
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dennis Douroumis | + 4236 word(s) | 4236 | 2021-05-07 05:32:31 | | | |
| 2 | Karina Chen | Meta information modification | 4236 | 2021-05-18 05:04:44 | | |
Video Upload Options
Twin-screw granulation (TSG) is a pharmaceutical process that has gained increased interest from the pharmaceutical industry for its potential for the development of oral dosage forms. The technology has evolved rapidly due to the flexibility of the equipment design, the selection of the process variables and the wide range of processed materials. Most importantly, TSG offers the benefits of both batch and continuous manufacturing for pharmaceutical products, accompanied by excellent process control, high product quality which can be achieved through the implementation of Quality by Design (QbD) approaches and the integration of Process Analytical Tools (PAT).
1. Twin Screw Granulation
TSG is an advanced processing technology that has been extensively used for granules production over the last couple of decades [1]. Several studies have been reported relating to TSG optimization by investigating the effect of the formulation composition and operational variables such as screw configuration, pitch and length of conveying element, thickness and angle of kneading element, or influence of kneading blocks. A schematic diagram of a TSG line is illustrated in Figure 1.
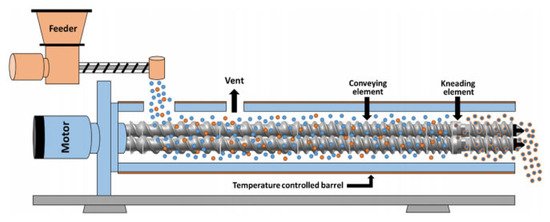
Figure 1. Schematic representation of a twin screw granulator (Reproduced with permission from [2], Elsevier, 2020).
The first study for the development of pharmaceutical granules at a laboratory scale using a single-screw extruder was reported by Goodhart et al. in 1973 [3]. The investigation was undertaken to evaluate various factors related to wet granulation such as the effect of granulating fluid, type of end plate, number of mixing anvils and screw speed. Another objective of these studies was to further understand the level of content uniformity during granulation. The use of water-isopropanol as granulating fluid reduced the sugar solubility in the granulating fluid, creating increased torque during highspeed processing. The granules prepared by using this granulating fluid resulted in the compression of less gritty and smoother tablets compared to water. It was concluded that the water- isopropanol granulating fluid created processing interruption but produced granules with low bulk densities.
Later on, in 1986, Gamlen and Eardley introduced the twin screw extruder in pharmaceutical research, to produce paracetamol extrudates with a combination of excipients i.e., Avicel, lactose and/ or hydroxypropyl methyl cellulose (HPMC) and using water as a granulating fluid [4]. The results revealed that addition of HPMC in the formulation influenced significantly on the extrusion properties. HPMC helped to retain water in its interstitial spaces, reducing frictional forces between extruder and the plate. Micrographs of the extruded formulation with and without HPMC showed similar appearances. Hence, the addition of HPMC improved the extrudability without affecting the extrudate quality. A year later, Lindberg (1987) and later Kleinebudde (1998) also employed twin-screw extruders for the formation of effervescent granules [5][6][7][8][9][10]. Furthermore, TSG has been implemented to perform dry, wet and melt granulation process [11] including scaling-up of continuous granulation coupled with Process Analytical Tools (PAT) by applying Quality by Design (QbD) approaches [12].
Despite the fact the TSG is an emerging technology the majority of the published studies are data-driven and (semi-) mechanistic which investigate the association between the process parameters and the granulation performance using multivariate data analysis. However, the effect of critical processing parameters (CPP) on the growth and kinetics of granule formation is not well established in comparison to batch granulation (e.g., high shear).
For this reason, theoretical [13][14] and experimental [15][16][17][18] approaches have been introduced to understand the transport, mixing and the fundamental mechanisms in twin-screw wet granulation. As shown in Figure 2, the dry powder is fed in the barrel through the feeding zone while the granulating liquid is added using two nozzles (for each screw). The granule formation takes place through a combination of capillary and viscous forces facilitating particle binding in the wet stage. Subsequently, the wet material is distributed, compacted and elongated in the kneading elements (mixing zone) transforming the particle morphology from small (microstructure) to large (macro structure) irregular and porous granules. Other sub -processes such as aggregation and breakage can also take place during granulation. Eventually, the formed granules leave the discharge zone and are pneumatically fed in a fluidised bed dryer.
