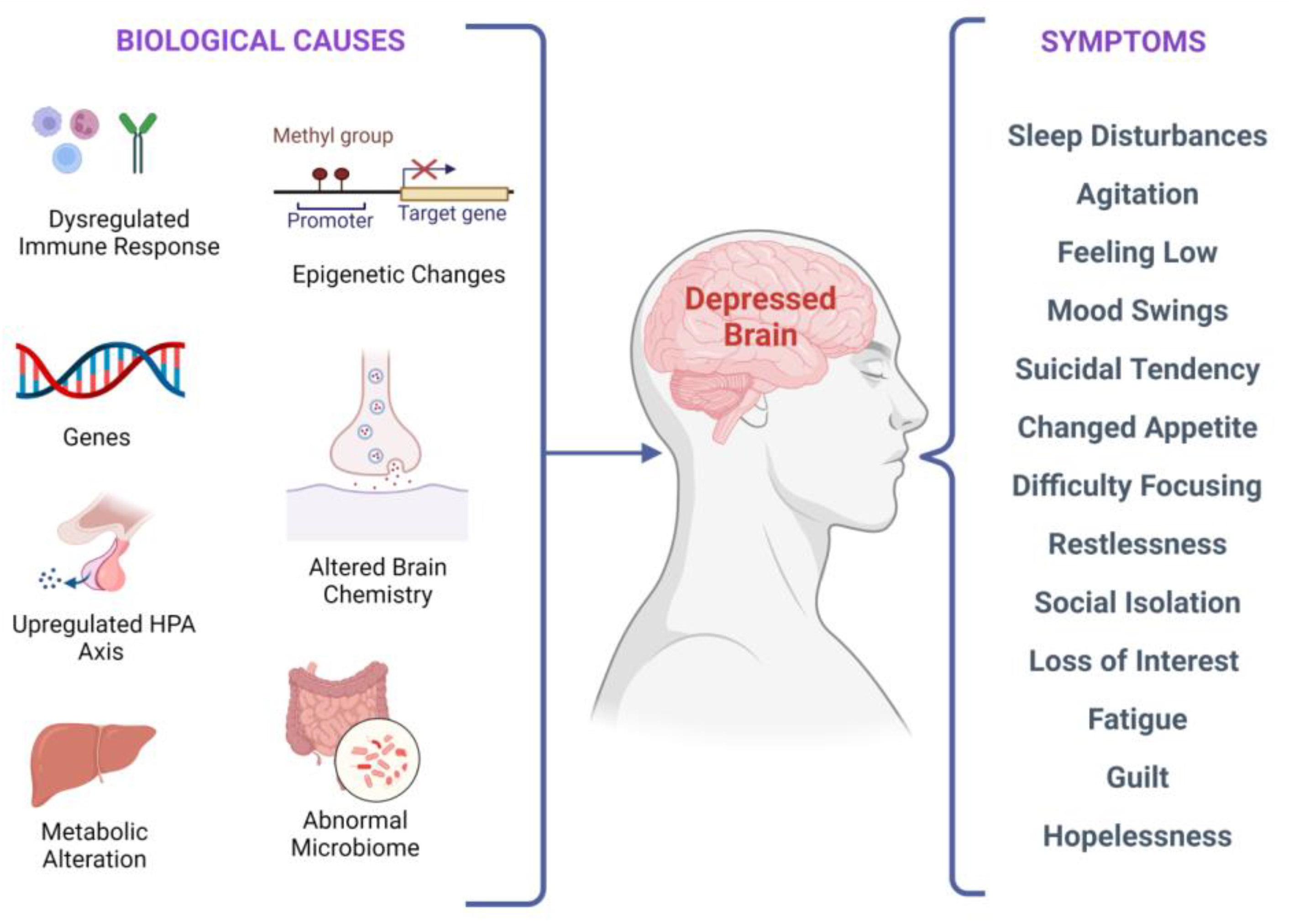
Major Depressive Disorder (MDD), colloquially known as depression, is a debilitating condition affecting an estimated 3.8% of the population globally, of which 5.0% are adults and 5.7% are above the age of 60. MDD is differentiated from common mood changes and short-lived emotional responses due to subtle alterations in gray and white matter, including the frontal lobe, hippocampus, temporal lobe, thalamus, striatum, and amygdala. It can be detrimental to a person’s overall health if it occurs with moderate or severe intensity. It can render a person suffering terribly to perform inadequately in their personal, professional, and social lives. Depression, at its peak, can lead to suicidal thoughts and ideation. Antidepressants manage clinical depression and function by modulating the serotonin, norepinephrine, and dopamine neurotransmitter levels in the brain.
