
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mariangela Hungria | + 4243 word(s) | 4243 | 2021-05-07 06:04:31 | | | |
| 2 | Bruce Ren | -21 word(s) | 4222 | 2021-05-13 10:59:36 | | |
Video Upload Options
Inoculants or biofertilizers aiming to partially or fully replace chemical fertilizers are becoming increasingly important in agriculture, as there is a global perception of the need to increase sustainability.
1. Introduction
Technologies and agricultural inputs currently applied for food production are essential for large-scale production and are mandatory to feed a population of more than 7 billion people [1]. Years of research and experiments continually performed into the challenges and technological evolution have resulted in progress in several fields of science. The 1950s was known as the “Green Revolution” period, marked by the intense modernization of agriculture [2][3]. Products including new machines and synthetic fertilizers, with an emphasis on nitrogen (N) fertilizers, pesticides, seeds of better quality, improvements in the water supply systems, breeding and genetic engineering are examples of technologies developed at that time and that have gained prominence in agriculture [4][5].
The main positive result of the Green Revolution was the global increase in food production, thus contributing to the reduction of hunger in the world. However, in the following years, the side-effect of this revolution unfolded [6]. The accumulation of pesticides and chemical fertilizers contributes to the pollution of groundwater and cultivated land, soil degradation, and reduction of biodiversity in different ecosystems [5][7]. Increased deforestation, soil degradation, and emission of polluting gases into the atmosphere have been increasingly observed [8][9][10]. Despite the undeniable benefits of the Green Revolution, many of the technologies and inputs generated during that period are broadly criticized today. Currently, efforts are being made towards the development of new technologies and inputs focused on more sustainable systems.
Contemporary scientists have pointed out that we are living in a “New Green Revolution” whose main characteristic, and which differs from that experienced in the 1950s, is the development of environmentally-friendly technologies and products [3][11][12][13]. Examples of this new concept include the development of products and techniques such as crop rotation, plant genetic engineering for resistance to pests, diseases, and abiotic stresses such as drought, the use of bio-inputs as activators of soil biota, biopesticides and microbial inoculants, also known as biofertilizers in some countries, with the purpose of partially or fully replacing the use of chemical fertilizers, favoring the growth of plants [14][15][16][17].
Although the current movement towards agricultural sustainability has force worldwide, the use of agrochemicals is and will continue to be the reality of most farmers [18]. As such, the common scenario towards improving agricultural sustainability with feasible yields to guarantee food security includes the increasing use of bioproducts, such as microbial inoculants, together with pesticides, which are still indispensable for controlling pests and diseases [4][5][11][14][17]. Therefore, the compatibility between inoculants and pesticides must be understood.
In general, pesticides contain molecules that are potentially toxic to living cells. Depending on the specificity, pesticides can cause toxicity to cells of microorganisms, animals, and plants, often resulting in death after contact with the product. In agriculture, they are commonly applied to the seeds, soil, and leaves of plants to prevent or control pests and diseases [7][18][19][20][21][22][23][24][25][26][27][28][29][30][31]. Usually, at least for large commercial crops such as is the case of soybean [Glycine max (L.) Merr.] [11][15][17][20] and maize (Zea mays L.) [26][27][28] in South America, pesticides and inoculants are added together on the seeds. Thus, it is necessary to verify whether microbial cells in the inoculants are affected by pesticides, impairing the benefits of inoculation.
We should also mention the increasing demand for anticipated inoculation or pre-inoculation [20], and in the pre-inoculation the most common adoption is to treat seeds with pesticides and inoculants several days before sowing [15][24]. However, the microorganisms are subjected to long-term exposure to pesticides, increasing the pernicious effect on the bacteria and resulting, for example, in decreased nodulation in legumes [21][22][23], lower N accumulation in grains [24][25], and negatively impacting root development of grasses [26]. These losses may be due to microbial cell death caused by pesticides, as demonstrated in some studies [25][26][27][28], in which the longer the contact between bacteria and pesticides, the greater the mortality. Moreover, changes in cell metabolism, such as formation of smaller colonies and decreased nitrogenase activity, have been reported [25].
2. Are Pesticides and Microbial Inoculants Compatible?
As the benefits of inoculation are closely related to the establishment of a plant-microorganism interaction, the survival and maintenance of microbial properties are crucial and mandatory. Therefore, the evaluation of microbial survival at the time of inoculation, and of the effectiveness on the inoculated crop are critical. The most common situation in commercial crops is the combination of several products for different purposes, such as soybean seed treatment with microbial inoculants for nitrogen fixation, fertilizers and pesticides for the prevention or treatment of pests and diseases. In many cases, depending on the mode of application, one product ends up being exposed to the other, or interacting with one another either on the seeds, propagated material, in the soil, or leaf surface. It is important to know whether the contact of pesticides with microorganisms in the inoculant can affect cell survival and metabolism. Concerns about the compatibility of agrochemicals with microbial inoculants have been raised for decades, and several studies have shown that the impact of chemicals on the inoculant depends on the active ingredient, the presence of other chemical substances that make up the formulation of pesticides, the mechanism of action (systemic or contact), and the method of application. The effects of incompatibility also depend on the bacterial species present in the inoculant, which may have different responses. However, few species have been evaluated for this purpose. The most recurrent ones belong to the genres Rhizobium spp., Bradyrhizobium spp. and Azospirillum sp.
