Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joshua D Nosanchuk | + 2000 word(s) | 2000 | 2021-05-13 12:34:24 | | | |
| 2 | Peter Tang | Meta information modification | 2000 | 2021-05-20 04:56:23 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Nosanchuk, J. Immunological interactions with fungal melanin. Encyclopedia. Available online: https://encyclopedia.pub/entry/9857 (accessed on 07 February 2026).
Nosanchuk J. Immunological interactions with fungal melanin. Encyclopedia. Available at: https://encyclopedia.pub/entry/9857. Accessed February 07, 2026.
Nosanchuk, Joshua. "Immunological interactions with fungal melanin" Encyclopedia, https://encyclopedia.pub/entry/9857 (accessed February 07, 2026).
Nosanchuk, J. (2021, May 19). Immunological interactions with fungal melanin. In Encyclopedia. https://encyclopedia.pub/entry/9857
Nosanchuk, Joshua. "Immunological interactions with fungal melanin." Encyclopedia. Web. 19 May, 2021.
Copy Citation
Melanins are ubiquitous complex polymers that are commonly known in humans to cause pigmentation of our skin. Melanins are also present in bacteria, fungi, and helminths.
melanin
fungus
yeast
immune response
1. Introduction
Melanins are a family of structurally complex dark pigment polymer present in all biological kingdoms [1]. The polymer is made up of covalently linked indoles, but the overall structure is granular; however, detailed structures are not well characterized because it is a collection of polymers with mixed pre-indole structures [2]. Moreover, melanins are amorphous and are not suitable for study by crystallography or cryo-electron microscopy, which has led to complicated efforts to solve the structure of these natural pigments. Mammalian melanin biogenesis happens by oxidation of L-tyrosine via tyrosinase in melanocytes, which are neural crest-derived dendritic cells [3][4]. However, melanins in bacteria, fungi, and helminths are produced through the polyketide synthase (PKS) pathway or catalyzed by phenoloxidase [5].
2. Melanin Synthesis
Fungi synthesize melanin via two main pathways, namely 1,8-dihydroxynaphthalene (DHN) and l-3,4-dihyroxyphenylalanine (L-DOPA) (Figure 1 and Table 1). In the DHN pathway, 1,3,6,8-tetrahydroxynaphthalene (1,3,6,8-THN) is first synthesized through PKS, which is a multi-domain enzyme complex that produces polyketides. Polyketides are products derived from acetyl-CoA or propionyl-CoA with malonyl-CoA or methylmalonyl-CoA. The condensation reactions are driven by decarboxylation, yielding a beta-keto functional group [6]. This is then followed by reduction and dehydration reactions that eventually produce DHN. It is the polymerization of DHN that leads to the formation of melanin [5][7][8]. Polyketides are a class of secondary metabolites mainly produced in bacteria, fungi, and plants, and they serve very different purposes in our society [9]. Polyketides such as macrolide, tetracycline, and amphotericin antimicrobials serve tremendous value, while aflatoxin can be lethal to mammals [10][11].
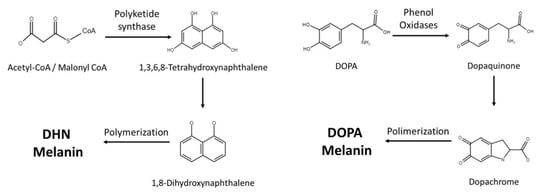
Figure 1. Current knowledge of melanin synthetic pathways in fungi.
|
Species |
Isolate Environment |
Melanin Types |
|---|---|---|
|
Aspergillus fumigatus |
Clinical |
DHN and pyo-melanin |
|
Aspergillus niger |
Industrial fermentation |
DHN and L-DOPA |
|
Blastomyces dermatitidis |
Clinical |
DHN |
|
Candida Albicans |
Clinical |
L-DOPA |
|
Cryptococcus neoformans |
Clinical |
L-DOPA |
|
Histoplasma capsulatum |
Clinical |
DHN and L-DOPA |
|
Paracoccidioides brasiliensis |
Clinical |
DHN and L-DOPA |
|
Fonsecaea monophora |
Clinical |
DHN and L-DOPA |
|
Fonsecaea pedrosoi |
Clinical |
DHN |
|
Sporothrix schenckii |
Clinical |
DHN |
Abbreviations: DHN is 1,8-dihydroxynaphthalene and L-DOPA is l-3,4-dihyroxyphenylalanine.