- depression
- stem cells
- molecular pathways
- neurogenesis
1. Introduction
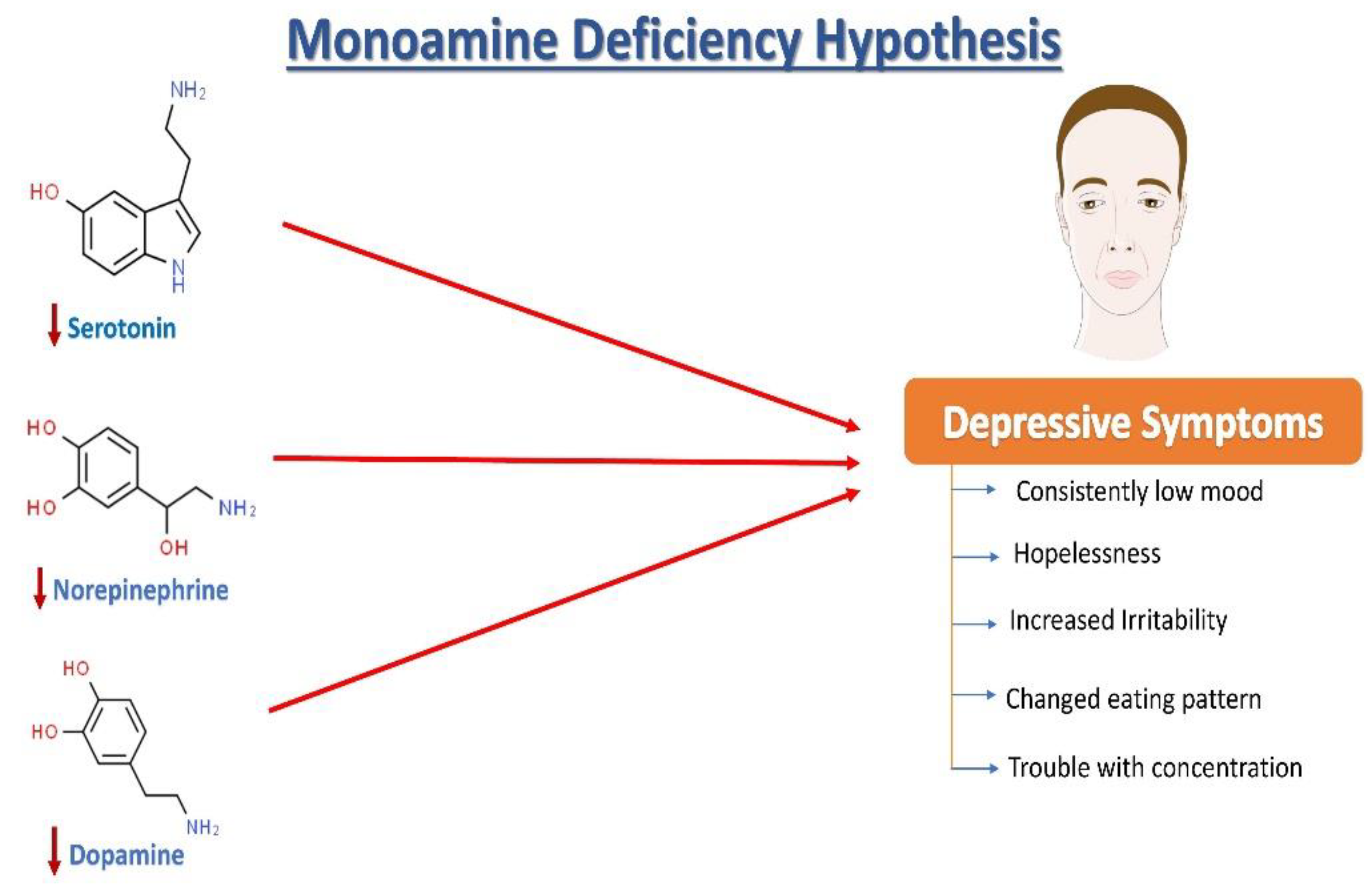
2. Neurochemistry of Depression: The Monoamine Hypothesis
3. Growth Factors Involved in Depression
4. Neural Stem Cells and Depression
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15030814
References
- Maj, M. When Does Depression Become a Mental Disorder? Br. J. Psychiatry 2011, 199, 85–86.
