Peyronie and Dupuytren are pathologies characterized by the appearance of localized fibrotic lesions in an organ. These disorders originate from an excessive production of collagen in the tissue provoking dysfunction and functional limitations to the patients. Local administration of collagenase is the most used treatment for these fibrotic-type diseases, but a high lability of the enzyme limits its therapeutic efficacy. Herein, we present a novel methodology for the preparation of collagenase nanocapsules without affecting its enzymatic activity and capable of releasing the enzyme in response to an ultraviolet A (UVA) light stimulus.
1. Introduction
Fibrosis is defined as the excessive overproduction and accumulation of collagens and other extracellular matrix (ECM) proteins in the connective tissue of different organs. This pathology usually leads to discomfort and malfunction or disablement of the affected organ [1,2]. Collagen is synthesized by fibroblasts and other mesenchymal cells, such as myofibroblasts. These cells are ultimately responsible for the overproduction of ECM-related proteins. This overproduction leads, in turn, to the accumulation of excessive amounts of collagens, and the contraction and remodeling of the connective tissue [3]. The inappropriate remodeling of ECM lead to the formation of fibrotic lesions fibrosis, which are characterized by scarring and thickening of the affected tissue [4,5]. The process may occur systemically (i.e., systemic sclerosis [6]) or locally (i.e., liver cirrhosis [7]) as an abnormal repairing process following inflammatory lesions. This can also be observed in pathological wound healing, leading to abnormal, hypertrophic or keloid scars, as consequence of autoimmune conditions. This process is observable in different fibrotic-type diseases, such as scleroderma (morphea) [8], and those with localized fibrotic lesions that frequently cause severe local dysfunction (i.e., painful erections in Peyronie disease [9,10] or hand contractures in Dupuytren disease [11,12]). These pathologies are characterized by an overproduction of collagen, which induces local discomfort and functional limitation to the patients.
These diseases are mainly treated by surgery or collagenase administration [13]. Surgical procedures are invasive interventions that frequently involve long recovery times for patients. Restoration of a proper functionality is challenging, and relapses are not rare [11]. Furthermore, several side effects as tendon, nerve, or artery injuries are commonly found. Since Food and Drug Administration (FDA) approved in 2010 the injection of Collagenase Clostridium Histolyticum (CCH) for the intra-lesional treatment of Peyronie’s disease and Dupuytren’s contracture [14], collagenase has become the preferred treatment for these localized pathologies. CCH is a matrix metalloproteinase that specifically cleaves type I and III fibrillar collagens without affecting type IV collagen. Type I and III are the major collagen fibers in ECM, while type IV are mainly present in basement membranes [3]. Since fibrotic lesions are produced in ECM, local administration of CCH promote a meaningful reduction of the excessive collagen fibers present in fibrotic tissues, alleviating thereby the painful symptoms of these diseases [13,15]. The main limitation of the use of this enzyme is its high liability in physiological environment; collagenase loses its activity in less than 24 h at physiological conditions. This issue may be solved by periodically injecting multiple collagenase doses but produces important local pain and is time consuming for patients and health professionals. On the other hand, there is a potential risk of adverse events, derived from overdose, leading to tissue damage.
Our research group has developed diverse methodologies to produce polymeric nanocapsules for a wide variety of enzymes; as peroxidase [16], catalase [17] or collagenase [18]. Encapsulation protects the enzyme from degradation by external factors, but do not affect its enzymatic activity, which are of interest in the delivery of therapeutic enzymes in various diseases. In this way, collagenase nanocapsules were designed to release enzymes in a controlled manner into the diseased tissue in response to a local stimulus, such as pH changes. This feature has been employed to degrade the ECM in tumoral-like tissues, which exhibit acidic environment. The pH-promoted collagenase release enhanced the penetration of a drug-loaded nanocarrier [19]. Latterly, the incorporation of a non-degradable crosslinker within nanocapsules structure reduced the hydrolysis rate. In this way, the release of controlled amounts of collagenase was achieved over more than 10 days, which was advantageous for the treatment of skin fibrosis [20]. Additionally, the dual-fluorescent labelling of nanocapsules allowed the monitorization of their penetration in a three-dimensional collagen gel [21].