The L-DOPA pathway is similar to mammalian melanin biosynthesis, where the pathway typically uses either L-DOPA or tyrosine as starting molecules. If the pathway starts with tyrosine, tyrosinase will perform a two-step oxidation, turning tyrosine into dopaquinone. Similarly, laccase is the enzyme responsible for converting L-DOPA into dopaquinone. Dopaquinone is then turned into leucodopachrome (cyclodopa) and then oxidized to dopachrome. Dopachrome then goes through tautomerization to form dihydroxyindoles, which are simultaneously oxidized and polymerized to produce DOPA melanin [8].
3. Cryptococcus neoformans
C. neoformans is one of the most well studied pathogens for melanization (Figure 2). Cryptococcus neoformans is unique among pathogenic fungi as it solely relies on the L-DOPA pathway and requires exogenous phenolic substrates to form melanin. After the polysaccharide capsule, melanin is the second most important virulence factor in C. neoformans as it has been calculated as contributing to 14% of the pathogen’s total virulence [16]. This notable aspect of cryptococcal melanization has led to extensive study of this polymer in Cryptococcus spp. [17]. Other species can produce melanin using endogenous compounds or exogenous substrates, and some can produce more than one type of melanin [12][18][19].
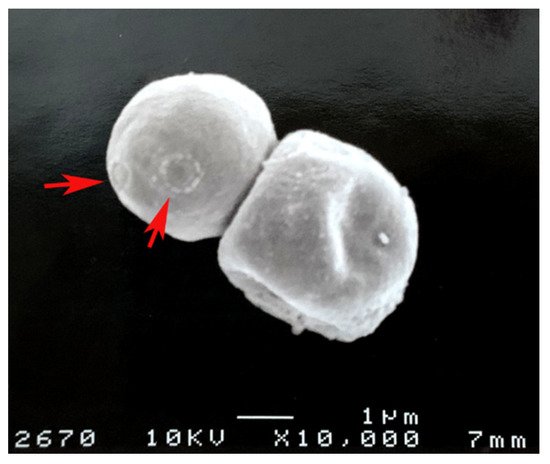
Figure 2. Cryptococcus neoformans melanin “ghosts” obtained from lungs of infected mice as described in [20]. The red arrows show bud scars on the melanin.
In the early 1980s, Kwon-Chung et al. produced melanin-deficient strains (Mel−) via UV irradiation and observed that the mutants lacked virulence as the cells were cleared from mouse organs, whereas wild-type (WT) cells expanded in numbers, especially in the brain [21]. The same study showed that Mel− mutants were defective in the active transport system for diphenolic compounds and phenoloxidase, which is the first enzyme needed in the L-DOPA melanogenesis pathway. A subsequent study confirmed that the loss of phenoloxidase activity was responsible for the Mel− mutant phenotype [22]. Williamson next discovered that laccase, a phenoloxidase, was encoded by the lac1 gene [23]. Laccase was linked to C. neoformans virulence in vivo through disruption of the 5′ end of the lac1 gene [24]. Interestingly, C. neoformans has two laccase genes, where lac2 is 75% similar to lac1, but basal transcript levels of lac2 are much lower, and mutation of the gene only induced a mild delay in melanin formation [25].
4. Aspergillus fumigatus
A. fumigatus is another pathogenic fungus that undergoes melanization, and pigment formation in this species has been the subject of extensive investigation. 1,8-dihydroxynaphthalene (DHN) melanin is the major melanin found in A. fumigatus [26]. A set of six genes are needed for melanin synthesis: pksP, ayg1, arp2, arp1, arb1, and arb2, ordered from upstream to downstream [27]. Melanin from A. fumigatus has many similarities to melanin from C. neoformans, as both provide protection from ultraviolet light and scavenge ROS generated by phagocytes [28]. Albino conidia incubated with phagocytes induced a 10-fold increase in ROS production compared with WT, which suggests that the melanin increased ROS scavenging abilities in the WT conidia. Moreover, the albino mutants were more effectively killed by monocytes than wild-types [29].
