
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alejandro Baeza | + 2806 word(s) | 2806 | 2021-05-12 04:24:59 | | | |
| 2 | Vivi Li | Meta information modification | 2806 | 2021-05-18 08:52:16 | | |
Video Upload Options
Peyronie and Dupuytren are pathologies characterized by the appearance of localized fibrotic lesions in an organ. These disorders originate from an excessive production of collagen in the tissue provoking dysfunction and functional limitations to the patients. Local administration of collagenase is the most used treatment for these fibrotic-type diseases, but a high lability of the enzyme limits its therapeutic efficacy. Herein, we present a novel methodology for the preparation of collagenase nanocapsules without affecting its enzymatic activity and capable of releasing the enzyme in response to an ultraviolet A (UVA) light stimulus.
1. Introduction
Fibrosis is defined as the excessive overproduction and accumulation of collagens and other extracellular matrix (ECM) proteins in the connective tissue of different organs. This pathology usually leads to discomfort and malfunction or disablement of the affected organ [1][2]. Collagen is synthesized by fibroblasts and other mesenchymal cells, such as myofibroblasts. These cells are ultimately responsible for the overproduction of ECM-related proteins. This overproduction leads, in turn, to the accumulation of excessive amounts of collagens, and the contraction and remodeling of the connective tissue [3]. The inappropriate remodeling of ECM lead to the formation of fibrotic lesions fibrosis, which are characterized by scarring and thickening of the affected tissue [4][5]. The process may occur systemically (i.e., systemic sclerosis [6]) or locally (i.e., liver cirrhosis [7]) as an abnormal repairing process following inflammatory lesions. This can also be observed in pathological wound healing, leading to abnormal, hypertrophic or keloid scars, as consequence of autoimmune conditions. This process is observable in different fibrotic-type diseases, such as scleroderma (morphea) [8], and those with localized fibrotic lesions that frequently cause severe local dysfunction (i.e., painful erections in Peyronie disease [9][10] or hand contractures in Dupuytren disease [11][12]). These pathologies are characterized by an overproduction of collagen, which induces local discomfort and functional limitation to the patients.
These diseases are mainly treated by surgery or collagenase administration [13]. Surgical procedures are invasive interventions that frequently involve long recovery times for patients. Restoration of a proper functionality is challenging, and relapses are not rare [11]. Furthermore, several side effects as tendon, nerve, or artery injuries are commonly found. Since Food and Drug Administration (FDA) approved in 2010 the injection of Collagenase Clostridium Histolyticum (CCH) for the intra-lesional treatment of Peyronie’s disease and Dupuytren’s contracture [14], collagenase has become the preferred treatment for these localized pathologies. CCH is a matrix metalloproteinase that specifically cleaves type I and III fibrillar collagens without affecting type IV collagen. Type I and III are the major collagen fibers in ECM, while type IV are mainly present in basement membranes [3]. Since fibrotic lesions are produced in ECM, local administration of CCH promote a meaningful reduction of the excessive collagen fibers present in fibrotic tissues, alleviating thereby the painful symptoms of these diseases [13][15]. The main limitation of the use of this enzyme is its high liability in physiological environment; collagenase loses its activity in less than 24 h at physiological conditions. This issue may be solved by periodically injecting multiple collagenase doses but produces important local pain and is time consuming for patients and health professionals. On the other hand, there is a potential risk of adverse events, derived from overdose, leading to tissue damage.
Our research group has developed diverse methodologies to produce polymeric nanocapsules for a wide variety of enzymes; as peroxidase [16], catalase [17] or collagenase [18]. Encapsulation protects the enzyme from degradation by external factors, but do not affect its enzymatic activity, which are of interest in the delivery of therapeutic enzymes in various diseases. In this way, collagenase nanocapsules were designed to release enzymes in a controlled manner into the diseased tissue in response to a local stimulus, such as pH changes. This feature has been employed to degrade the ECM in tumoral-like tissues, which exhibit acidic environment. The pH-promoted collagenase release enhanced the penetration of a drug-loaded nanocarrier [19]. Latterly, the incorporation of a non-degradable crosslinker within nanocapsules structure reduced the hydrolysis rate. In this way, the release of controlled amounts of collagenase was achieved over more than 10 days, which was advantageous for the treatment of skin fibrosis [20]. Additionally, the dual-fluorescent labelling of nanocapsules allowed the monitorization of their penetration in a three-dimensional collagen gel [21].