2. Synthesis of UVA Sensitive Photolinker
In order to correctly incorporate ONV moiety in the structure of nanocapsules, acrylate groups in both ends of the molecule were required. The presence of an α,β-unsaturated carbonyl in the photolabile compound provides the capacity to react intermolecularly between monomers and crosslinkers during the free-radical polymerization reaction.
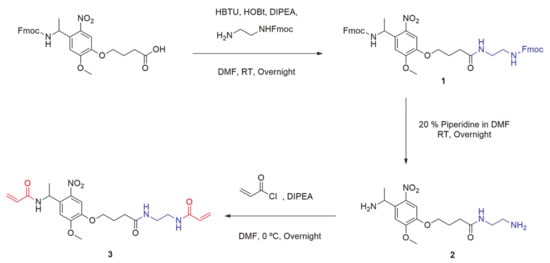
The commercially available Fmoc-photolinker carboxylic acid was employed as ONV source for the obtention of this crosslinker. The synthetic steps necessaries to obtain the molecule are summarized in
Scheme 1. HBTU and HOBt benzotriazoles in basic conditions were employed to incorporate a nucleophilic amine group at the other end of the molecule. In this way, the correspondent activated ester for the amide formation was obtained by coupling the carboxylic end of the photolinker molecule and amine from mono-Fmoc diamine compound. The resulting double-Fmoc protected amide (product
1) was analyzed by
1H NMR (
Supplementary Materials, Figure S1) and Mass Spectrometry (
Supplementary Materials, Figure S2). Then, both amine ends were deprotected by removing Fmoc groups in basic conditions employing a large excess of a solution of Piperidine/DMF at a 2:8 ratio, and the resulting mixture was evaporated under vacuum to obtain a crude containing the diamine-Photolinker (product 2). Due to the high polar and basic properties of this compound, the purification was easily carried out using a strong cation exchange retention technique with a solid phase extraction (SPE) SCX-2 cartridge. As the crude containing the diamine is passed through the Si-propylsulfonic acid (SCX-2) resin, the amine is retained by the strong cation exchange (SCX) column. By washing the column with MeOH in mild acidic conditions, non-basic impurities are not retained and are further removed. The product was subsequently released from the cartridge by elution with a 2 M solution of Ammonia in MeOH and solvent was dried, filtered, and evaporated. The obtained product 2 was analyzed by
1H NMR (
Supplementary Materials, Figure S3).
Scheme 1. Synthetic pathway employed for the obtention of bisacrylamide photolinker, PL (product 3).
Finally, product 2 was dissolved in anhydrous DMF under Nitrogen atmosphere, and DIPEA was added to keep the basicity of amine groups. Significant excess of acryloyl chloride was slowly added dropwise at 0 °C to this solution and kept overnight. Then, crude was evaporated under vacuum and dissolved in DCM. Then, the organic layer was extracted with a 5% aqueous solution of LiCl (to remove the remained DMF), brine and water, and evaporated under vacuum. The bisacrylamide photolinker (PL) product 3 was obtained as a pale-yellow solid. This novel compound was fully characterized by
1H NMR (
Supplementary Materials, Figure S4), Mass spectrometry (MS) (
Supplementary Materials, Figure S5), FTIR (
Supplementary Materials, Figure S6),
13C NMR (
Supplementary Materials, Figure S7), COSY 2D-NMR (
Supplementary Materials, Figure S8), HMQC 2D-NMR (
Supplementary Materials, Figure S9).
3. Photo-Lability Evaluation of PL
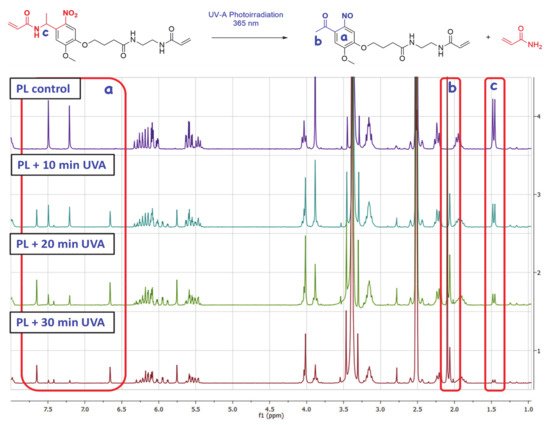
UVA irradiation (365 nm) of the molecule containing the ONV photolabile moiety produces an intramolecular rearrangement through a complex three-step mechanism [
35] (-Upper image). This intramolecular photo-redox reaction of the nitroaromatic compound involves the overall reduction of the nitro group to nitroso, in concomitance with the oxidation of the heteroatom present in the ortho-benzyl position. Initiation is produced by the attacking nitro-group oxygen, which rapidly leads to the elimination of acrylamide and formation of the correspondent nitroso-benzaldehyde derivate. The incorporation of a methyl group on the benzyl position has been proved that enhances the cleavage kinetics by a 20-fold rate [
36].