Melanin regulates host pro-inflammatory cytokine responses by physically masking fungal pathogen-associated molecular patterns (PAMPs) from immune recognition such as that observed by the rodlets layer. Chai et al. demonstrated that albino conidia were able to generate much higher IL-6, IL-10, and TNF-α levels compared to WT. IL-6 and IL-10 had roughly 12-fold and 5-fold higher levels, respectively. The authors pinpointed that albino conidia had β-glucan and other PAMPs such as mannans more readily available to bind to dectin-1, Toll-like receptor 4 (TLR4) and Mannose receptors on peripheral blood mononuclear cells (PBMCs) [30]. As conidia mature, they swell and germinate, and this process exposes β-1,3-glucan on their surface, which induces an immune response [31].
Jahn et al. observed that ΔpksP melanin-deficient conidia were more effectively phagocytosed and killed by macrophages when compared with WT conidia [32]. Thywissen et al. next demonstrated that the albino ΔpksP mutant conidia had a 3.5-fold increase in vacuolar-type ATPase-dependent phagosomal acidification observed in alveolar and monocyte-derived macrophages (~20% acidified conidia vs. 70%). There were virtually no acidified phagosomes containing WT conidia in granulocytes, but ~50% in the mutant group. The pH within the mutant-containing phagolysosome was 5, while the WT had a pH of 6, and the lower pH results in more effective actions by phagosomal enzymes. Interestingly, synthetic DOPA melanin did not prevent acidification, only DHN melanin did [33]. However, among Aspergillus spp., A. flavus was the most effective in suppressing acidification, closely followed by A. fumigatus. Microtubule-associated protein 1A/1B-light chain 3 (LC3)-associated phagocytosis (LAP) is a non-canonical autophagy pathway that is linked to certain pattern recognition receptors that trigger phagosome formation [34]. A. fumigatus melanin inhibits calcium-calmodulin signaling on the protein Rubicon, a key regulator in the LAP pathway [35][36], and Rubicon directly interacts with the p22phox subunit and facilitates NADPH oxidase activation during phagocytosis [37]. Melanin ultimately blocks the p22phox NADPH oxidase subunit from localizing on the phagosome membrane, thus blocking the assembly of the oxidase complex. Melanin’s effect on the NADPH oxidase complex is conserved in A. nidulans as well [38].
The effects of melanin vary on different immune cells. Dendritic cells (DCs) are not stimulated by A. fumigatus melanin. Bayry et al. demonstrated that DCs failed to produce cytokines TNF-α, IL-1β, IL-6, and IL-10 in response to melanized A. fumigatus conidia, and DCs treated with WT melanin ghost also failed to activate T cells [39]. The group interestingly found that ΔpksP, Δayg1, and Δarp2 mutants increased different amounts of acetyl-CoA, malonyl-CoA, and 1,3,6,8-THN, respectively. These mutants displayed altered cell walls with unmasked surface structures and were able to activate DCs.
Mammals have evolved various systems to combat melanized fungal pathogens. On the surface of mouse endothelial cells, there is a melanin-sensing C-type lectin receptor (MelLec) that recognizes DHN melanin in the conidial spores of A. fumigatus and other DHN-melanized fungi, such as Cladosporium cladosporioides and Fonsecaea pedrosoi [40]. The expression of this C-lectin is essential for protection against disseminated aspergillosis, and albino mutants are not recognized by the receptor. Macrophages change their metabolism in the presence of melanin, especially glycolysis metabolism, which is required for defense against Aspergillus. Goncalves et al. elucidated that DHN melanin blocks endoplasmic reticulum calcium/calmodulin signaling, which activates glycolysis and mammalian target of rapamycin (mTOR)-mediated defense against Aspergillus conidia [41]. Consequentially, this impairment of glycolysis, mediated by mammalian target of rapamycin (mTOR) and hypoxia-inducible factor 1 subunit alpha (HIF-1α), decreases macrophages’ conidicidal ability by lowering ROS concentration and inflammatory cytokines IL-1β, IL-6, IL-17A, TNF-α, and IFN-γ production [42].
A. fumigatus can be cleared by activating the complement system [43][44]. Tsai et al. further demonstrated that disruption of the arp1 gene leads to increased C3 deposition on the conidial cell surface [45]. Similarly, disrupting alb1/pksP that encodes polyketide synthase resulted in a significant increase in C3 binding on conidial surfaces, with an expected increase in phagocytosis by neutrophils and a decrease in virulence [26][46]. Direct binding of C3 fragments in normal human serum has been shown with A. niger melanin [47].