- Lang, U.E.; Borgwardt, S. Molecular Mechanisms of Depression: Perspectives on New Treatment Strategies. Cell Physiol. Biochem. 2013, 31, 761–777.
- Ramasubbu, R.; Patten, S.B. Effect of Depression on Stroke Morbidity and Mortality. Can. J. Psychiatry 2003, 48, 250–257.
- Van der Kooy, K.; van Hout, H.; Marwijk, H.; Marten, H.; Stehouwer, C.; Beekman, A. Depression and the Risk for Cardiovascular Diseases: Systematic Review and Meta Analysis. Int. J. Geriat. Psychiatry 2007, 22, 613–626.
- Green, R.C.; Cupples, L.A.; Kurz, A.; Auerbach, S.; Go, R.; Sadovnick, D.; Duara, R.; Kukull, W.A.; Chui, H.; Edeki, T.; et al. Depression as a Risk Factor for Alzheimer Disease: The MIRAGE Study. Arch. Neurol. 2003, 60, 753.
- Hesdorffer, D.C.; Hauser, W.A.; Annegers, J.F.; Cascino, G. Major Depression Is a Risk Factor for Seizures in Older Adults. Ann. Neurol. 2000, 47, 246–249.
- Nouwen, A.; Lloyd, C.E.; Pouwer, F. Depression and Type 2 Diabetes Over the Lifespan: A Meta-Analysis. Diabetes Care 2009, 32, e56.
- Penninx, B.W.J.H.; Guralnik, J.M.; Havlik, R.J.; Pahor, M.; Ferrucci, L.; Cerhan, J.R.; Wallace, R.B. Chronically Depressed Mood and Cancer Risk in Older Persons. JNCI J. Natl. Cancer Inst. 1998, 90, 1888–1893.
- Kaur, J.; Ghosh, S.; Singh, P.; Dwivedi, A.K.; Sahani, A.K.; Sinha, J.K. Cervical Spinal Lesion, Completeness of Injury, Stress, and Depression Reduce the Efficiency of Mental Imagery in People With Spinal Cord Injury. Am. J. Phys. Med. Rehabil. 2022, 101, 513–519.
- Maniam, J.; Antoniadis, C.P.; Youngson, N.A.; Sinha, J.K.; Morris, M.J. Sugar Consumption Produces Effects Similar to Early Life Stress Exposure on Hippocampal Markers of Neurogenesis and Stress Response. Front. Mol. Neurosci. 2015, 8, 86.
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am. J. Psychiatry 2006, 163, 1905–1917.
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment Resistant Depression: A Multi-Scale, Systems Biology Approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288.
- Salzman, C.; Wong, E.; Wright, B.C. Drug and ECT Treatment of Depression in the Elderly, 1996–2001: A Literature Review. Biol. Psychiatry 2002, 52, 265–284.
- Keller, M.B.; Hirschfeld, R.M.A.; Demyttenaere, K.; Baldwin, D.S. Optimizing Outcomes in Depression: Focus on Antidepressant Compliance. Int. Clin. Psychopharmacol. 2002, 17, 265–271.
- Nestler, E.J.; Carlezon, W.A. The Mesolimbic Dopamine Reward Circuit in Depression. Biol. Psychiatry 2006, 59, 1151–1159.
