therapies for trauma and perfusion issues such as stroke
[58], ischemia, or neurodegeneration-related conditions
[58][59][60]. Not unexpectedly, the prospects of NSCs in mental health care are being hotly debated. Numerous psychiatric illnesses are likely to possess genetic variants and specific cellular and anatomical correlations that are mostly unknown
[58].
In depression, a reduction of neurogenesis is often seen in the hippocampus
[61]. This further implies that neurogenesis deficiencies might cause the symptoms associated with depression, while enhanced neurogenesis can mediate antidepressant action and ease symptoms. However, various conflicting reports regarding the role of neurogenesis in alleviating depression must be first reconciled before this bidirectional concept’s complete legitimacy is established
[62]. The activation of adult hippocampal neurogenesis leads to the transformation of neural somatic cell progeny to mature CNS neurons. These CNS neurons then acquire functional and morphological qualities to integrate into existing neural networks or replace various other brain cells that have died
[63][64].