5. Other Melanotic Fungi and Their Interactions with the Immune System
Fonseca spp. are causative agents of chromoblastomycosis, and these fungi produce large quantities of melanin. Melanized Fonsecaea monophora and cell wall-containing extracted melanin significantly decrease the expression of inducible nitric oxide synthase gene and the production of nitric oxide and enhanced non-protective Th2 responses [48]. Macrophages infected with pigmented F. monophora enhanced the differential expression of genes related to immune responses, including the MAPK signaling pathway, demonstrating how melanization modifies pathogenesis [49]. As with C. neoformans and A. niger melanin, melanized Fonsecaea pedrosoi was quickly labeled with C3, C4, and C9 complement components [50].
Several endemic dimorphic pathogenic fungi produce melanin, including Histoplasma capsulatum [51], Paracoccidoides spp. [52], Coccidioides immitis [53], Blastomyces dermatitidis [15], and Talaromyces marneffei [54]. Melanin production is associated with pathogenesis in Paracoccidioides spp. through complex processes that extend beyond pigment production. Different Paracoccidioides species resist phagocytosis of yeast cells by macrophages, and this effect is associated with the degree of melanization in each strain [55][56]. Melanized P. brasiliensis is also highly resistant to NO, ROS, hypochlorite, and H2O2 [57]. A recent proteomic analysis comparing melanized and non-melanized P. brasiliensis and P. lutzii revealed that melanization leads to an abundance of virulence-associated proteins, including heat-shock proteins, vesicular transport proteins, adhesins, superoxide dismutases, proteases, and phospholipases, which further underscores the complex mechanisms that occur along with melanin production to subvert the host [58]. As with cryptococcosis, melanin-binding antibodies are generated during murine as well as human infection with P. brasiliensis [55].
T. marneffei (formerly Penicillium marneffei) also utilizes melanin to avoid host defenses. Remarkably, the T. marneffei genome has 23 polyketide synthase genes and additional non-ribosomal polyketide synthase hybrid genes [59]. Mutants unable to form the polymer are more sensitive to antifungals, H2O2, and sodium dodecyl sulfate (SDS). Furthermore, melanized cells were significantly more resistant to phagocytosis and killing compared to melanin-deficient mutants [60]. A second study corroborated the capacity of melanized fungal cells to resist antifungals [61]. There is an interesting link to melanin in T. marneffei with tyrosine catabolism, which is essential for survival in the host cells [62].
Melanin is well described in Sporothrix spp., and the production of the polymer is closely linked to virulence [13][63][64]. Melanization of S. globosa leads to a reduction in antigen presentation by macrophages and facilitates the dissemination of the pathogen [65]. Melanin formation has been linked to increased dissemination in several Sporothrix species [66]. However, melanization of some S. schenckii strains may, instead, induce the formation of granuloma, which facilitates the survival of the yeast [67]. Melanin production in Sporothrix complex species is protective against diverse antifungal compounds [68][69].
Melanin is purported to be a major factor in the pathogenicity of Mucorales spp. A recent paper from the Ibrahim laboratory identified compounds that could selectively inhibit eumelanin production by Rhizopus sp. [70]. Moreover, the inhibition of melanin by one blocking compound, UOSC-2, led to the formation of spores that were more efficiently phagocytosed and killed in mouse lungs compared to melanized spores, and the albino spores were similarly more efficiently killed by human macrophages, verifying the importance of melanin in protection against host effector responses against this pigmented species.
References
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187.
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239.
- Eisen, T.G. The control of gene expression in melanocytes and melanomas. Melanoma Res. 1996, 6, 277–284.
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1995, 1247, 1–11.
- Wheeler, M.H.; Bell, A.A. Melanins and their importance in pathogenic fungi. In Current Topics in Medical Mycology; Springer: Berlin/Heidelberg, Germany, 1988; pp. 338–387.
- Staunton, J.; Weissman, K.J. Polyketide biosynthesis: A millennium review. Nat. Prod. Rep. 2001, 18, 380–416.