2.1. Compatibility with Fungicides
Fungicides are chemical products formulated to prevent the infection of plant tissues by phytopathogenic fungi, and in some cases, capable of extending the control of diseases caused by bacteria and viruses. The control exerted by fungicides can be mediated by killing the pathogen, temporarily inhibiting its germination and growth, or by affecting the production of spores [32]. Over the years, several fungicides have made it to the market; some have stood out and remained at the top in the list of the most used fungicides until today, more effective products have replaced others, and some have been banned. Fungicides of contact generally do not have a specific mode of action, are highly toxic, and when applied to seeds, soil, and plant leaves limit the pathogen survival. The most common are thiram (dithiocarbamate), captan (quinone and heterocyclic), exon (aromatic), and guazatine. Upon entering microbial cells, the molecules promote a series of chemical reactions in nucleic acids and their precursors, and metabolic routes, affecting cell survival [32].
The majority of studies on compatibility have been performed with fungicides and microbial inoculants carrying rhizobia. Fungicides may affect several steps of the symbiosis, from the survival of the rhizobia on the seed to nodule formation and N2 fixation efficiency; in general, studies have been performed with soybean (e.g., [33][34][35][36]). The use of pesticides intensified in the past two decades, and so did concerns about their compatibility with inoculants [17].
Brikol et al. [21] evaluated the effects of applying different concentrations of the fungicide Thiram (10 to 750 µg mL−1) on soybean seeds inoculated with B. japonicum, which were grown under greenhouse conditions for 75 days. They observed that concentrations above 100 µg mL−1 reduced nodule number and dry weight, as well as the activity of the bacterial enzyme nitrogenase, responsible for the nitrogen fixation process. Similarly, there are reports [24][37] of decrease in soybean nodulation and N accumulation in plants when seeds were inoculated with B. elkanii (SEMIA 5019) and B. japonicum (SEMIA 5079) and treated with different fungicides, benomyl, captan, carbendazin, carboxin, difenoconazole, thiabendazole, thiram, and tolylfluanid. Changes in nodule number were also observed in a field trial by Zilli et al. [38] when soybean seeds were treated with either carbendazin + thiram or carboxin + thiram.
Interestingly, results of some studies indicate differences between strains in their tolerance to fungicides. For example, in soybean seeds treated with carbendazim + thiram, the lowest tolerance was observed in B. elkanii amongst four soybean Bradyrhizobium strains used in commercial inoculants in Brazil [B. elkanii (SEMIA 5019 and SEMIA 587), B. japonicum (SEMIA 5079), and B. diazoefficiens (SEMIA 5080)] [38]. In another study, Gomes et al. [39] observed no effects on nodulation when seeds were inoculated with B. japonicum SEMIA 5079 + B. diazoefficiens SEMIA 5080 and treated with carbendazin + thiram. More recently, when the compatibility of B. japonicum SEMIA 5079 and B. elkanii SEMIA 587 was verified with Standak® Top, composed of a mixture of two fungicides and one insecticide (piraclostrobin, thiophanatemethyl, and fipronil), the effects were also more drastic for B. elkanii [25]. Altogether, indications are that B. elkanii is less tolerant to fungicides than B. japonicum or B. diazoefficiens.
It is reasonable to postulate that the main effects of the fungicides used in seed treatment would be the decrease of rhizobial survival or inhibition of the root infection process, consequently affecting nodulation and BNF, and as a result grain yield, as observed by Zilli et al. [38]. In that study, the grain yield reduced by 20%, in addition to a decrease in the N content in grains when seeds were treated with B. elkanii SEMIA 587 + carbendazin + thiram (Figure 2). However, some reports have indicated that the effects of fungicides may appear later. For example, in a study by Gomes et al. [39], although fungicides (carbendazin + thiram) did not affect nodulation, plants had lower number of pods per plant, grains per plant, and yield. Intriguingly, in two field experiments performed with soybean in Brazil, seed treatment with Standak® Top affected the total N accumulated in the grains of plants relying on both BNF and N fertilizer, indicating the negative impact of the pesticide on N metabolism [25]. In another study, a decrease in both protein and oleic acid contents was observed in soybean inoculated and treated with mefenoxan + fludioxonil [40].
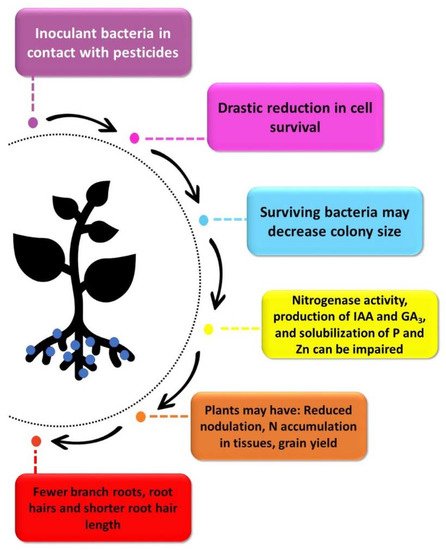
Figure 2. Representation of the effects reported on the incompatibility between pesticides and inoculants, from the moment of contact with microbial cells to the damage to plant development.