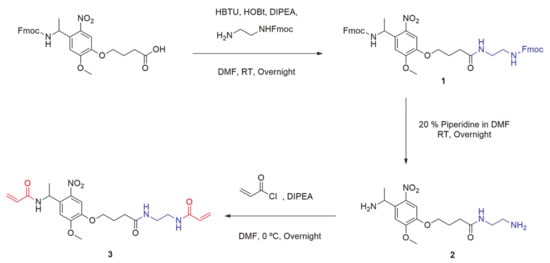
2. Synthesis of UVA Sensitive Photolinker
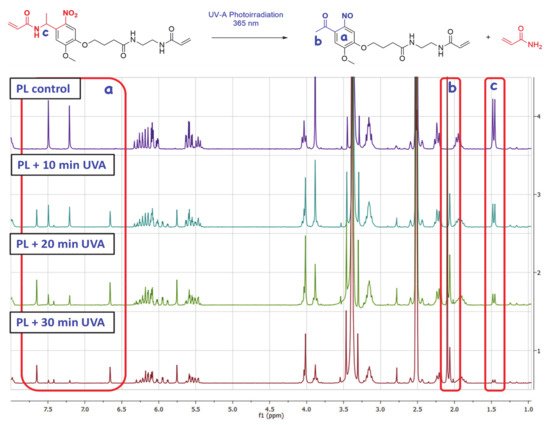
3. Photo-Lability Evaluation of PL
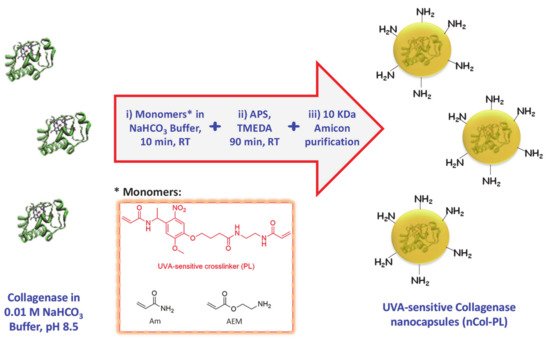
4. Synthesis of UVA-Sensitive Collagenase Nanocapsules (nCol-PL)
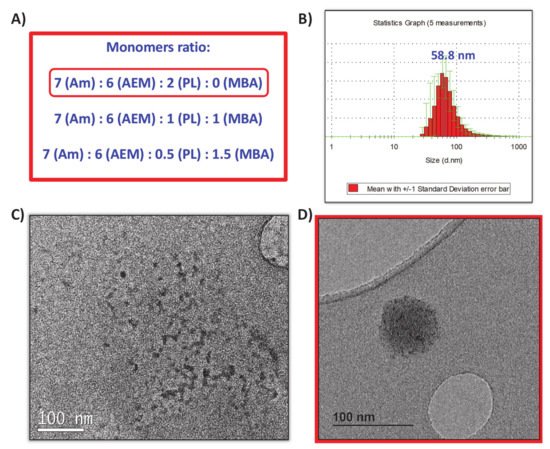
5. In Vitro Cleavage Evaluation of nCol-PL and Enzymatic Activity Measurement
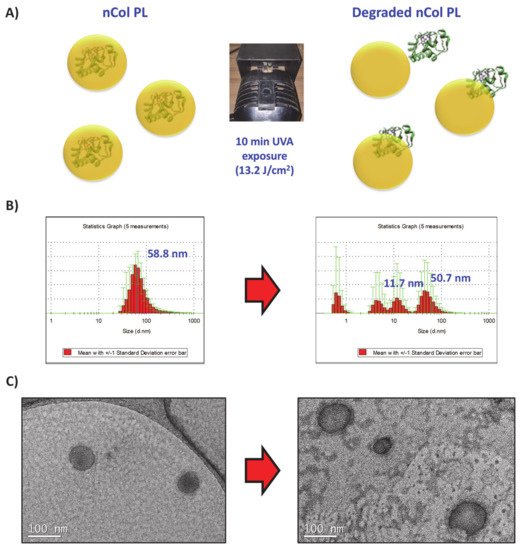
6. Conclusions
A new approach for treating fibrotic diseases in localized areas through the release of collagenase from within UVA-degradable polymeric nanocapsules has been presented. The novel compound Bisacrylamide-Photolinker has been synthetized and deeply characterized, with the aim of providing UVA-sensitivity properties to nanocapsules. These nanocapsules have been successfully prepared through free-radicals polymerization using acrylamide-type monomers. The employ of UVA-sensitive collagenase nanocapsules provides a triple advantage in comparison with the conventional administration of free enzymes. Firstly, the use of these capsules for transporting the proteolytic enzymes solves the problem associated with a lower stability of the enzymes in physiological conditions. Polymeric nanocapsule protect the enzymes against external factors, present in the diseased tissue, such as other proteolytic enzymes, oxidative agents and temperature increases. Secondly, the capacity to release the housed enzyme only if UVA radiation is applied allows a precise control of the administered dose along the time. Higher enzymatic activities were observed when additional UVA pulse were applied to nCol-PL. This fact avoids the apparition of side effects, which usually accompany the use of proteolytic enzymes, provoked by an excessive tissue degradation. Therefore, it would maximize the efficacy of the therapy in a focused area. Finally, the choice of UVA radiation for triggering the release of collagenase might provide additional benefits for the therapy, due to its potential anti-inflammatory effects and its capacity to inhibit the collagen synthesis in the irradiated area of the diseased tissue.
The administration of nanocapsules can be carried out locally, providing greater advantages for the treatment of localized lesions caused by fibrosis. Their systemic use (i.e., intravenously) followed by local activation in patients with other fibrotic diseases, such as localized or systemic scleroderma, is a potential application to be explored. In all cases, the fact that the collagenase release takes place only if UVA is applied, will focus the therapeutic effect exclusively in the diseased zones, thereby reducing the apparition of side effects in healthy tissues. A significant therapeutic improvement is expected, as a result of the the synergy between these capacities and the easily-controlled release of the proteolytic enzyme.
