
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Muhammad Humayun | + 2044 word(s) | 2044 | 2021-04-27 08:35:39 | | | |
| 2 | Camila Xu | Meta information modification | 2044 | 2021-05-19 03:07:05 | | |
Video Upload Options
Covalent organic frameworks (COFs) are emerging crystalline polymeric materials with highly ordered intrinsic and uniform pores.
1. Introduction
Purification processes such as distillation, evaporation, concentration, and crystallization are important in basic research as well as playing an important role in industries. These processes, however, operate at the expense of environmental deterioration by consuming an enormous amount of energy, further promoting global warming. Industrial effluents also contain a large amount of chemical and bio-chemical hazardous ingredients polluting the already scarce freshwater resources. Moreover, many industries waste a large amount of precious chemical compounds and organic solvents due to the lack of economical separation/purification materials. Therefore, purification processes with low energy requirements may benefit the environment by saving energy on one hand and saving important capital costs on the other. In recent years, adsorption- (entrapment) and membrane (size exclusion)-based purification have attracted immense research and industrial interest due to their low energy consumption as well as simple and environmentally friendly operation. Various amorphous materials such as hyper-cross-linked polymers (HCPs) [1][2], porous organic polymers (POPs) [3][4], conjugated microporous polymers (CMPs) [5][6][7], and activated carbon [8][9][10]; and crystalline materials such as metal-organic frameworks (MOFs) [11][12][13][14] and zeolites [15][16][17][18][19], have shown excellent preliminary separation performance. Covalent organic frameworks (COFs) are a class of crystalline framework material synthesized from purely organic building blocks. Their synthesis involves reticular chemistry, which gives immense freedom of pre-design. Their pore geometry, size, and functionalities can be pre-determined by choosing building units from a large bank. Yaghi and co-workers first reported COFs based on boroxine and boronate ester rings [20]. These COFs, however, are prone to deformation in the presence of even trace amounts of humidity rendering them unsuitable in aqueous conditions. Later, imine-based COFs were reported to have superior chemical and solvent stability. Banerjee and co-workers prepared β-ketoenamine COFs with exceptional stability at high temperatures and in extreme acidic and basic conditions [21]. Similarly, Thomas and co-workers reported triazine-based COFs prepared at 400 °C, exhibiting excellent thermo-chemical stabilities [22]. Some of the important applications and various types of COFs are shown in Scheme 1 and Figure 1, respectively. Among many important aspects, COFs offer pore post-functionalization due to their organic nature, for specific desirable applications such as gas storage [23][24][25], catalysis [26][27], electronic devices [11][28], electrode material for batteries [29][30][31][32], etc.
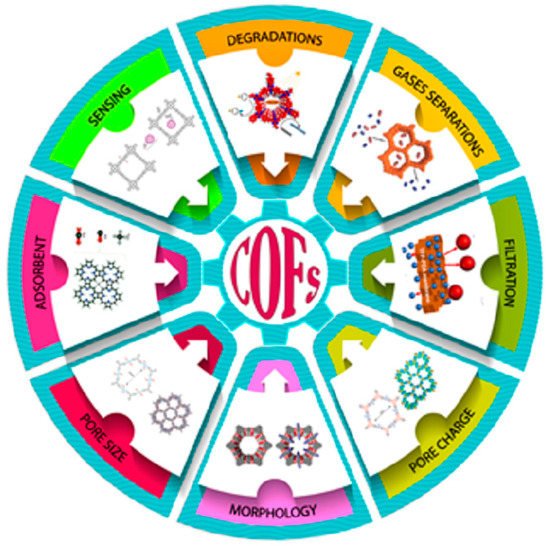
Scheme 1. The applications of COFs in various technologies.
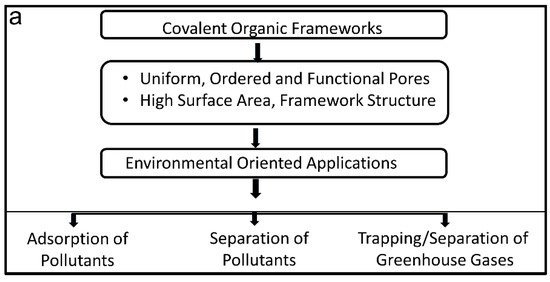
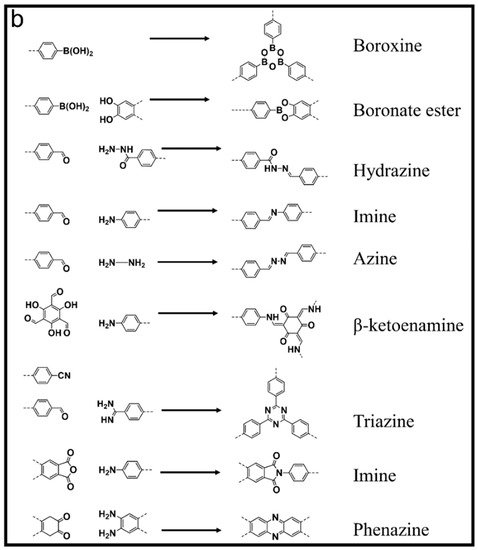
Figure 1. (a) Illustration of COFs towards environmental applications and (b) important classes of COFs and their pore geometry by binding different linkers.