- Roy, A. Cerebrospinal Fluid Monoamine Metabolites and Suicidal Behavior in Depressed Patients: A 5-Year Follow-up Study. Arch. Gen. Psychiatry 1989, 46, 609.
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J. Neurosci. 1997, 17, 8451–8458.
- Marshe, V.S.; Maciukiewicz, M.; Rej, S.; Tiwari, A.K.; Sibille, E.; Blumberger, D.M.; Karp, J.F.; Lenze, E.J.; Reynolds, C.F.; Kennedy, J.L.; et al. Norepinephrine Transporter Gene Variants and Remission From Depression With Venlafaxine Treatment in Older Adults. Am. J. Psychiatry 2017, 174, 468–475.
- Dunn, A.J. Effects of Cytokines and Infections on Brain Neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68.
- Anacker, C.; Cattaneo, A.; Musaelyan, K.; Zunszain, P.A.; Horowitz, M.; Molteni, R.; Luoni, A.; Calabrese, F.; Tansey, K.; Gennarelli, M.; et al. Role for the Kinase SGK1 in Stress, Depression, and Glucocorticoid Effects on Hippocampal Neurogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 8708–8713.
- Jokinen, J.; Nordström, A.-L.; Nordström, P. The Relationship Between CSF HVA/5-HIAA Ratio and Suicide Intent in Suicide Attempters. Arch. Suicide Res. 2007, 11, 187–192.
- Pizzagalli, D.A.; Berretta, S.; Wooten, D.; Goer, F.; Pilobello, K.T.; Kumar, P.; Murray, L.; Beltzer, M.; Boyer-Boiteau, A.; Alpert, N.; et al. Assessment of Striatal Dopamine Transporter Binding in Individuals With Major Depressive Disorder: In Vivo Positron Emission Tomography and Postmortem Evidence. JAMA Psychiatry 2019, 76, 854.
- Cassano, P.; Lattanzi, L.; Fava, M.; Navari, S.; Battistini, G.; Abelli, M.; Cassano, G.B. Ropinirole in Treatment-Resistant Depression: A 16-Week Pilot Study. Can. J. Psychiatry 2005, 50, 357–360.
- Descarries, L.; Watkins, K.C.; Garcia, S.; Beaudet, A. The Serotonin Neurons in Nucleus Raphe Dorsalis of Adult Rat: A Light and Electron Microscope Radioautographic Study. J. Comp. Neurol. 1982, 207, 239–254.
- Bunin, M.A.; Wightman, R.M. Quantitative Evaluation of 5-Hydroxytryptamine (Serotonin) Neuronal Release and Uptake: An Investigation of Extrasynaptic Transmission. J. Neurosci. 1998, 18, 4854–4860.
- Steinbusch, H.W.M. Distribution of Serotonin-Immunoreactivity in the Central Nervous System of the Rat—Cell Bodies and Terminals. Neuroscience 1981, 6, 557–618.
- Mann, J. Role of the Serotonergic System in the Pathogenesis of Major Depression and Suicidal Behavior. Neuropsychopharmacology 1999, 21, 99S–105S.
- Chaouloff, F. Serotonin and Stress. Neuropsychopharmacology 1999, 21, 28S–32S.
- Andrews, P.W.; Bharwani, A.; Lee, K.R.; Fox, M.; Thomson, J.A. Is Serotonin an Upper or a Downer? The Evolution of the Serotonergic System and Its Role in Depression and the Antidepressant Response. Neurosci. Biobehav. Rev. 2015, 51, 164–188.
- Bot, M.; Chan, M.K.; Jansen, R.; Lamers, F.; Vogelzangs, N.; Steiner, J.; Leweke, F.M.; Rothermundt, M.; Cooper, J.; Bahn, S.; et al. Serum Proteomic Profiling of Major Depressive Disorder. Transl. Psychiatry 2015, 5, e599.
- Quintana, J. Platelet Serotonin and Plasma Tryptophan Decreases in Endogenous Depression. Clinical, Therapeutic, and Biological Correlations. J. Affect. Disord. 1992, 24, 55–62.