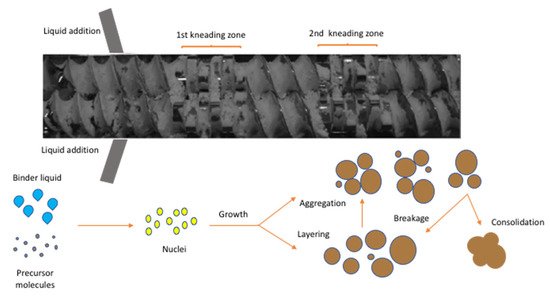
Figure 2. Screw configuration with 12 kneading disks illustrating the formation of granules in TSG (Reproduced with permission from [19], John Wiley and Sons, 2017).
A very interesting study was conducted by Dhenge et al. [20] who replaced the top metal barrel of the extruder and replace it with an acrylic glass barrel to visualize the granulation process and identify the possible mechanisms. The authors concluded that there are five regions of granulation behaviour or finished granules classified as under-wetted (dry), nuclei, crumb, granules and over wetted or paste. The under wetted granules were obtained when low amount of granulating liquid was used resulting in un-granulated material. The nuclei refers to the initial wet mass which comprises of small granulating liquid amounts and are loosely bound with weak mechanical properties. The “crumb” denotes again ungranulated or poorly granulated materials which present different strength depending on the type of used elements (conveying of kneading). To the contrary, the “granules” are robust consolidated agglomerates which have a similar behaviour compared to classical granules and can be turned to wetted agglomerates or paste if additional granulating liquid is added. The study included the use of particle image velocimetry and discrete element modelling to prove that the feed rate and viscosity greatly affect the surface velocity of dry materials and wet granules. The actual mechanisms of granulation have been further investigated and elaborated by published work from other researchers [18]. These studies provide information on how the processing parameters can significantly affect the aggregation and breakage mechanisms including the consolidation of the particles.
Furthermore, modelling approaches, for example, using novel multi-component population balance model have investigated TSG processing by taking into account the rate processes of aggregation, breakage, liquid addition and consolidation. The linkage of the granulation mechanism with experimental data were used to develop predictive tools [14]. Kumar et al., introduced conceptual model in order to understand and simulate the residence time distribution (RTD) for a particular TSG formulation that could be easily adopted for other excipients [13]. The comparison of the experimental findings and best conceptual model were used to estimate and predict the influence of kneading elements, stagger angle, screw speed and powder feed on RTD (Figure 9).
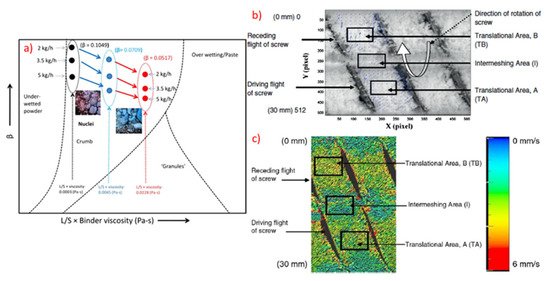
Figure 9. (a) Granule regime map for TSG using conveying screws, (b) Representation of different areas in the screw channel in 2D and (c) Snapshot showing different areas in the screw channel with surface velocity vectors (Reproduced with permission from [20], Elsevier, 2013).
In 2010, Dhenge et al. conducted an empirical study on a twin – screw extruder TSE with model pharmaceutical formulations focused on the physical properties of the final granules [21]. The authors investigated the effect of screw speed, powder feed rate and liquid to solid (L/S) ratio on the residence time and torque which was found to affect the particle size, the strength, shape and structure of the granules. The most pronounced effect on granule properties was observed with a L/S ratio of 0.4, showing a monomodal distribution of granule sizes with a peak around the 1000 µm mark. At higher L/S, the extra liquid caused an increase in the residence time of material in the granulator, resulting a reduction of undersized and oversized granules. The granules shape and hence the flow was improved due to the increased amount of granulating fluid which helped to achieve stronger liquid bridges between the particles. The powder feed rate influenced the transition and final state of granule properties such as size, shape, structure, porosity, strength, and dissolution time. At a low feed rate the residence time became long, resulted in strong granules with an increased average granule size whereas higher feed rate reduced the granule size. At a powder feed rate of 3.5 and 5 kg/h, the sphericity of the granules was found to be increased. The improved sphericity of granule was related to the increased filling at high powder feed rate which led to increase in shearing forces within the barrel and turned the processed powder to spherical agglomerates. The surface morphology of the granules became smooth by increasing the length of the screw while porosity was decreased. The high feed rate not only increased the granules morphological strength and stability but also affected the dissolution rates. It was concluded that TSG optimisation is a complex process, and the granule properties (size distribution, strength and structure) are affected significantly by the critical processing parameters.