The most attractive feature of COFs is their framework structure with uniform and extended porous channels, attracting the interest of scientists in the purification/separation field. Earth’s environment is under threat from the increasing disposal of hazardous ingredients such as heavy metals and poisonous organic and bio-organic chemicals [33][34][35][36][37][38][39]. Due to their distinct features, COFs represent themselves as viable alternative materials to address these issues [40][41]. Suitable pore designs and functionalities can render COFs as adsorbents for trapping hazardous metals, organic and bio-pollutants, and greenhouse gases [39][42][43][44][45][46][47]. As adsorbents, COF pores attract and trap pollutants based on their affinity towards functional COFs. COFs are also touted as excellent robust materials to fabricate separation membranes [48][49]. In the membrane form, pollutants are separated based on diffusion rates relying on the size, geometry, or charge of permeate and retentate. COFs have been reported to remove toxic components from both gases and liquids. Moreover, functionally decorated COFs can facilitate catalytic degradation of pollutants and convert them to clean energy. Our group has extensively worked on metal oxides and their composites for the application of photo- and electro-catalytic process [50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67], but recently we have shifted our focus towards COFs due to their excellent characteristic properties. Although COFs were first reported in 2005, their environmental applications have only attracted interest very recently [68]. Since then, COFs have been extensively explored for various environmentally related applications. We believe that an up-to-date account is urgently required for scientists in this field.
2. Evolution of COF Synthesis through Time
A brief evolution overview of various COF synthetic methods since their first report will be discussed here. The first COF was based on boroxine and boronate-ester linkages [20]. Although highly crystalline, these COFs are not suitable for an aqueous environment as the crystallinity and framework nature is destroyed even in the presence of a trace amount of water due to the electron-deficient boron. Later on, various other COFs based on imine [27][69], triazine [70], hydrazine [71], and keto-enol [29][72] linkages were reported, exhibiting excellent stability in organic, aqueous environments as well as harsh acidic and basic conditions. The synthesis of COFs intended for environmental applications generally follows bottom-up and top-down approaches. The former involves solvothermal [20], interfacial polymerization [73][74], in-situ growth [75], micro-wave assisted [76], and on-surface crystallization [77][78] methods, and the latter involves delamination of COF powders into mono/few-layer sheets for further applications [79]. COF pore geometry can be pre-determined by choosing suitable linker symmetry (Figure 1) or modified through post-functionalization. Further elaboration of these synthetic and post-functionalization methods is beyond the scope of this review. Many other reviews have covered various aspects of COF reticular synthesis and properties in detail [80][81][82][83][84][85][86][87][88][89].
3. Important Aspects of COF Materials towards Cleaner Environment
Industries are polluting our environment in two ways: (i) disposal of hazardous chemicals in water reservoirs; (ii) and greenhouse gases into the air. Materials with advanced functionalities to trap these hazardous chemicals and gases are urgently required to address these issues [90]. COFs are materials that have intrinsic, uniform, ordered and tailorable pores along with high surface area, making them very attractive for trapping and separating these hazardous molecules. COFs with multi-functional pores have extended their applicability in environmental cleansing. Due to their high surface area and ordered porous channels, they can not only be used to trap/store gas molecules but can also function as separating media to remove unwanted chemicals from the waste solution. This application can reduce environmental pollution as well as help in the recovery of precious solvents to be re-used in industries, rendering the whole operation environmentally and economically friendly. Recent development has yielded COFs with extraordinary stability in harsh acidic and basic conditions, rendering them highly desirable in industrial purification and trapping applications [21][91]. The rational design of COFs needs careful consideration of many aspects for intended purification applications. In this section, we will discuss some important aspects of COFs such as structure, morphology, and charge of their pores, as well as their stability, which makes them ideal alternative materials for environmental applications.