- Butler, M.; Day, A. Fungal melanins: A review. J. Can. J. Microbiol. 1998, 44, 1115–1136.
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158.
- Katz, L. Manipulation of Modular Polyketide Synthases. Chem. Rev. 1997, 97, 2557–2576.
- Huffman, J.; Gerber, R.; Du, L. Recent advancements in the biosynthetic mechanisms for polyketide-derived mycotoxins. Biopolymers 2010, 93, 764–776.
- Hopwood, D.A. Complex enzymes in microbial natural product biosynthesis, part B: Polyketides, aminocoumarins and carbohydrates. Preface. Methods Enzymol. 2009, 459, xvii–xix.
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257.
- Morris-Jones, R.; Youngchim, S.; Gomez, B.L.; Aisen, P.; Hay, R.J.; Nosanchuk, J.D.; Casadevall, A.; Hamilton, A.J. Synthesis of Melanin-Like Pigments by Sporothrix schenckiix In Vitro and during Mammalian Infection. Infect. Immun. 2003, 71, 4026–4033.
- Walker, C.A.; Gómez, B.L.; Mora-Montes, H.M.; Mackenzie, K.S.; Munro, C.A.; Brown, A.J.P.; Gow, N.A.R.; Kibbler, C.C.; Odds, F.C. Melanin externalization in Candida albicans depends on cell wall chitin structures. Eukaryot Cell 2010, 9, 1329–1342.
- Nosanchuk, J.D.; Van Duin, D.; Mandal, P.; Aisen, P.; Legendre, A.M.; Casadevall, A. Blastomyces dermatitidis produces melanin in vitro and during infection. FEMS Microbiol. Lett. 2004, 239, 187–193.
- McClelland, E.E.; Bernhardt, P.; Casadevall, A. Estimating the relative contributions of virulence factors for pathogenic microbes. Infect. Immun. 2006, 74, 1500–1504.
- Chatterjee, S.; Prados-Rosales, R.; Frases, S.; Itin, B.; Casadevall, A.; Stark, R.E. Using solid-state NMR to monitor the molecular consequences of Cryptococcus neoformans melanization with different catecholamine precursors. Biochemistry 2012, 51, 6080–6088.
- Almeida-Paes, R.; Frases, S.; Fialho Monteiro, P.C.; Gutierrez-Galhardo, M.C.; Zancope-Oliveira, R.M.; Nosanchuk, J.D. Growth conditions influence melanization of Brazilian clinical Sporothrix schenckii isolates. Microbes Infect. 2009, 11, 554–562.
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Rosas, A.L.; Nosanchuk, J.D.; Feldmesser, M.; Cox, G.M.; McDade, H.C.; Casadevall, A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect. Immun. 2000, 68, 2845–2853.
- Kwon-Chung, K.J.; Polacheck, I.; Popkin, T.J. Melanin-lacking mutants of Cryptococcus neoformans and their virulence for mice. J. Bacteriol. 1982, 150, 1414–1421.
- Rhodes, J.C.; Polacheck, I.; Kwon-Chung, K.J. Phenoloxidase activity and virulence in isogenic strains of Cryptococcus neoformans. Infect. Immun. 1982, 36, 1175–1184.
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J. Bacteriol. 1994, 176, 656–664.
- Salas, S.D.; Bennett, J.E.; Kwon-Chung, K.J.; Perfect, J.R.; Williamson, P.R. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J. Exp. Med. 1996, 184, 377–386.
- Pukkila-Worley, R.; Gerrald, Q.D.; Kraus, P.R.; Boily, M.-J.; Davis, M.J.; Giles, S.S.; Cox, G.M.; Heitman, J.; Alspaugh, J.A. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell 2005, 4, 190–201.
- Tsai, H.F.; Chang, Y.C.; Washburn, R.G.; Wheeler, M.H.; Kwon-Chung, K.J. The developmentally regulated alb1 gene of Aspergillus fumigatus: Its role in modulation of conidial morphology and virulence. J. Bacteriol. 1998, 180, 3031–3038.
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477.
- Abad, A.; Fernández-Molina, J.V.; Bikandi, J.; Ramírez, A.; Margareto, J.; Sendino, J.; Hernando, F.L.; Pontón, J.; Garaizar, J.; Rementeria, A. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev. Iberoam. Micol. 2010, 27, 155–182.