- Park, C.; Rosenblat, J.D.; Brietzke, E.; Pan, Z.; Lee, Y.; Cao, B.; Zuckerman, H.; Kalantarova, A.; McIntyre, R.S. Stress, Epigenetics and Depression: A Systematic Review. Neurosci. Biobehav. Rev. 2019, 102, 139–152.
- Ghosh, S.; Sinha, J.K.; Raghunath, M. “Obesageing”: Linking Obesity & Ageing. Indian J. Med. Res. 2019, 149, 610–615.
- Campbell, S.; MacQueen, G. An Update on Regional Brain Volume Differences Associated with Mood Disorders. Curr. Opin. Psychiatry 2006, 19, 25–33.
- Videbech, P. Hippocampal Volume and Depression: A Meta-Analysis of MRI Studies. Am. J. Psychiatry 2004, 161, 1957–1966.
- Mishra, P.; Mittal, A.K.; Kalonia, H.; Madan, S.; Ghosh, S.; Sinha, J.K.; Rajput, S.K. SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan. Curr. Neuropharmacol. 2021, 19, 1019–1037.
- Moroi, K.; Sato, T. Comparison between Procaine and Isocarboxazid Metabolism in Vitro by a Liver Microsomal Amidase-Esterase. Biochem. Pharmacol. 1975, 24, 1517–1521.
- Pittenger, C.; Duman, R.S. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology 2008, 33, 88–109.
- Aydemir, O.; Deveci, A.; Taneli, F. The Effect of Chronic Antidepressant Treatment on Serum Brain-Derived Neurotrophic Factor Levels in Depressed Patients: A Preliminary Study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2005, 29, 261–265.
- Ricken, R.; Adli, M.; Lange, C.; Krusche, E.; Stamm, T.J.; Gaus, S.; Koehler, S.; Nase, S.; Bschor, T.; Richter, C.; et al. Brain-Derived Neurotrophic Factor Serum Concentrations in Acute Depressive Patients Increase During Lithium Augmentation of Antidepressants. J. Clin. Psychopharmacol. 2013, 33, 806–809.
- Bauer, M.; Adli, M.; Bschor, T.; Pilhatsch, M.; Pfennig, A.; Sasse, J.; Schmid, R.; Lewitzka, U. Lithium’s Emerging Role in the Treatment of Refractory Major Depressive Episodes: Augmentation of Antidepressants. Neuropsychobiology 2010, 62, 36–42.
- Coradduzza, D.; Garroni, G.; Congiargiu, A.; Balzano, F.; Cruciani, S.; Sedda, S.; Nivoli, A.; Maioli, M. MicroRNAs, Stem Cells in Bipolar Disorder, and Lithium Therapeutic Approach. Int. J. Mol. Sci. 2022, 23, 10489.
- Mondal, A.C.; Fatima, M. Direct and Indirect Evidences of BDNF and NGF as Key Modulators in Depression: Role of Antidepressants Treatment. Int. J. Neurosci. 2019, 129, 283–296.
- de Miranda, A.S.; de Barros, J.L.V.M.; Teixeira, A.L. Is Neurotrophin-3 (NT-3): A Potential Therapeutic Target for Depression and Anxiety? Expert Opin. Ther. Targets 2020, 24, 1225–1238.
- Diniz, B.S.; Teixeira, A.L.; Miranda, A.S.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Circulating Glial-Derived Neurotrophic Factor Is Reduced in Late-Life Depression. J. Psychiatr. Res. 2012, 46, 135–139.
- Evans, S.J.; Choudary, P.V.; Neal, C.R.; Li, J.Z.; Vawter, M.P.; Tomita, H.; Lopez, J.F.; Thompson, R.C.; Meng, F.; Stead, J.D.; et al. Dysregulation of the Fibroblast Growth Factor System in Major Depression. Proc. Natl. Acad. Sci. USA 2004, 101, 15506–15511.
- Beaulieu, J.-M. A Role for Akt and Glycogen Synthase Kinase-3 as Integrators of Dopamine and Serotonin Neurotransmission in Mental Health. J. Psychiatry Neurosci. 2012, 37, 7–16.