3.1. Pore Structure
Trapping or separation processes involve distinct characters of pores, the most important being their size. The pore geometry and structure can be pre-designed by choosing monomers with appropriate symmetry, as shown in Figure 1. The size of environmental pollutants ranges from sub-nanometer (metals) to several nanometers (dyes) and even up to micrometer (bio-pollutants such as bacteria, etc.) [92][93]. Therefore, careful consideration should be given to designing COFs for specific purification applications. COFs with pore sizes ranging from 0.5–5 nm have been reported so far, making them highly desirable in size-dependent separation processes. Banerjee et al. exhibited control over pore size by cross-linking precursors of different lengths and obtained COFs with pores in the range of 1.4–2.6 nm. 1,3,5-trifromylphloroglucinol (TFP) was chosen as the aldehyde-bearing monomer, whereas four amine-bearing monomers with different lengths were chosen as linkers (Figure 2) [73].
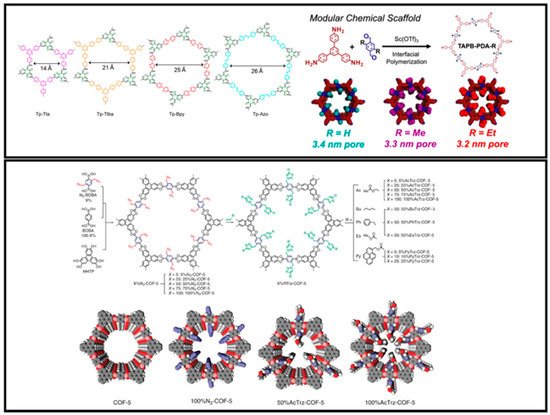
Figure 2. Control of pore size through linker selection (top) and post-functionalization (bottom). Adapted with permission from references [73][94], copyright 2017 and 2019 American Chemical Society.
Similarly, Ditchel et al. efficiently reduced the pore size of COFs by choosing linkers with six methyl or ethyl groups directing into the pores of the framework [94]. Pore surface engineering or pore post-functionalization is another strategy to tune the pore structure of COFs. Jiang et al., for the first time, reported an interesting strategy to first synthesize COFs with azide functionalities [95]. The COFs were synthesized through the condensation reaction between azide-appended benzene diboronic acid (N3-BDBA) and benzene diboronic acid (BDBA) with hexahydroxytriphenylene (HHTP). The azide could be easily cross-linked with many moieties such as propyl acetate, –COOH, –NH2, –COOMe, –OH, and –C≡C through click chemistry. The pore size was controlled between 1.2 and 3 nm by employing this strategy. Moreover, introducing these functional groups through post-functionalization also rendered COFs with desirable wettability and charge surfaces.
3.2. Hydrophobicity/Hydrophilicity of COFs
The morphology of COFs, such as their surface area and hydrophilic/hydrophobic nature, is very important for purification processes. COFs with a surface area of >2000 m2·g−1 have already been reported [72][96]. Ordered porous channels along with such high surface area are highly desired for purification/trapping applications. Hydrophilic/hydrophobic properties of COFs are another important controllable aspect, which are exploited by merely choosing desired linkers or through post-functionalization. COFs with hydrophobic nature [97] will enhance their applications in organic media, whereas hydrophilic COFs [98] will work better in aqueous media. Zhang et al. prepared a superhydrophobic COF through pore surface functionalization and evaluated their application in harsh conditions [99]. They first synthesized the COF and the pores were grafted with fluoride. The contact angle was increased from 0° to 150° by varying fluoride grafting. The modified COF retained its crystallinity and hydrophobicity under extremely harsh conditions such as in boiling water and in solutions with pH ranging from 1 to 14. Similarly, Hu et al. synthesized a hydrophilic triazine-based COF (Figure 3) and used it as a sensor for the detection of gallic acid (GA) and uric acid (UA) [100].
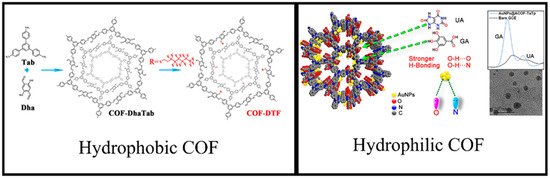
Figure 3. Examples of hydrophobic and hydrophilic COFs. Adapted with permission from references [99], copyright 2020 American Chemical Society and [100], copyright 2021 Elsevier.