- Jahn, B.; Boukhallouk, F.; Lotz, J.; Langfelder, K.; Wanner, G.; Brakhage, A.A. Interaction of human phagocytes with pigmentless Aspergillus conidia. Infect. Immun. 2000, 68, 3736–3739.
- Chai, L.Y.; Netea, M.G.; Sugui, J.; Vonk, A.G.; Van de Sande, W.W.; Warris, A.; Kwon-Chung, K.J.; Kullberg, B.J. Aspergillus fumigatus conidial melanin modulates host cytokine response. Immunobiology 2010, 215, 915–920.
- Luther, K.; Torosantucci, A.; Brakhage, A.A.; Heesemann, J.; Ebel, F. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell. Microbiol. 2007, 9, 368–381.
- Jahn, B.; Langfelder, K.; Schneider, U.; Schindel, C.; Brakhage, A.A. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell. Microbiol. 2002, 4, 793–803.
- Thywißen, A.; Heinekamp, T.; Dahse, H.M.; Schmaler-Ripcke, J.; Nietzsche, S.; Zipfel, P.F.; Brakhage, A.A. Conidial Dihydroxynaphthalene Melanin of the Human Pathogenic Fungus Aspergillus fumigatus Interferes with the Host Endocytosis Pathway. Front. Microbiol. 2011, 2, 96.
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257.
- Kyrmizi, I.; Ferreira, H.; Carvalho, A.; Figueroa, J.A.L.; Zarmpas, P.; Cunha, C.; Akoumianaki, T.; Stylianou, K.; Deepe, G.S.; Samonis, G.; et al. Calcium sequestration by fungal melanin inhibits calcium–calmodulin signalling to prevent LC3-associated phagocytosis. Nat. Microbiol. 2018, 3, 791–803.
- Martinez, J.; Malireddi, R.S.; Lu, Q.; Cunha, L.D.; Pelletier, S.; Gingras, S.; Orchard, R.; Guan, J.-L.; Tan, H.; Peng, J. Molecular characterization of LC3-associated phagocytosis reveals distinct roles for Rubicon, NOX2 and autophagy proteins. Nat. Cell Biol. 2015, 17, 893–906.
- Yang, C.-S.; Lee, J.-S.; Rodgers, M.; Min, C.-K.; Lee, J.-Y.; Kim, H.J.; Lee, K.-H.; Kim, C.-J.; Oh, B.; Zandi, E. Autophagy protein Rubicon mediates phagocytic NADPH oxidase activation in response to microbial infection or TLR stimulation. Cell Host Microbe 2012, 11, 264–276.
- Akoumianaki, T.; Kyrmizi, I.; Valsecchi, I.; Gresnigt, M.S.; Samonis, G.; Drakos, E.; Boumpas, D.; Muszkieta, L.; Prevost, M.-C.; Kontoyiannis, D.P. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 2016, 19, 79–90.
- Bayry, J.; Beaussart, A.; Dufrêne, Y.F.; Sharma, M.; Bansal, K.; Kniemeyer, O.; Aimanianda, V.; Brakhage, A.A.; Kaveri, S.V.; Kwon-Chung, K.J.; et al. Surface structure characterization of Aspergillus fumigatus conidia mutated in the melanin synthesis pathway and their human cellular immune response. Infect. Immun. 2014, 82, 3141–3153.
- Stappers, M.H.T.; Clark, A.E.; Aimanianda, V.; Bidula, S.; Reid, D.M.; Asamaphan, P.; Hardison, S.E.; Dambuza, I.M.; Valsecchi, I.; Kerscher, B.; et al. Recognition of DHN-melanin by a C-type lectin receptor is required for immunity to Aspergillus. Nature 2018, 555, 382–386.
- Gonçalves, S.M.; Duarte-Oliveira, C.; Campos, C.F.; Aimanianda, V.; Ter Horst, R.; Leite, L.; Mercier, T.; Pereira, P.; Fernández-García, M.; Antunes, D.; et al. Phagosomal removal of fungal melanin reprograms macrophage metabolism to promote antifungal immunity. Nat. Commun. 2020, 11, 2282.
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat. Med. 2004, 10, 594–601.