Figure 1. Upper image: Elimination reaction involved in the photodegradation of the synthetized photolinker (PL). Bottom image: 1H NMR comparative spectra for the three consecutive 10-min cycles of UVA irradiation.
To confirm the UVA-sensitivity of synthetized product 3, it was diluted in DMSO-d6 (concentration of 2.5 mg/mL) and was transferred to a 1×1 cm quartz translucent cuvette. Then, the solution was exposed to three cycles of UVA irradiation (10 min of exposure each) using an irradiation wavelength of 365 nm at a distance of ~20 cm from the lamp focus. The energy registered at this point was 22.4 mW/cm2. After the three irradiation exposure times [Initial (PL + 10 min UVA) 10 min of irradiation (energy of 13.2 J/cm2), second 10 min of irradiation (PL + 20 min UVA) (accumulated energy of 26.4 J/cm2) and final 10 min of irradiation (PL + 30 min UVA) (accumulated energy of 39.6 J/cm2)], the solution was analyzed by 1H NMR, as can be observed in -Bottom image. The same procedure was performed with an aluminum covered cuvette as a blank, with no variation in product 1H NMR spectrum after the three UVA exposure cycles (PL control).
Regarding initial (PL + 10 min UVA) and final NMR spectra (PL + 30 min UVA) of the compound, it is observable the apparition and progressive area increase of two peaks at ~6.66 ppm and ~7.65 ppm in the aromatic chemical shifts area (, area a), due to the influence of ketone and NO electron withdrawing groups present in the aromatic ring of the degraded photolinker (dPL). It is also remarkable that a new peak at ~2.06 ppm (, area b) corresponding with the methyl group of newly formed aryl ketone. In parallel, it is also observable the disappearance of the doublet peak at ~1.46 ppm (, area c) corresponding to the α-methyl group of PL, confirming the elimination of acrylamide moiety to yield the ketone. The NMR calculated degradation ratio by the comparison of peak area from both compounds PL and dPL showed that the “degradation” yield for every irradiation pulse was ~45% for first 10 min, 72% for 20 min and 87% for 30 min (See
Supplementary Materials, Figure S10).
This degradation was also confirmed by MS analysis (See
Supplementary Materials, Figure S11), obtaining a m/z of 482.9 for the pre-irradiation sample (PL) and a m/z of 411.8 corresponding to PL after irradiation (dPL). A decrease of 71 units in the mass of the irradiated product was consistent with the mass of acrylamide sub product, which was generated in the degradation.
4. Synthesis of UVA-Sensitive Collagenase Nanocapsules (nCol-PL)
As we have introduced, this methodology is based on the method reported in previous works for the construction of a polymeric shell around collagenase enzyme [
18,
20,
21]. In this method, nanocapsules were formed by in situ free-radicals polymerization of acrylamide-type monomers which were adsorbed on the macromolecule surface [
37]. This interaction is favored by electrostatic interactions between monomers and collagenase surface, mainly hydrogen bonds. However, the relationship between electrostatics, protein interaction, and dynamic adsorption is an important one, but questions remain unanswered related to the physicochemical determinants of charge interactions. Moreover, these favourable interactions also positively contribute to the formation of the polymeric coating trough of the free-radicals polymerization reaction. The existing electron-rich hydroxyl and amino groups in the protein structure (i.e., in form of hydroxyproline, serine or lysine) can react with the free radicals formed during the decomposition of APS. These newly formed hydroxyl and amino radicals react, in turn, with the monomers that surround the protein, leading to the formation of the polymeric coating by the generation of covalent bonds.