3.3. Structural Stability
The first reported COFs based on boroxine and boronate ester linkages had poor stability in even a small amount of water. This phenomenon arises from the fact that the boron sites are electron deficient and can undergo nucleophilic reaction, resulting in structural degradation through hydrolysis. The effect is more severe as water is produced as a by-product during COF synthesis, which can facilitate the backward reaction and therefore severely affect their industrial applications. For environmental applications, COFs need to retain their ordered structure in practically harsh conditions. Linkers with strong covalent bonding to extend the framework, along with hydrogen bonding between interlayers, can overcome this shortcoming to some extent. Imine, azines, hydrazine, imides, and triazine-based COFs exhibit exceptional stability in harsh conditions because they are synthesized through acidic catalyst-based reversible reactions. Therefore, the backward reaction will be facilitated in an acidic environment but the COF should be stable in water as well as organic solvents [101]. Banerjee et al. explored a reversible/irreversible approach to improve COF stability one step further in solvents with pH ranging from 1 to 14 [21]. The same group also exhibited that increasing hydrogen bonding in the framework can also improve the stability of COFs [102]. They incorporated –OH functionalities adjacent to the –C=N bonds to introduce –OH–N=C hydrogen bonds, which ultimately safeguarded the imine nitrogen from hydrolysis in the presence of both acids and water. Similarly, other groups have improved COF stability through increasing intra-molecular hydrogen bonding to improve the COFs overall thermo-chemical stability [103][104].
3.4. Pore Charge
Pore charge is a crucial factor in the separation or trapping of hazardous chemicals through electrostatic interactions. According to Donnan’s theory, a negative charge-separating barrier will repulse divalent anions whereas divalent cations will be attracted. Therefore, if the negatively charged separating barrier is an adsorption agent then the cations will be trapped inside the matrix, but if the barrier is a membrane then the positively charged ions will transport more efficiently leaving anions in the feed. The same phenomenon is applied to the oppositely charged separating barrier. So far, very little attention has been given to design-charged COFs. Ma and co-workers introduced cationic sites in the COF (EB-COF:Br) by reacting 1,3,5-triformylphloroglucnol and ethidium bromide (EB) (3,8-diamino-5-ethyl-6-phenylphenanthridinium bromide) [105]. Similarly, Oakey et al. prepared a negatively charged COF by introducing carboxyl functional groups in the COF’s backbone and introduced them as fillers in the preparation of mixed matrix membranes [106]. In addition to the narrow size distribution of COF pores, the deprotonated -COOH− enhanced the rejection of bovine serum albumin (negatively charged protein) to as high as 81% at a COF loading of 0.8%. A similar approach was adopted to prepare a COF with modifiable carboxyl functional groups to prepare 12 COFs with variable aperture size and was self-assembled as continuous membranes [107]. It is expected that the synthesis of COFs based on charged pores for environmental application will attract more attention in coming years by incorporating functional groups such as hydroxyl, sulfonic acid, amine, etc.
References
- Sheng, Y.; Chen, Q.; Mahurin, S.M.; Mayes, R.T.; Zhan, W.; Zhang, J.; Liu, H.; Dai, S. Fibers with Hyper-Crosslinked Functional Porous Frameworks. Macromol. Rapid Commun. 2018, 39, 1700767.
- Li, H.; Meng, B.; Mahurin, S.M.; Chai, S.-H.; Nelson, K.M.; Baker, D.C.; Liu, H.; Dai, S. Carbohydrate based hyper-crosslinked organic polymers with -OH functional groups for CO2 separation. J. Mater. Chem. A 2015, 3, 20913–20918.
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563.
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375.
- Li, A.; Sun, H.-X.; Tan, D.-Z.; Fan, W.-J.; Wen, S.-H.; Qing, X.-J.; Li, G.-X.; Li, S.-Y.; Deng, W.-Q. Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ. Sci. 2011, 4, 2062–2065.
- Liang, B.; Wang, H.; Shi, X.; Shen, B.; He, X.; Ghazi, Z.A.; Khan, N.A.; Sin, H.; Khattak, A.M.; Li, L.; et al. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration. Nat. Chem. 2018, 10, 961–967.
- He, X.; Sin, H.; Liang, B.; Ghazi, Z.A.; Khattak, A.M.; Khan, N.A.; Alanagh, H.R.; Li, L.; Lu, X.; Tang, Z. Controlling the Selectivity of Conjugated Microporous Polymer Membrane for Efficient Organic Solvent Nanofiltration. Adv. Funct. Mater. 2019, 29, 1900134.
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403.
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379.
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and efficient nitrogen modified porous carbon derived from sugarcane bagasse for CO2 capture: Experimental and DFT investigation of nitrogen atoms on carbon frameworks. Chem. Eng. J. 2020, 382, 122964.