Vercruysse et al. (2012) investigated the operational parameters related to TSG and their effect on the manufacturing process using theophylline as model API [22]. There was no significant relationship of screw configuration and screw speed to the granules morphology, but high number of kneading elements and increased throughput resulted higher torque during granulation. The high torque raised the temperature in the barrel which led to reduced number of fines and less friable granules. The binder was found to be more effective when it was dissolved in the granulating fluid. Increasing the number of kneading elements yielded denser granules with a longer disintegration time and dissolution rate. The findings of this work suggested that the granule and tablet quality can be optimised by adjusting specific process variables. Khorsheed et al. (2017) also investigated the effect of TSG processing parameters on the powder and granule and tablet properties using microcrystalline cellulose (MCC) or mannitol C160 [23]. Their study showed increasing MCC granule size and strength can reduce tabletability and vice versa. Although, mannitol C160 granules did not affect tabletability, particle size reduction had shown significant compactibility improvements. They found a correlation between the yield pressure, plastic and elastic work of the initial powders and changes in tabletability performance as a result of the granulation process. The authors mentioned that increasing the strength and size of granules may cause reduction of the tabletability.
The granule structure is closely related to tablet’s quality attributes and it can be controlled by the selection of appropriate excipients as it was recently reported by Megarry et al. [24]. Allopurinol granulated formulations prepared by wet granulation via both twin-screw and high shear processing. By using different mannitol grades (200SD and 160 °C) a polymorphic transition during processing, containing mostly β-mannitol, was found. The 200SD granules had a needle-shaped morphology with high porosity and specific surface area, which led to poorer flow properties but higher tablet tensile strength. Furthermore, 200SD granules presented lower d10 values compared to 160C while the L/S ratio did not affect the size as it was observed for the 160 °C. The study suggested that the understanding of specific excipient grade and their effect during processing is crucial to optimise tablet manufacturability.
Lute et al. (2018) investigated the influence of varying barrel fill levels on the mean residence time, granule properties and tensile strength of tables using MCC and lactose [25]. They reported that specific feed load or powder feed volume directly affects the granule size and shape. Increasing fill levels of MCC inside the extruder barrel caused shorter residence times along with decreased granules size. On the other hand, lactose maintained its granule size at all fill levels. Again, the usage of a specific pharma excipient and their processing parameters are crucial parts of the granulation optimisation process.
Twin screw dry granulation is considered more effective for granule manufacturing as it limits heat exposure to only one-barrel zone, much shorter than melt granulation. Lui et al. (2017) studied formulations containing different polymeric binders (AF15, Kollidon VA 64®, Soluplus®, Kollidon SR®), with glass transition temperature less than 130 °C [26]. Granulation of the primary powders with some degree of moisture was found to be beneficial for processed polymers with high glass transition temperatures. Selected polymer particles are more prone to soften and flow under frictional forces if their Tg was closer to the barrel zone temperature in the kneading section. Because of this, the Barrel temperature is often set to match the Tg of the processing polymer to allow for successful granulation. Screw speed was a major cause for friction heating while the kneading block offset was only minor in its influence on the granulation process. According to their results, a higher screw speed tended to increase the particles size, producing bigger chunks (>3350 µm). Conversely, an increase of moisture content in the excipient resulted in smaller particle size distribution. So, successful granulation can be achieved by varying the processing parameters and pre-formulation studies will allow one to quickly optimise the process.
Ye et al. (2019) used twin screw dry granulation to improve the flow property of moisture sensitive materials [27]. They produced dry granule formulations using four different APIs processed with KlucelTM, EthocelTM, and magnesium stearate for sustained release. A Design of Experiment (DoE) was employed to determine the effect of different processing parameters i.e., screw speed, feeding rate, barrel temperature and screw configuration on the product properties (flow properties, particle size distribution and dissolution time). It was revealed that an increased screw speed related to a higher percentage of medium size granules while a negative correlation was found between the amount of large size granules and screw speed. This was attributed to the decrease of the mean residence time due to the high screw speeds and the subsequent reduction of the kneading effect on primary powders and the formation of smaller granules. Higher feeding rates improved the flow properties of the powders and decreased angles of repose were obtained. The morphology of granules affected the powder flow where long stripe shape exhibited poor flowability compared to round shape particles. Finally, drug release was affected by the binder content in the granule and presented significant variations i.e., large stronger granules with more binder showed relatively slow release. On the other hand, small amount soft binder provided less particle adhesion which resulted in faster disintegration and hence faster dissolution rates. The continuous processing, simplicity of operation, absence of milling, suggest that twin-screw dry granulation TSDG is more effective compared to other conventional dry granulation approaches.