- Miskowiak, K.W.; Vinberg, M.; Harmer, C.J.; Ehrenreich, H.; Knudsen, G.M.; Macoveanu, J.; Hansen, A.R.; Paulson, O.B.; Siebner, H.R.; Kessing, L.V. Effects of Erythropoietin on Depressive Symptoms and Neurocognitive Deficits in Depression and Bipolar Disorder. Trials 2010, 11, 97.
- Eden Evins, A.; Demopulos, C.; Yovel, I.; Culhane, M.; Ogutha, J.; Grandin, L.D.; Nierenberg, A.A.; Sachs, G.S. Inositol Augmentation of Lithium or Valproate for Bipolar Depression. Bipolar Disord. 2006, 8, 168–174.
- Cattaneo, A.; Sesta, A.; Calabrese, F.; Nielsen, G.; Riva, M.A.; Gennarelli, M. The Expression of VGF Is Reduced in Leukocytes of Depressed Patients and It Is Restored by Effective Antidepressant Treatment. Neuropsychopharmacology 2010, 35, 1423–1428.
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848.
- Kukekov, V.G.; Laywell, E.D.; Suslov, O.; Davies, K.; Scheffler, B.; Thomas, L.B.; O’Brien, T.F.; Kusakabe, M.; Steindler, D.A. Multipotent Stem/Progenitor Cells with Similar Properties Arise from Two Neurogenic Regions of Adult Human Brain. Exp. Neurol. 1999, 156, 333–344.
- Alvarez-Buylla, A.; García-Verdugo, J.M. Neurogenesis in Adult Subventricular Zone. J. Neurosci. 2002, 22, 629–634.
- Liu, Z.; Martin, L.J. Olfactory Bulb Core Is a Rich Source of Neural Progenitor and Stem Cells in Adult Rodent and Human. J. Comp. Neurol. 2003, 459, 368–391.
- Kempermann, G. Regulation of Adult Hippocampal Neurogenesis—Implications for Novel Theories of Major Depression 1: Regulation of Adult Hippocampal Neurogenesis. Bipolar Disord. 2002, 4, 17–33.
- Mansoor, A.K.; Thomas, S.; Sinha, J.K.; Alladi, P.A.; Ravi, V.; Raju, T.R. Olfactory tract transection reveals robust tissue-level plasticity by cellular numbers and neurotrophic factor expression in olfactory bulb. Indian J. Exp. Biol. 2012, 50, 765–770.
- Feldmann, R.E.; Mattern, R. The Human Brain and Its Neural Stem Cells Postmortem: From Dead Brains to Live Therapy. Int. J. Leg. Med. 2006, 120, 201–211.
- Lindvall, O.; Kokaia, Z. Recovery and Rehabilitation in Stroke: Stem Cells. Stroke 2004, 35, 2691–2694.
- Sachdeva, P.; Ghosh, S.; Ghosh, S.; Han, S.; Banerjee, J.; Bhaskar, R.; Sinha, J.K. Childhood Obesity: A Potential Key Factor in the Development of Glioblastoma Multiforme. Life 2022, 12, 1673.
- Ghosh, S.; Manchala, S.; Raghunath, M.; Sharma, G.; Singh, A.K.; Sinha, J.K. Role of Phytomolecules in the Treatment of Obesity: Targets, Mechanisms and Limitations. Curr. Top. Med. Chem. 2021, 21, 863–877.
- Goldman, S. Stem and Progenitor Cell–Based Therapy of the Human Central Nervous System. Nat. Biotechnol. 2005, 23, 862–871.
- Lipska, B.K. Using Animal Models to Test a Neurodevelopmental Hypothesis of Schizophrenia. J. Psychiatry Neurosci. 2004, 29, 282–286.
- Feldmann, R.E.; Sawa, A.; Seidler, G.H. Causality of Stem Cell Based Neurogenesis and Depression—To Be or Not to Be, Is That the Question? J. Psychiatr. Res. 2007, 41, 713–723.
- Zhao, C. Distinct Morphological Stages of Dentate Granule Neuron Maturation in the Adult Mouse Hippocampus. J. Neurosci. 2006, 26, 3–11.