- Yildirim, O.; Bonomo, M.; Barbero, N.; Atzori, C.; Civalleri, B.; Bonino, F.; Viscardi, G.; Barolo, C. Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies. Energies 2020, 13, 5602.
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504.
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly Efficient Permeation and Separation of Gases with Metal–Organic Frameworks Confined in Polymeric Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001.
- Ghanem, A.S.; Ba-Shammakh, M.; Usman, M.; Khan, M.F.; Dafallah, H.; Habib, M.A.; Al-Maythalony, B.A. High gas permselectivity in ZIF-302/polyimide self-consistent mixed-matrix membrane. J. Appl. Polym. Sci. 2020, 137, 48513.
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites 2. J. Am. Chem. Soc. 1947, 69, 2836–2848.
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24.
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. Catalytic Flash Pyrolysis of Biomass Using Different Types of Zeolite and Online Vapor Fractionation. Energies 2016, 9, 187.
- Luis Miguez, J.; Porteiro, J.; Perez-Orozco, R.; Angel Gomez, M. Technology Evolution in Membrane-Based CCS. Energies 2018, 11, 3153.
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919.
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170.
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527.
- Katekomol, P.; Roeser, J.; Bojdys, M.; Weber, J.; Thomas, A. Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548.
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883.
- Alahakoon, S.B.; Thompson, C.M.; Occhialini, G.; Smaldone, R.A. Design Principles for Covalent Organic Frameworks in Energy Storage Applications. Chemsuschem 2017, 10, 2116–2129.
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317.
- Royuela, S.; Gil-San Millan, R.; Mancheno, M.J.; Mar Ramos, M.; Segura, J.L.; Navarro, J.A.R.; Zamora, F. Catalytically Active Imine-based Covalent Organic Frameworks for Detoxification of Nerve Agent Simulants in Aqueous Media. Materials 2019, 12, 1974.
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki-Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822.
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Loebermann, F.; Trauner, D.; et al. Extraction of Photogenerated Electrons and Holes from a Covalent Organic Framework Integrated Heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807.
- DeBlase, C.R.; Silberstein, K.E.; Thanh-Tam, T.; Abruna, H.D.; Dichtel, W.R. beta-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage. J. Am. Chem. Soc. 2013, 135, 16821–16824.
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of Covalent Organic Frameworks into Few-Layer Redox-Active Nanosheets as Cathode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261.
- Sun, T.; Xie, J.; Guo, W.; Li, D.-S.; Zhang, Q. Covalent-Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199.
- Ghazi, Z.A.; Zhu, L.; Wang, H.; Naeem, A.; Khattak, A.M.; Liang, B.; Khan, N.A.; Wei, Z.; Li, L.; Tang, Z. Efficient Polysulfide Chemisorption in Covalent Organic Frameworks for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 6, 1601250.
- Delrue, F.; Alvarez-Diaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132.
- Song, W.; Deng, X. Effects of Urbanization-Induced Cultivated Land Loss on Ecosystem Services in the North China Plain. Energies 2015, 8, 5678–5693.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182.
- Raju, K.A.; Ramakrishna, C. The effects of heavy metals on the anatomical structures of Avicennia marina (Forssk.) Vierh. Braz. J. Bot. 2021, 54, 1–9.
- Abrham, F.; Gholap, A.V. Analysis of heavy metal concentration in some vegetables using atomic absorption spectroscopy. Pollution 2021, 7, 205–216.
- Ghazi, Z.A.; Khattak, A.M.; Iqbal, R.; Ahmad, R.; Khan, A.A.; Usman, M.; Nawaz, F.; Ali, W.; Felegari, Z.; Jan, S.U.; et al. Adsorptive removal of Cd2+ from aqueous solutions by a highly stable covalent triazine-based framework. New J. Chem. 2018, 42, 10234–10242.
- Liu, L.; Wang, X.-X.; Wang, X.; Xu, G.-J.; Zhao, Y.-F.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S.; Wu, Y. Triazine-cored covalent organic framework for ultrasensitive detection of polybrominated diphenyl ethers from real samples: Experimental and DFT study. J. Hazard. Mater. 2021, 403, 123917.
- Zhang, Y.; Li, H.; Chang, J.; Guan, X.; Tang, L.; Fang, Q.; Valtchev, V.; Yan, Y.; Qiu, S. 3D Thioether-Based Covalent Organic Frameworks for Selective and Efficient Mercury Removal. Small 2021, e2006112.
- Huang, L.; Shen, R.; Liu, R.; Xu, S.; Shuai, Q. Facile fabrication of magnetic covalent organic frameworks for magnetic solid-phase extraction of diclofenac sodium in milk. Food Chem. 2021, 347, 129002.