Kallakunta et al. (2019) applied heat assisted dry granulation using a twin screw extruder to formulate sustained release granules [28]. Granulation feasibility was studied with different binders (e.g., Klucel™ EF, Kollidon® VA64), sustained release agents (e.g., Klucel™ MF, Eudragit® RSPO) and diluents at various drug loads. The processing conditions were below the melting point or glass transition temperature of the formulation ingredients. They found a size correlation to the binding capacity of the excipient. Formulations with Klucel prepared granules with a size of around 250 µm whereas Kollidon® VA64 promoted the formation of finer granules related to its own particle size (20 µm). Hence, the good binding properties of the ingredients in the formulation, facilitated easier granule formation and larger granule size. On the other hand, formulations consisting of only Klucel™ MF showed minimal erosion (approximately 5%) over 24 h. The drug release was found to be incomplete in these formulations, due to the high viscosity of Klucel™ MF. However, the excellent binding properties and viscosity of Klucel™ MF resulted in dosage forms with good matrix integrity, which then became one of the controlling factors for drug release. Conversely, formulations with Kollidon® VA64 reported to undergo greater erosion which destabilised the tablet matrix and led fast drug release. In summary, heat-assisted dry granulation could be applied for continuous twin-screw granulation, which may ameliorate the process constraints and stability problems in conventional granulation techniques. However, the careful polymer selection is crucial to achieving the desired physiochemical properties for the end product.
Unlike wet and dry granulation, melt granulation offers several advantages for processing pharmaceutical actives. Twin-screw melt granulation is carried out at higher temperatures than traditional batch melt granulation and thermoplastic polymers can be used as binders. This has a clear benefit, considering the limited number of traditional binders that are suitable for use in conventional granulation processes. The process of twin-screw melt granulation is also advantageous as it eliminates the need for organic solvents and water, while it is cost effective and environmentally friendly. As a totally water-free process, twin-screw melt granulation is suitable for drugs that undergo hydrolysis or degradation in the presence of water but may not be suitable for some heat-labile API.
Ven Melkebeke et al. (2006) studied twin screw melt granulation for the manufacturing of immediate release formulation using two grades of polyethylene glycol (PEG 400 and 4000) as a binder [29]. The authors described the importance of granulation temperature on its dissolution properties. A high yield and fast dissolution rate were obtained only at a processing temperature near the melting point of PEG. Post granulation characterisations showed that a homogeneous dispersion of the BCS class II drug within the polymeric matrix created a micro-environment around the drug particles enhancing the dissolution rate. The addition of small surfactant amounts (polysorbate 80 or Cremophor) helped to achieve a complete drug release within 10 minutes. A high drug content required greater PEG and surfactant content to obtain 100% drug release.
Batra et al. (2017) investigated polymeric binders with high melting points (180 °C) for improving tabletability of APIs using twin screw melt granulation [30]. For the purposes of the study metformin hydrochloride and acetaminophen were used as active ingredients and several polymers i.e., hydroxypropyl cellulose, hydroxypropyl methyl- cellulose, polyvinylpyrrolidone and methacrylate-based polymers, including Klucel® EXF, Eudragit® EPO, and Soluplus® as binders. The prepared granules demonstrated good tensile strength during tableting even with polymer concentrations as low as 10% w/w. As the melting temperature of acetaminophen is below 180 °C, its TSG temperature was achieved at 130 °C, and even at that temperature, extruded granules provided acceptable compatibility of >2 MPa, suggesting that compressed tablets could withstand manufacturing and end-use stresses during coating, packaging, transportation, and handling. The work demonstrated that a pool of polymeric binders can be successfully use for twin-screw melt granulation and further exploited in the future.
Unlike wet or dry granulation, in melt granulation the growth mechanism involves an additional nucleation mechanism known as immersion or distribution as shown in Figure 3
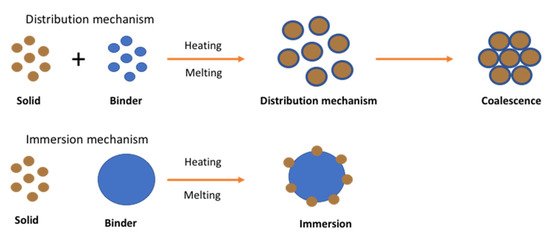
Figure 3. Nucleation mechanism of melt granulation (Reproduced with permission from [31], Elsevier, 1996).