References
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040.
- Distler, J.H.W.; Györfi, A.H.; Ramanujam, M.; Whitfield, M.L.; Königshoff, M.; Lafyatis, R. Shared and distinct mechanisms of fibrosis. Nat. Rev. Rheumatol. 2019, 15, 705–730.
- Trojanowska, M.; LeRoy, E.C.; Eckes, B.; Krieg, T. Pathogenesis of fibrosis: Type 1 collagen and the skin. J. Mol. Med. 1998, 76, 266–274.
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. DMM Dis. Model. Mech. 2011, 4, 165–178.
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566.
- Ho, Y.Y.; Lagares, D.; Tager, A.M.; Kapoor, M. Fibrosis-A lethal component of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 390–402.
- Šmíd, V. Liver fibrosis. Vnitr. Lek. 2020, 66, e36–e41.
- Arias, D.; Borbujo-Martínez, J. Localized scleroderma (Morphea). FMC Form. Med. Contin. Aten. Primaria 2005, 12, 680.
- Levine, L.A.; Larsen, S.M. Surgical correction of persistent peyronie’s disease following collagenase clostridium histolyticum treatment. J. Sex. Med. 2015, 12, 259–264.
- Patel, D.P.; Christensen, M.B.; Hotaling, J.M.; Pastuszak, A.W. A review of inflammation and fibrosis: Implications for the pathogenesis of Peyronie’s disease. World J. Urol. 2020, 38, 253–261.
- Soreide, E.; Murad, M.H.; Denbeigh, J.M.; Dudakovic, A.; Kakar, S.; Lewallen, E.A.; Nordsletten, L.; Van Wijnen, A.J. Treatment of Dupuytren’s contracture: A systematic review. Bone Jt. J. 2018, 100B, 1138–1145.
- Al-Qattan, M.M. Factors in the pathogenesis of Dupuytren’s contracture. J. Hand Surg. Am. 2006, 31, 1527–1534.
- Nellas, C.L.; Crawford, N.; Scherbel, A.L. Pancreatic collagenase therapy for severe, progressive systemic sclerosis: Effect on skin and on hydroxyproline content in urine. Clin. Pharmacol. Ther. 1965, 6, 367–371.
- Mills, S.A.; Gelbard, M.K. Sixty years in the making: Collagenase Clostridium histolyticum, from benchtop to FDA approval and beyond. World J. Urol. 2020, 38, 269–277.
- Gilpin, D.; Coleman, S.; Hall, S.; Houston, A.; Karrasch, J.; Jones, N. Injectable collagenase clostridium histolyticum: A new nonsurgical treatment for Dupuytren’s disease. J. Hand Surg. Am. 2010, 35, 2027–2038.e1.
- Baeza, A.; Guisasola, E.; Torres-Pardo, A.; González-Calbet, J.M.; Melen, G.J.; Ramirez, M.; Vallet-Regí, M. Hybrid enzyme-polymeric capsules/mesoporous silica nanodevice for in situ cytotoxic agent generation. Adv. Funct. Mater. 2014, 24, 4625–4633.
- Simmchen, J.; Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Improving catalase-based propelled motor endurance by enzyme encapsulation. Nanoscale 2014, 6, 8907–8913.
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Hybrid collagenase nanocapsules for enhanced nanocarrier penetration in tumoral tissues. ACS Appl. Mater. Interfaces 2015, 7, 24075–24081.
- Villegas, M.R.; Baeza, A.; Noureddine, A.; Durfee, P.N.; Butler, K.S.; Agola, J.O.; Brinker, C.J.; Vallet-Regí, M. Multifunctional protocells for enhanced penetration in 3D extracellular tumoral matrices. Chem. Mater. 2018, 30, 112–120.
- Villegas, M.R.; Baeza, A.; Usategui, A.; Ortiz-Romero, P.L.; Pablos, J.L.; Vallet-Regí, M. Collagenase nanocapsules: An approach to fibrosis treatment. Acta Biomater. 2018, 74, 430–438.
- Moreno, V.M.; Baeza, A.; Vallet-regí, M. Evaluation of the penetration process of fluorescent collagenase nanocapsules in a 3D collagen gel. Acta Biomater. 2021, 121, 263–274.
- Šolomek, T.; Mercier, S.; Bally, T.; Bochet, C.G. Photolysis of ortho-nitrobenzylic derivatives: The importance of the leaving group. Photochem. Photobiol. Sci. 2012, 11, 548–555.
- Kim, M.S.; Diamond, S.L. Photocleavage of o-nitrobenzyl ether derivatives for rapid biomedical release applications. Bioorg. Med. Chem. Lett. 2006, 16, 4007–4010.
- Villegas, M.R.; Baeza, A.; Vallet-Regí, M. Nanotechnological strategies for protein delivery. Molecules 2018, 23, 1008.