- Jiang, H.-L.; Lin, Y.-L.; Li, N.; Wang, Z.-W.; Liu, M.; Zhao, R.-S.; Lin, J.-M. Application of magnetic N-doped carbon nanotubes in solid-phase extraction of trace bisphenols from fruit juices. Food Chem. 2018, 269, 413–418.
- Li, X.; Cui, Y.-Y.; Yang, C.-X. Covalent coupling fabrication of microporous organic network bonded capillary columns for gas chromatographic separation. Talanta 2021, 224, 121914.
- Xiao, Y.; Ma, C.; Jin, Z.; Wang, J.; He, L.; Mu, X.; Song, L.; Hu, Y. Functional covalent organic framework for exceptional Fe2+, Co2+ and Ni2+ removal: An upcycling strategy to achieve water decontamination and reutilization as smoke suppressant and flame retardant simultaneously. Chem. Eng. J. 2020, 127837.
- Xin, J.; Zhou, Y.; Wang, X.; Xu, G.; Xie, M.; Liu, L.; Zhao, R.; Wu, Y.; Wang, M. Room-temperature synthesis of magnetic covalent organic frameworks for analyzing trace benzoylurea insecticide residue in tea beverages. Food Chem. 2021, 347, 129075.
- Garba, M.D.; Usman, M.; Mazumder, M.A.J.; Al-Ahmed, A. Inamuddin, Complexing agents for metal removal using ultrafiltration membranes: A review. Environ. Chem. Lett. 2019, 17, 1195–1208.
- Turangan, N.; Xu, Y.; Spratt, H.; Rintoul, L.; Bottle, S.; MacLeod, J. Self-supporting covalent organic framework membranes synthesized through two different processes: Solvothermal annealing and solvent vapor annealing. Nanotechnology 2021, 32, 075604.
- Zhang, L.; Li, Y.; Wang, Y.; Ma, S.; Ou, J.; Shen, Y.; Ye, M.; Uyama, H. Integration of covalent organic frameworks into hydrophilic membrane with hierarchical porous structure for fast adsorption of metal ions. J. Hazard. Mater. 2021, 407, 124390.
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4 (001) thin film for the applications of photocatalysis. Appl. Catal. B 2018, 224, 895–903.
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B 2017, 241, 1161–1169.
- Ullah, H.; Tahir, A.A.; Bibi, S.; Mallick, T.K.; Karazhanov, S.Z. Electronic properties of β-TaON and its surfaces for solar water splitting. Appl. Catal. B 2018, 229, 24–31.
- Ullah, H.; Loh, A.; Trudgeon, D.P.; Li, X. Density Functional Theory Study of NiFeCo Trinary Oxy-Hydroxides for an Efficient and Stable Oxygen Evolution Reaction Catalyst. ACS Omega 2020, 5, 20517–20524.
- Ullah, H.; Bibi, S.; Tahir, A.A.; Mallick, T.K. Donor-acceptor polymer for the design of all-solid-state dye-sensitized solar cells. J. Alloy. Compd. 2017, 696, 914–922.
- Ullah, H.; Bibi, S.; Tahir, A.A.; Mallick, T.K. Density functional theory study of selenium-substituted low-bandgap donor–acceptor–donor polymer. J. Phys. Chem. C 2016, 120, 27200–27211.
- Nasir, S.N.F.M.; Ullah, H.; Ebadi, M.; Tahir, A.A.; Sagu, J.S.; Mat Teridi, M.A. New insights into Se/BiVO4 heterostructure for photoelectrochemical water splitting: A combined experimental and DFT study. J. Phys. Chem. C 2017, 121, 6218–6228.
- Humayun, M.; Ullah, H.; Cao, J.; Pi, W.; Yuan, Y.; Ali, S.; Tahir, A.A.; Yue, P.; Khan, A.; Zheng, Z. Experimental and DFT Studies of Au Deposition Over WO 3/gC 3 N 4 Z-Scheme Heterojunction. Nano-Micro Lett. 2020, 12, 1–18.
- Samsudin, M.F.R.; Ullah, H.; Tahir, A.A.; Li, X.; Ng, Y.H.; Sufian, S. Superior photoelectrocatalytic performance of ternary structural BiVO4/GQD/g-C3N4 heterojunction. J. Colloid Interface Sci. 2021, 586, 785–796.
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT insights on microflower g-C3N4/BiVO4 photocatalyst for enhanced photoelectrochemical hydrogen generation from lake water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403.