The mechanism was studied by Monteyne et al. (2016) for the development of immiscible drug-binder formulations [32]. They implemented thermal analysis, rheological characterisation and microscopic images to reach an in-depth understanding of material behaviour during agglomeration. They reported that the distribution of the binder in the immiscible blends caused a double Tg and a clear loss peak in the thermogram. The binder was found to act as a separate phase favouring efficient binder distribution where a thin binder layer with restricted mobility is formed on the surface of the primary drug particles during granulation. Then it is covered by a second layer with improved mobility when the binder concentration is sufficiently high. The granules manufactured with 20% (w/w) Soluplus® or higher became smaller and more spherical as a function of temperature whereas a lower binder concentration resulted in larger and more needle-shaped granules. The study showed strong evidence of the binder distribution during TSG which strongly affected the granule characteristics.
In another study, the same group carried out TSG studies with Soluplus (SOL) and metoprolol (MTP) or caffeine [33]. In this case, thermal analysis showed only one Tg, indicating a highly miscible system. A high barrel temperature was used to obtain granules larger than 500 µm which also showed minimum torque fluctuation during granulation. Surprisingly, granules prepared with 20% or more binder concentration resulted large aggregates at processing temperatures <90 °C. Prepared granules with caffeine-Soluplus blends displayed a broad particle size distribution over the different binder concentrations and was not affected by the process temperatures whereas the granule size distribution of the MPT/SOLblends appeared narrower and shifted towards lower particle sizes with an elevated granulation temperature.
5. Twin Screw Granulation and Continuous Manufacturing
The traditional manufacturing of medicinal products in the pharmaceutical industry uses batch processing, where every single unit operation is conducted separately. The adoption of continuous manufacturing (CM) from pharmaceutical industry is relatively new and it takes place in a stepwise manner. On the other hand food, petrochemical, polymer and oil refining industries have been undertaking CM operations for decades and produce large volume production at a cost effective manner [34][35][36][37]. The term “continuous” means a required process may run for an extended period, with raw material constantly fed into the process. The pharmaceutical industry has faced many obstacles in attempting such a continuous production method on a day-to-day basis. The main perceived issue is the industry’s rigid structure, because of the strict supervision of regulatory agencies such as the U.S. Food and Drug Administration FDA in the U.S. or the European Medicines Agency (EMA) in Europe [38][39][40].
Despite the fact that there are no regulatory hurdles for implementing continuous processes pharmaceutical industry needs to reform regulatory framework, pharmaceutical equipment design and operation. Some pharmaceutical companies plan to convert 70% of their production lines to continuous manufacturing to allow a more efficient product and process development as part of the product operation. CM processes are usually operating at or near steady state which allows a clsosed-loop control of the entire process and thus to a robust and reliable operation. They usually reach steady state in a few minutes enabling the application of a true QbD manufacturing approach.
Nevertheless, TSG is an ideal example of such a process that has successfully been introduced for CM production. Recently, GEA introduced a continuous platform for oral solid dosage forms which integrates multiple operation units comprising of granulation, drying and tablet compression. As shown in Figure 4, the process involves the dosing and mixing of raw materials suing multiple feeders, followed by the high shear granulator for mixing, wetting and granulation through the coupling with a highly accurate dosing system (e.g., water, solvents, binder solutions). Subsequently the granules are continuously transferred (e.g., vacuum or gravity) to a fluidized bed dryer for drying and milled to achieve the desired particle size and content uniformity. The drying process is monitored with an on-line moisture LightHouseTM probe. Prior to tablet compression in a rotary tablet press the dried powders are blended with the external phase (e.g., lubricants, disintegrants, fillers). The tablet press comprises of 6 compression modes such as compression to equal porosity, Exchangeable Compression Module with a wash-off-line capability which are coupled with advanced in-line PAT sensors and a control system. The technology is versatile and processed amounts vary from 500 g for R&D purposes up to 100 Kg/h.
Figure 4. ConsiGmaTM continuous high shear granulation process by GEA Group (images provided by the manufacturer).