- Sturtevant, J.; Latgé, J.P. Participation of complement in the phagocytosis of the conidia of Aspergillus fumigatus by human polymorphonuclear cells. J. Infect. Dis. 1992, 166, 580–586.
- Behnsen, J.; Hartmann, A.; Schmaler, J.; Gehrke, A.; Brakhage, A.A.; Zipfel, P.F. The Opportunistic Human Pathogenic Fungus Aspergillus fumigatus Evades the Host Complement System. J. Infect. Immun. 2008, 76, 820–827.
- Tsai, H.F.; Washburn, R.G.; Chang, Y.C.; Kwon-Chung, K.J. Aspergillus fumigatus arp1 modulates conidial pigmentation and complement deposition. Mol. Microbiol. 1997, 26, 175–183.
- Langfelder, K.; Jahn, B.; Gehringer, H.; Schmidt, A.; Wanner, G.; Brakhage, A.A. Identification of a polyketide synthase gene (pksP) of Aspergillus fumigatus involved in conidial pigment biosynthesis and virulence. Med. Microbiol. Immunol. 1998, 187, 79–89.
- Rosas, A.L.; MacGill, R.S.; Nosanchuk, J.D.; Kozel, T.R.; Casadevall, A. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn Lab. Immunol. 2002, 9, 144–148.
- Zhang, J.; Wang, L.; Xi, L.; Huang, H.; Hu, Y.; Li, X.; Huang, X.; Lu, S.; Sun, J. Melanin in a meristematic mutant of Fonsecaea monophora inhibits the production of nitric oxide and Th1 cytokines of murine macrophages. Mycopathologia 2013, 175, 515–522.
- Shi, M.; Sun, J.; Lu, S.; Qin, J.; Xi, L.; Zhang, J. Transcriptional profiling of macrophages infected with Fonsecaea monophora. Mycoses 2019, 62, 374–383.
- Pinto, L.; Granja, L.F.Z.; Almeida, M.A.d.; Alviano, D.S.; Silva, M.H.d.; Ejzemberg, R.; Rozental, S.; Alviano, C.S. Melanin particles isolated from the fungus Fonsecaea pedrosoi activates the human complement system. Memórias do Instituto Oswaldo Cruz 2018, 113.
- Nosanchuk, J.D.; Gómez, B.L.; Youngchim, S.; Díez, S.; Aisen, P.; Zancopé-Oliveira, R.M.; Restrepo, A.; Casadevall, A.; Hamilton, A.J. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect. Immun. 2002, 70, 5124–5131.
- Gómez, B.L.; Nosanchuk, J.D.; Díez, S.; Youngchim, S.; Aisen, P.; Cano, L.E.; Restrepo, A.; Casadevall, A.; Hamilton, A.J. Detection of Melanin-Like Pigments in the Dimorphic Fungal Pathogen Paracoccidioides brasiliensis In Vitro and during Infection. J. Infect. Immun. 2001, 69, 5760–5767.
- Nosanchuk, J.D.; Yu, J.-J.; Hung, C.-Y.; Casadevall, A.; Cole, G.T. Coccidioides posadasii produces melanin in vitro and during infection. Fungal Genet. Biol. 2007, 44, 517–520.
- Youngchim, S.; Hay, R.J.; Hamilton, A.J. Melanization of Penicillium marneffei in vitro and in vivo. Microbiology 2005, 151, 291–299.
- Uran, M.E.; Nosanchuk, J.D.; Restrepo, A.; Hamilton, A.J.; Gomez, B.L.; Cano, L.E. Detection of antibodies against Paracoccidioides brasiliensis melanin in in vitro and in vivo studies during infection. Clin. Vaccine Immunol. 2011, 18, 1680–1688.
- Emidio, E.C.P.; Uran, M.E.; Silva, L.B.R.; Dias, L.S.; Doprado, M.; Nosanchuk, J.D.; Taborda, C.P. Melanin as a Virulence Factor in Different Species of Genus Paracoccidioides. J. Fungi. 2020, 6, 291.
- Silva, M.B.; Thomaz, L.; Marques, A.F.; Svidzinski, A.E.; Nosanchuk, J.D.; Casadevall, A.; Travassos, L.R.; Taborda, C.P. Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Memórias do Instituto Oswaldo Cruz 2009, 104, 644–648.