- Noh, M.F.M.; Ullah, H.; Arzaee, N.A.; Ab Halim, A.; Rahim, M.A.F.A.; Mohamed, N.A.; Safaei, J.; Nasir, S.N.F.M.; Wang, G.; Teridi, M.A.M. Rapid fabrication of oxygen defective α-Fe2O3 (110) for enhanced photoelectrochemical activities. Dalton Trans. 2020, 49, 12037–12048.
- Mohamed, N.A.; Ullah, H.; Safaei, J.; Ismail, A.F.; Mohamad Noh, M.F.; Soh, M.F.; Ibrahim, M.A.; Ludin, N.A.; Mat Teridi, M.A. Efficient Photoelectrochemical Performance of γ Irradiated g-C3N4 and Its Heterojunction for Solar Water Splitting. J. Phys. Chem. C 2019, 123, 9013–9026.
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B 2018, 234, 296–310.
- Ola, O.; Ullah, H.; Chen, Y.; Thummavichai, K.; Wang, N.; Zhu, Y. DFT and Experimental Studies of Iron Oxide-based Nanocomposites for Efficient Electrocatalysis. J. Mater. Chem. C 2021.
- Lin, Y.; Wang, P.; Loh, A.; Wan, L.; Habib, U.; Xu, Z.; Li, X.; Wang, B. Assembling flower-on-sheet CoP–NiCoP nanohybrids as efficient self-supported electrocatalysts for hydrogen evolution reaction in both acidic and alkaline media. J. Mater. Sci. 2021, 56, 3375–3386.
- Sookhakian, M.; Ullah, H.; Teridi, M.A.M.; Tong, G.B.; Basirun, W.J.; Alias, Y. Boron-doped graphene-supported manganese oxide nanotubes as an efficient non-metal catalyst for the oxygen reduction reaction. Sustain. Energy Fuels 2020, 4, 737–749.
- Kim, H.P.; Vasilopoulou, M.; Ullah, H.; Bibi, S.; Gavim, A.E.X.; Macedo, A.G.; da Silva, W.J.; Schneider, F.K.; Tahir, A.A.; Teridi, M.A.M. A hysteresis-free perovskite transistor with exceptional stability through molecular cross-linking and amine-based surface passivation. Nanoscale 2020, 12, 7641–7650.
- Bin Mohd Yusoff, A.R.; Mahata, A.; Vasilopoulou, M.; Ullah, H.; Hu, B.; da Silva, W.J.; Schneider, F.K.; Gao, P.; Ievlev, A.V.; Liu, Y. Observation of large Rashba spin–orbit coupling at room temperature in compositionally engineered perovskite single crystals and application in high performance photodetectors. Mater. Today 2021, in press.
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037.
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klock, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571.
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453.
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481.
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862.
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091.
- Khan, N.A.; Zhang, R.; Wu, H.; Shen, J.; Yuan, J.; Fan, C.; Cao, L.; Olson, M.A.; Jiang, Z. Solid–Vapor Interface Engineered Covalent Organic Framework Membranes for Molecular Separation. J. Am. Chem. Soc. 2020, 142, 13450–13458.
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087.
- Diaz de Grenu, B.; Torres, J.; Garcia-Gonzalez, J.; Munoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amoros, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233.
- Liu, X.-H.; Guan, C.-Z.; Ding, S.-Y.; Wang, W.; Yan, H.-J.; Wang, D.; Wan, L.-J. On-Surface Synthesis of Single-Layered Two-Dimensional Covalent Organic Frameworks via Solid–Vapor Interface Reactions. J. Am. Chem. Soc. 2013, 135, 10470–10474.
- Xu, L.; Zhou, X.; Tian, W.Q.; Gao, T.; Zhang, Y.F.; Lei, S.; Liu, Z.F. Surface-Confined Single-Layer Covalent Organic Framework on Single-Layer Graphene Grown on Copper Foil. Angew. Chem. Int. Ed. 2014, 53, 9564–9568.
- Khan, N.A.; Yuan, J.; Wu, H.; Huang, T.; You, X.; Olson, M.A.; Azad, C.S.; Rahman, A.U.; Jiang, Z. Covalent Organic Framework Nanosheets as Reactive Fillers to Fabricate Free-Standing Polyamide Membranes for Efficient Desalination. ACS Appl. Mater. Interfaces 2020, 12, 27777–27785.
- Bian, G.; Yin, J.; Zhu, J. Recent Advances on Conductive 2D Covalent Organic Frameworks. Small 2021, e2006043.
- Liu, Y.; Zhou, W.; Teo, W.L.; Wang, K.; Zhang, L.; Zeng, Y.; Zhao, Y. Covalent-Organic-Framework-Based Composite Materials. Chem 2020, 6, 3172–3202.