A similar processing technology known as Modular Continuous System MODCOS has been developed by Glatt for the conversion of the batch mode to a continuous fluid bed system. As shown in Figure 5, loss in weight feeders is used for the dosing of the active drug substance and excipients through vacuum conveyor in a twin-screw extruder to produce medium to high density granules. The wet granules are pneumatically conveyed into the process insert of the fluid bed via a specially designed transfer line, where they are continuously dried to the required moisture level. Before the granules are compressed in tablets with other ingredients a dry mixer is used to achieve homogeneous mixing. A key advantage of the MODCOS line is the narrow retention time distribution during drying while a sophisticated discharge system facilitates complete emptying of the process chambers and thus preventing any cross-contamination with consistent product quality. The continuous line is coupled with a range of PAT tools including two Near–Infrared (NIR) probes for determine content uniformity at the end of the extruder or the discharge system. A moisture probe is used at the fluid bed dryer discharge point and a particle sizer probe is inserted at the powder discharge points (feeders).
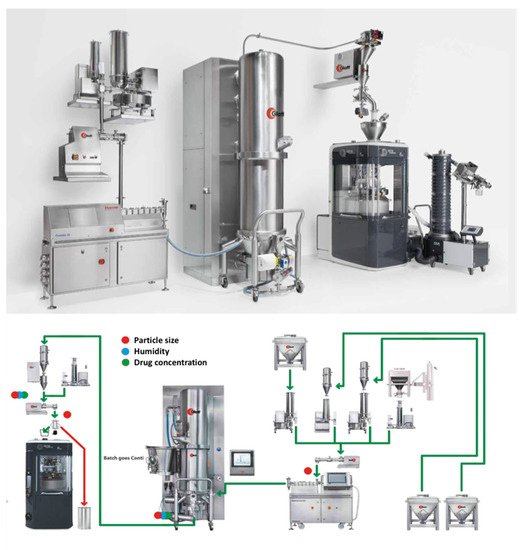
Figure 5. MODCOS continuous high shear granulation process by Glatt (images provided by the manufacturer).
A unique feature of the continuous line is the intelligent control system where all the process parameters are controlled together and continuously monitored to provide the basis for automatic process control. The manufacturing process is controlled by recipes which include the distribution of residence times while the data for all process unit can be displayed as tables of graphs.
In batch manufacturing of solid dosage form there is a limited process understanding and control which requires the application of more intensive and advanced manufacturing processes. Continuous granulation processing is fully automated and thus scale up issues related to batch manufacturing are no longer encountered. Often, in batch granulation, the active substances tend to agglomerate especially when they present highly cohesive properties. This problem can be easily mitigated in continuous granulation by combining high shear co-milling of the drug substances and excipients followed by low -shear blending. The powder blends are immediately compressed to produce tablets and, unlike in batch processing, do not allow time for particles to re-agglomerate. CM lines can be built in a flexible way for the processing of multiple formulations (products) through careful consideration and optimal design. Continuous granulation lines can be easily adopted for both dry and wet processing which eventually results a good return on the initial investment. Other advantages of continuous granulation processing include the following [41]:
-
-
-
Enhanced development approach by implementing QbD approaches and incorporating PAT tools
-
-
-
Reduce risk of manufacturing failure and prevent drug shortages
-
-
-
Decreased risk of out of specification failures both for intermediates and finished products
-
-
-
Flexibility by using the same system to develop a manufacturing process
-
-
-
Effect on supply chain by increasing supply speed and react to market demands
-
-
-
Agility and reduced scale-up efforts
-
-
-
Real time quality assurance-secure quality attributes and measure critical quality parameters
-
-
-
Reduction of capital and operational costs, environmentally friendly
-
-
-
Cost reductions in R&D, product transfer and productivity
-
-
-
Reduced system’s footprint
References
- Seem, T.C.; Rowson, N.A.; Ingram, A.; Huang, Z.; Yu, S.; de Matas, M.; Gabbott, I.; Reynolds, G.K. Twin screw granulation—A literature review. Powder Technol. 2015, 276, 89–102.
- Kittikunakorn, N.; Liu, T.; Zhang, F. Twin-screw melt granulation: Current progress and challenges. Int. J. Pharm. 2020, 588, 119670.
- Goodhart, F.W.; Draper, J.R.; Ninger, F.C. Design and use of a laboratory extruder for pharmaceutical granulations. J. Pharm. Sci. 1973, 62, 133–136.
- Gamlen, M.J.; Eardley, C. Continuous extrusion using a raker perkins MP50 (multipurpose) extruder. Drug Dev. Ind. Pharm. 1986, 12, 1701–1713.
- Kleinebudde, P.; Lindner, H. Experiments with an instrumented twin-screw extruder using a single-step granulation/extrusion process. Int. J. Pharm. 1993, 94, 49–58.