- Almeida-Paes, R.; Almeida, M.A.; Baeza, L.C.; Marmello, L.A.M.; Trugilho, M.R.O.; Nosanchuk, J.D.; Soares, C.M.A.; Valente, R.H.; Zancopé-Oliveira, R.M. Beyond Melanin: Proteomics Reveals Virulence-Related Proteins in Paracoccidioides brasiliensis and Paracoccidioides lutzii Yeast Cells Grown in the Presence of L-Dihydroxyphenylalanine. J. Fungi. 2020, 6, 328.
- Tam, E.W.; Tsang, C.C.; Lau, S.K.; Woo, P.C. Polyketides, toxins and pigments in Penicillium marneffei. Toxins 2015, 7, 4421–4436.
- Sapmak, A.; Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Andrianopoulos, A.; Pruksaphon, K.; Youngchim, S. Talaromyces marneffei laccase modifies THP-1 macrophage responses. Virulence 2016, 7, 702–717.
- Kaewmalakul, J.; Nosanchuk, J.D.; Vanittanakom, N.; Youngchim, S. Melanization and morphological effects on antifungal susceptibility of Penicillium marneffei. Antonie Van Leeuwenhoek 2014, 106, 1011–1020.
- Boyce, K.J.; McLauchlan, A.; Schreider, L.; Andrianopoulos, A. Intracellular growth is dependent on tyrosine catabolism in the dimorphic fungal pathogen Penicillium marneffei. PLoS Pathog. 2015, 11, e1004790.
- Almeida-Paes, R.; Frases, S.; Araújo Gde, S.; De Oliveira, M.M.; Gerfen, G.J.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Biosynthesis and functions of a melanoid pigment produced by species of the Sporothrix complex in the presence of L-tyrosine. Appl. Environ. Microbiol. 2012, 78, 8623–8630.
- Cruz, I.L.R.; Figueiredo-Carvalho, M.H.G.; Zancopé-Oliveira, R.M.; Almeida-Paes, R. Evaluation of melanin production by Sporothrix luriei. Memórias do Instituto Oswaldo Cruz 2018, 113, 68–70.
- Song, Y.; Yao, L.; Zhen, Y.; Cui, Y.; Zhong, S.; Liu, Y.; Li, S. Sporothrix globosa melanin inhibits antigen presentation by macrophages and enhances deep organ dissemination. Braz J. Microbiol. 2020.
- Almeida-Paes, R.; de Oliveira, L.C.; Oliveira, M.M.; Gutierrez-Galhardo, M.C.; Nosanchuk, J.D.; Zancope-Oliveira, R.M. Phenotypic characteristics associated with virulence of clinical isolates from the Sporothrix complex. BioMed Res. Int. 2015, 2015, 212308.
- Madrid, I.M.; Xavier, M.O.; Mattei, A.S.; Fernandes, C.G.; Guim, T.N.; Santin, R.; Schuch, L.F.; Nobre Mde, O.; Araújo Meireles, M.C. Role of melanin in the pathogenesis of cutaneous sporotrichosis. Microbes Infect. 2010, 12, 162–165.
- Mario, D.A.; Santos, R.C.; Denardi, L.B.; Vaucher Rde, A.; Santurio, J.M.; Alves, S.H. Interference of melanin in the susceptibility profile of Sporothrix species to amphotericin B. Rev. Iberoam. Micol. 2016, 33, 21–25.
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.; Brito-Santos, F.; Almeida-Silva, F.; Oliveira, M.M.; Zancopé-Oliveira, R.M. Melanins Protect Sporothrix brasiliensis and Sporothrix schenckii from the Antifungal Effects of Terbinafine. PLoS ONE 2016, 11, e0152796.
- Soliman, S.S.M.; Hamdy, R.; Elseginy, S.A.; Gebremariam, T.; Hamoda, A.M.; Madkour, M.; Venkatachalam, T.; Ershaid, M.N.; Mohammad, M.G.; Chamilos, G.; et al. Selective inhibition of Rhizopus eumelanin biosynthesis by novel natural product scaffold-based designs caused significant inhibition of fungal pathogenesis. Biochem. J. 2020, 477, 2489–2507.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
956
Revisions:
2 times
(View History)
Update Date:
04 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