- Ren, D.; Ren, S.; Lin, Y.; Xu, J.; Wang, X. Recent developments of organic solvent resistant materials for membrane separations. Chemosphere 2020, 271, 129425.
- Zhang, Y.; Jin, X.; Ma, X.; Wang, Y. Chiral porous organic frameworks and their application in enantioseparation. Anal. Methods 2021, 13, 8–33.
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585.
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568.
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19.
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671.
- Waller, P.J.; Gandara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063.
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311.
- Halder, A.; Karak, S.; Addicoat, M.; Bera, S.; Chakraborty, A.; Kunjattu, S.H.; Pachfule, P.; Heine, T.; Banerjee, R. Ultrastable Imine-Based Covalent Organic Frameworks for Sulfuric Acid Recovery: An Effect of Interlayer Hydrogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 5797–5802.
- Ehsan, M.F.; Fazal, A.; Hamid, S.; Arfan, M.; Khan, I.; Usman, M.; Shafiee, A.; Ashiq, M.N. CoFe2O4 decorated g-C3N4 nanosheets: New insights into superoxide anion mediated photomineralization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104556.
- Ehsan, M.F.; Shafiq, M.; Hamid, S.; Shafiee, A.; Usman, M.; Khan, I.; Ashiq, M.N.; Arfan, M. Reactive oxygen species: New insights into photocatalytic pollutant degradation over g-C3N4/ZnSe nanocomposite. Appl. Surf. Sci. 2020, 532, 147418.
- Corcos, A.R.; Levato, G.A.; Jiang, Z.; Evans, A.M.; Livingston, A.G.; Mariñas, B.J.; Dichtel, W.R. Reducing the Pore Size of Covalent Organic Frameworks in Thin-Film Composite Membranes Enhances Solute Rejection. ACS Mater. Lett. 2019, 1, 440–446.
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536.
- Zhuang, S.; Liu, Y.; Wang, J. Covalent organic frameworks as efficient adsorbent for sulfamerazine removal from aqueous solution. J. Hazard. Mater. 2020, 383, 121126.
- Mullangi, D.; Shalini, S.; Nandi, S.; Choksi, B.; Vaidhyanathan, R. Super-hydrophobic covalent organic frameworks for chemical resistant coatings and hydrophobic paper and textile composites. J. Mater. Chem. A 2017, 5, 8376–8384.
- Liu, Z.; Su, Q.; Ju, P.; Li, X.; Li, G.; Wu, Q.; Yang, B. A hydrophilic covalent organic framework for photocatalytic oxidation of benzylamine in water. Chem. Commun. 2020, 56, 766–769.
- Han, N.; Zhang, Z.; Gao, H.; Qian, Y.; Tan, L.; Yang, C.; Zhang, H.; Cui, Z.; Li, W.; Zhang, X. Superhydrophobic Covalent Organic Frameworks Prepared via Pore Surface Modifications for Functional Coatings under Harsh Conditions. ACS Appl. Mater. Interfaces 2020, 12, 2926–2934.
- Lin, X.; Deng, Y.; He, Y.; Chen, J.; Hu, S. Construction of hydrophilic N, O-rich carboxylated triazine-covalent organic frameworks for the application in selective simultaneous electrochemical detection. Appl. Surf. Sci. 2021, 545, 149047.
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681.
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056.
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912.
- Li, Z.; Zhang, Y.; Xia, H.; Mu, Y.; Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 2016, 52, 6613–6616.
- Zhang, W.; Zhang, L.; Zhao, H.; Li, B.; Ma, H. A two-dimensional cationic covalent organic framework membrane for selective molecular sieving. J. Mater. Chem. A 2018, 6, 13331–13339.
- Duong, P.H.H.; Kuehl, V.A.; Mastorovich, B.; Hoberg, J.O.; Parkinson, B.A.; Li-Oakey, K.D. Carboxyl-functionalized covalent organic framework as a two-dimensional nanofiller for mixed-matrix ultrafiltration membranes. J. Membr. Sci. 2019, 574, 338–348.
- Kuehl, V.A.; Yin, J.; Duong, P.H.H.; Mastorovich, B.; Newell, B.; Li-Oakey, K.D.; Parkinson, B.A.; Hoberg, J.O. A Highly Ordered Nanoporous, Two-Dimensional Covalent Organic Framework with Modifiable Pores, and Its Application in Water Purification and Ion Sieving. J. Am. Chem. Soc. 2018, 140, 18200–18207.