- Kleinebudde, P.; Sølvberg, A.J.; Lindner, H. The power-consumption-controlled extruder: A tool for pellet production. J. Pharm. Pharmacol. 1994, 46, 542–546.
- Lindberg, N.-O.; Tufvesson, C.; Olbjer, L. Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50 D. Drug Dev. Ind. Pharm. 1987, 13, 1891–1913.
- Lindberg, N.-O.; Tufvesson, C.; Holm, P.; Olbjer, L. Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50 D. Influence on intragranular porosity and liquid saturation. Drug Dev. Ind. Pharm. 1988, 14, 1791–1798.
- Lindberg, N.-O.; Myrenas, M.; Tufvesson, C.; Olbjer, L. Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50 D. Determination of mean residence time. Drug Dev. Ind. Pharm. 1988, 14, 649–655.
- Schmidt, C.; Kleinebudde, P. Comparison between a twin-screw extruder and a rotary ring die press. Part II: Influence of process variables. Eur. J. Pharm. Biopharm. 1998, 45, 173–179.
- Thompson, M.R. Twin screw granulation-review of current progress. Drug Dev. Ind. Pharm. 2015, 41, 1223–1231.
- Parker, F.R. Department of Health and Human Services, US Food and Drug Administration: Authority and Responsibility. In FDA Administrative Enforcement Manual; CRC Press: Boca Raton, FL, USA, 2005; pp. 21–60.
- Barrasso, D.; El Hagrasy, A.; Litster, J.D.; Ramachandran, R. Multi-dimensional population balance model development and validation for a twin screw granulation process. Powder Technol. 2015, 270, 612–621.
- Barrasso, D.; Walia, S.; Ramachandran, R. Multi-component population balance modeling of continuous granulation processes: A parametric study and comparison with experimental trends. Powder Technol. 2013, 241, 85–97.
- Sayin, R.; El Hagrasy, A.S.; Litster, J.D. Distributive mixing elements: Towards improved granule attributes from a twin screw granulation process. Chem. Eng. Sci. 2015, 125, 165–175.
- Vercruysse, J.; Toiviainen, M.; Fonteyne, M.; Helkimo, N.; Ketolainen, J.; Juuti, M.; Delaet, U.; Assche, I.V.; Remon, J.P.; Vervaet, C.; et al. Visualization and understanding of the granulation liquid mixing and distribution during continuous twin screw granulation using NIR chemical imaging. Eur. J. Pharm. Biopharm. 2014, 86, 383–392.
- Kumar, A.; Vercruysse, J.; Bellandi, G.; Gernaey, K.V.; Vervaet, C.; Remon, J.P.; De Beer, T.; Nopens, I. Experimental investigation of granule size and shape dynamics in twin-screw granulation. Int. J. Pharm. 2014, 475, 485–495.
- Kumar, A.; Vercruysse, J.; Toiviainen, M.; Panouillot, P.-E.; Juuti, M.; Vanhoorne, V.; Vervaet, C.; Remon, J.P.; Gernaey, K.V.; De Beer, T.; et al. Mixing and transport during pharmaceutical twin-screw wet granulation: Experimental analysis via chemical imaging. Eur. J. Pharm. Biopharm. 2014, 87, 279–289.
- Kleinebudde, P.; Khinast, J.; Rantanen, J. Continuous Manufacturing of Pharmaceuticals; John Wiley & Sons: Hoboken, NJ, USA, 2017.
- Dhenge, R.M.; Washino, K.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 2013, 238, 77–90.
- Dhenge, R.M.; Fyles, R.S.; Cartwright, J.J.; Doughty, D.G.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Granule properties. Chem. Eng. J. 2010, 164, 322–329.
- Vercruysse, J.; Díaz, D.C.; Peeters, E.; Fonteyne, M.; Delaet, U.; Van Assche, I.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous twin screw granulation: Influence of process variables on granule and tablet quality. Eur. J. Pharm. Biopharm. 2012, 82, 205–211.
- Khorsheed, B.; Gabbott, I.; Reynolds, G.K.; Taylor, S.C.; Roberts, R.J.; Salman, A.D. Twin-screw granulation: Understanding the mechanical properties from powder to tablets. Powder Technol. 2018, 341, 104–115.
- Megarry, A.; Taylor, A.; Gholami, A.; Wikström, H.; Tajarobi, P. Twin-screw granulation and high-shear granulation: The influence of mannitol grade on granule and tablet properties. Int. J. Pharm. 2020, 590, 119890.