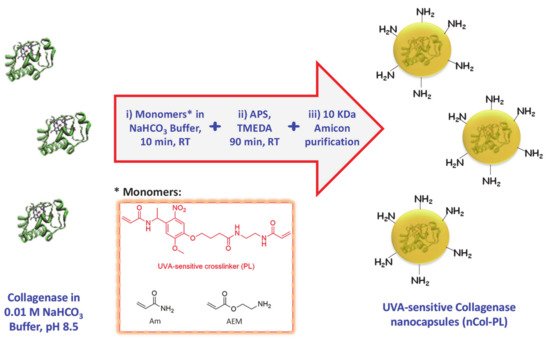
Collagenase nanocapsules were synthesized using the free-radicals polymerization method, previously reported in our research works [
18,
19,
20]. The incorporation of PL
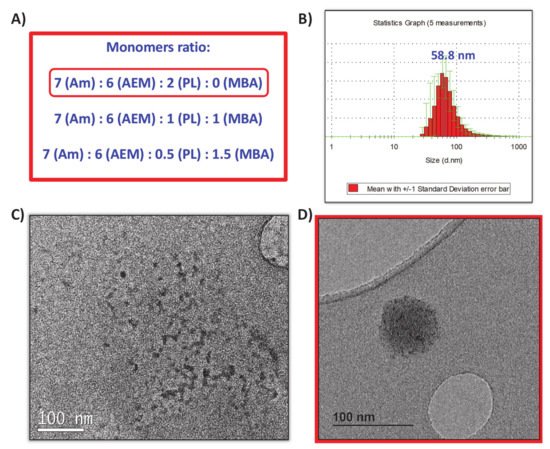
(3) as a crosslinker provide the capacity to respond to UVA radiation exposition. In this method, the monomers and proteins were mixed in a molar ratio of 2025:1 (monomers to protein) in aqueous solution at pH = 8.5 and then, after a short period of time required for the adsorption of the monomers on the protein surface, a radical initiator was added for inducing the formation of the protein capsule around the protein (). Each monomer plays different role in the capsule formation. Am is a structural monomer, AEM provides colloidal stability to the capsule in physiological conditions, and PL is the monomer that provides the light-responsive capacity. A complete set of different nanocapsules were synthesized varying the ratio between monomers to produce capsules with variable degradability in the presence of UVA light. For this, a combination of both photolabile PL and non-degradable crosslinker N,N’-Methylenebisacrylamide (MBA) was employed (A). Good results were achieved for the monomers ratio of 7 (Am):6 (AEM):2 (PL), thus discarding the employ of MBA as monomer.
Figure 2. Schematic procedure for UVA-sensitive Collagenase nanocapsules synthesis. Asterisk indicates the monomers employed in the synthetic step i).
Figure 3. (A) Monomers ratio tested for the nanocapsules synthesis. (B) DLS obtained for nanocapsules synthetized with the 7:6:2 ratio. (C) TEM micrography of free collagenase before encapsulation. (D) TEM micrography of obtained nanocapsules.
Once the free-radicals reaction was finished, nanocapsules (nCol-PL) were purified by centrifugation in 10 kDa Amicon and they were preserved from light at 4 °C. The DLS analysis showed an average hydrodynamic size of ∼58 nm, as can be observed in B. Z potential revealed a value of −11.0 mV, which was consistent with the presence of free amine groups in the polymeric framework of nanocapsules. Free collagenase and nCol-PL were also analyzed by TEM, showing that free collagenase (C) had a ∼10 nm size, and then, when nanocapsules were synthetized (D) they presented a size of ∼60 nm, consistent with DLS analysis. This suggest that nanocapsules are certainly “polymeric clusters” formed by the encapsulation of several collagenase enzymes.
5. In Vitro Cleavage Evaluation of nCol-PL and Enzymatic Activity Measurement
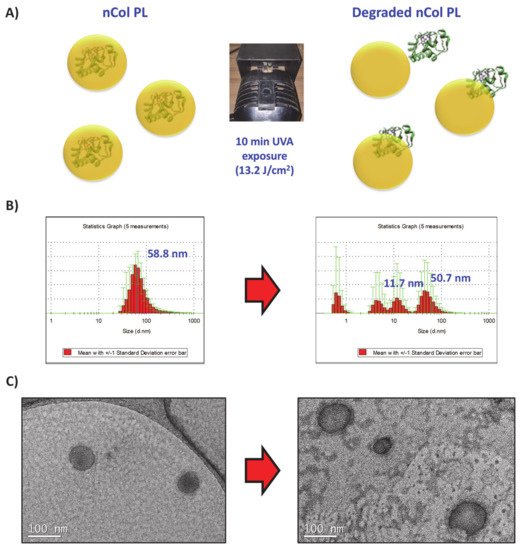
Firstly, the UVA-sensitive behaviour of the produced nCol-PL was evaluated in an acellular aqueous media exposing the nanocapsules to UVA light. For this, a solution of nanocapsules with a protein concentration (calculated according to the protocol described in
Section 2.3) of 50 µg/mL (corresponds to a theoretical collagenase activity of 14.5 U/mL) was introduced in a 1 × 1 cm quartz translucent cuvette and it was exposed to 10 min of UVA irradiation using an irradiation lamp with a wavelength of 365 nm at a distance of ∼20 cm from the lamp focus to the cuvette. Then, their size variation was evaluated by DLS and TEM. A summary scheme is shown in A. The change in the hydrodynamic size of nanocapsules was observable from an initial size of ∼58 nm to a heterogeneous sizes distributions DLS diagram after 10 min of UVA exposure (B). This result suggests that the crosslinker PL present in nCol-PL was degraded by UVA irradiation. TEM images showed that the polymeric framework that surrounds the enzyme was broken since free collagenase was also observable (C). This breakage allowed the release of collagenase trapped within the nanocapsule, giving thus hydrodynamic sizes of an average ∼50 nm (related to non-degraded nCol-PL), ∼11 nm (corresponding to released collagenases) and smaller that correspond to polymeric slices derived from degraded capsules. These results indicated that 10 min UVA exposure was enough to degrade many of the nanocapsules present in the sample, but not the whole population of nanocapsules.
Figure 4. (A) UVA-sensitive nanocapsules exposed 10 min to UVA irradiation. (B) DLS analysis showed initial 58 nm size of nanocapusules (Left side), which after irradiation showed several size distributions that correspond with collagenase nanocapsules and free collagenase (Right side). (C) TEM micrographs also signaled the breakage of the nanocapsule framework.
6. Conclusions
A new approach for treating fibrotic diseases in localized areas through the release of collagenase from within UVA-degradable polymeric nanocapsules has been presented. The novel compound Bisacrylamide-Photolinker has been synthetized and deeply characterized, with the aim of providing UVA-sensitivity properties to nanocapsules. These nanocapsules have been successfully prepared through free-radicals polymerization using acrylamide-type monomers. The employ of UVA-sensitive collagenase nanocapsules provides a triple advantage in comparison with the conventional administration of free enzymes. Firstly, the use of these capsules for transporting the proteolytic enzymes solves the problem associated with a lower stability of the enzymes in physiological conditions. Polymeric nanocapsule protect the enzymes against external factors, present in the diseased tissue, such as other proteolytic enzymes, oxidative agents and temperature increases. Secondly, the capacity to release the housed enzyme only if UVA radiation is applied allows a precise control of the administered dose along the time. Higher enzymatic activities were observed when additional UVA pulse were applied to nCol-PL. This fact avoids the apparition of side effects, which usually accompany the use of proteolytic enzymes, provoked by an excessive tissue degradation. Therefore, it would maximize the efficacy of the therapy in a focused area. Finally, the choice of UVA radiation for triggering the release of collagenase might provide additional benefits for the therapy, due to its potential anti-inflammatory effects and its capacity to inhibit the collagen synthesis in the irradiated area of the diseased tissue.
The administration of nanocapsules can be carried out locally, providing greater advantages for the treatment of localized lesions caused by fibrosis. Their systemic use (i.e., intravenously) followed by local activation in patients with other fibrotic diseases, such as localized or systemic scleroderma, is a potential application to be explored. In all cases, the fact that the collagenase release takes place only if UVA is applied, will focus the therapeutic effect exclusively in the diseased zones, thereby reducing the apparition of side effects in healthy tissues. A significant therapeutic improvement is expected, as a result of the the synergy between these capacities and the easily-controlled release of the proteolytic enzyme.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics13040499