- Lute, S.V.; Dhenge, R.M.; Salman, A.D. Twin screw granulation: An investigation of the effect of barrel fill level. Pharmaceutics 2018, 10, 67.
- Liu, Y.; Thompson, M.R.; O’Donnell, K.P.A.S. Heat Assisted Twin Screw Dry Granulation. AIChE J. 2017, 63, 4748–4760.
- Ye, X.; Kallakunta, V.; Kim, D.W.; Patil, H.; Tiwari, R.V.; Upadhye, S.B.; Vladyka, R.S.; Repka, M.A. Effects of Processing on a Sustained Release Formulation Prepared by Twin-Screw Dry Granulation. J. Pharm. Sci. 2019, 108, 2895–2904.
- Kallakunta, V.R.; Patil, H.; Tiwari, R.; Ye, X.; Vladyka, R.S.; Sarabu, S.; Kim, D.-W.; Bandari, S.; Repka, M.A. Exploratory studies in heat-assisted continuous twin-screw dry granulation: A novel alternative technique to conventional dry granulation. HHS Public Access. 2019, 555, 380–393.
- Van Melkebeke, B.; Vermeulen, B.; Vervaet, C.; Remon, J.P. Melt granulation using a twin-screw extruder: A case study. Int. J. Pharm. 2006, 326, 89–93.
- Batra, A.; Desai, D.; Serajuddin, A.T.M. Investigating the Use of Polymeric Binders in Twin Screw Melt Granulation Process for Improving Compactibility of Drugs. J. Pharm. Sci. 2017, 106, 140–150.
- Schfer, T.; Mathiesen, C. Melt pelletization in a high shear mixer. VIII Eff Bind Viscosity Int. J. Pharm. 1996, 139, 125–138.
- Monteyne, T.; Heeze, L.; Mortier, S.T.F.C.; Oldörp, K.; Cardinaels, R.; Nopens, I.; Vervaet, C.; Remon, J.P.; De Beer, T. The use of Rheology Combined with Differential Scanning Calorimetry to Elucidate the Granulation Mechanism of an Immiscible Formulation During Continuous Twin-Screw Melt Granulation. Pharm. Res. 2016, 33, 2481–2494.
- Monteyne, T.; Heeze, L.; Mortier, S.T.F.C.; Oldörp, K.; Nopens, I.; Remon, J.P.; Vervaet, C.; De Beer, T. The use of rheology to elucidate the granulation mechanisms of a miscible and immiscible system during continuous twin-screw melt granulation. Int. J. Pharm. 2016, 510, 271–284.
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326.
- Funakoshi, Y.; Asogawa, T.; Satake, E. The use of a novel roller compactor with a concavo-convex roller pair to obtain uniform compacting pressure. Drug Dev. Ind. Pharm. 1977, 3, 555–573.
- Bruce, L.D.; Shah, N.H.; Malick, A.W.; Infeld, M.H.; McGinity, J.W. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. Eur. J. Pharm. Biopharm. 2005, 59, 85–97.
- Martin, C. Twin screw extrusion for pharmaceutical processes. In Melt Extrusion; Springer: Berlin/Heidelberg, Germany, 2013; pp. 47–79.
- Byrn, S.; Futran, M.; Thomas, H.; Jayjock, E.; Maron, N.; Meyer, R.F.; Myerson, A.S.; Thien, M.P.; Trout, B.L. Achieving continuous manufacturing for final dosage formation: Challenges and how to meet them. 20–21 May 2014 continuous manufacturing symposium. J. Pharm. Sci. 2015, 104, 792–802.
- Lee, S.L.; O’Connor, T.F.; Yang, X.; Cruz, C.N.; Chatterjee, S.; Madurawe, R.D.; Moore, C.M.V.; Yu, L.X.; Woodcock, J. Modernizing pharmaceutical manufacturing: From batch to continuous production. J. Pharm. Innov. 2015, 10, 191–199.
- Portier, C.; Pandelaere, K.; Delaet, U.; Vigh, T.; Kumar, A.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Influence of process and formulation variables on granule quality attributes of model formulations. Int. J. Pharm. 2020, 576, 118981.
- Kumar, A.; Vercruysse, J.; Vanhoorne, V.; Toiviainen, M.; Panouillot, P.-E.; Juuti, M.; Vervaet, C.; Remon, J.P.; Gernaey, K.V.; De Beer, T.; et al. Conceptual framework for model-based analysis of residence time distribution in twin-screw granulation. Eur. J. Pharm. Sci. 2015, 71, 25–34.