As mentioned previously, negative effects may be related to the toxicity of fungicides on microbial cells, followed by impacts on microbial metabolism, reducing the effectiveness of the inoculant. Ahmed et al. [41] evaluated the growth of Bradyrhizobium sp. and Rhizobium sp. in Petri dishes containing solid culture medium and different concentrations of fungicides (captan, thiram, luxan, milcurb, and frernasan-D), soaked in discs placed on the medium. The bacteria were tolerant to concentrations below 100 µg L−1, but at 1000 µg L−1 inhibition of growth and a decrease in the colony diameter of the surviving cells were observed (Figure 2). Rathjen et al. [42] also reported that Rhizobium leguminosarum bv. viciae (WSM1455) was sensitive to the fungicides Thiram 600 and P-Pickel T (PPT), and their active ingredients (thiram and thiabendazole) at concentrations above 200 µg disc−1, with growth halos greater than 10 mm around the disks containing the fungicide.
To verify the survival of B. elkanii (SEMIA 5019) and B. japonicum (SEMIA 5079) on seeds treated with fungicides, Campo et al. [24] recovered and counted living cells from seeds inoculated and treated with benomyl, captan, carbendazin, carboxin, difenoconazole, thiabendazole, thiram, or tolylfluanid. After only 2 h of contact with carbendazin + thiram, viable bacterial cells were reduced by up to 64%, and reached 83% after 24 h (Table 1). Mortality was verified with all other fungicides, reaching 95% with the mixture of thiabendazole and tolylfluanid after 24 h of contact. In addition to rhizobia, fungicides can also impair the contribution of other PGPB. The toxic effect of the combination of carbendazin + thiram was also verified for A. brasilense strains Ab-V5 and Ab-V6 by Santos et al. [27]. In that study, a decrease in the recovery of viable cells from seeds only after 2 h of contact was observed, and the viable cell count drastically decreased after 24 h, in comparison with the seeds not treated with fungicides (Figure 2).
Table 1. Effect of pesticides on cell viability and/or morphology and on the metabolism of microorganisms used as inoculants.
| Pesticide | Concentration | Microorganism | Effect | Reference |
|---|---|---|---|---|
| Monocrotophos i, Malathion i, Chlorpyripho i, Dichlorvos i, Lindano i e Endosulphan i | Recommended dose of each product | Gluconacetobacter diazotrophicus | With the exception of Malathion, all insecticides reduced cell viability. Nitrogenase activity was totally inhibited by Monocrotophos, Dichlorvos and Lindano. Production of IAA and GA3, and solubilization of P and Zn were impaired | [43] |
| Butachlor h, Alachlor h Atrazine h and 2,4-D h | Recommended dose of each product | Cell growth was hindered by 2,4-D. All herbicides reduced the activity of nitrogenase, the production of IAA and GA3, and the solubilization of P and Zn | ||
| Captan f, Thiram f, Luxan f and Fernasan-D f | 1000 µg L−1 | Bradyrhizobium sp. and Rhizobium sp. | Decreased colony diameter and inhibited growth in areas close to the fungicide application site | [41] |
| Benomyl f, Captan f, Carbendazin f, Carboxin f, Difenoconazole f, Thiabendazole f, Thiram f, Tolylfluanid f | Recommended dose for soybean | B. elkanii and B. japonicum | All fungicides caused mortality of microorganisms | [24] |
| Imidacloprid i, Fipronil i, Thiamethoxam i, Endosulphan i e Carbofuran i | 250 g ha−1, 400 g ha−1, 480 g ha−1, 2.800 g ha−1, 1.650 g ha−1, respectively | Herbaspirillum seropedicae | Endosulphan increased the lag phase. Carbofuran increased generation time and reduced lag phase | [44] |
| Pyraclostrobin f, thiophanato-methyl f e fipronil i | 2 mL kg−1 maize seed | A. brasilense (strains Ab-V5 and Ab-V6) | Drastic reduction in cell concentration 24 h after inoculation in treated seeds | [26] |
| Carbendazin f + Thiram f | 40–60 mL 20 kg−1 maize seed | Drastic reduction in cell concentration 24 h after inoculation in treated seeds | [27] | |
| metalaxil-m + fludioxonil + tiametoxame + abamectin | Recommended dose for maize | Drastic reduction in cell concentration 12 h after inoculation in treated seeds | [28] | |
| Thiram f, Thiram + Thiabendazole f and PPT f | >200 µg L−1 | Rhizobium leguminosarum bv. viciae | Formation of growth inhibition halos greater than 10 mm around the fungicide | [42] |
| Imidacloprid i | 0, 100, 200 e 300 µg L−1 | Formation of growth inhibition halos greater than 10 mm around the insecticide for all concentrations evaluated | ||
| Pyraclostrobin f, thiophanato-methyl f e fipronil i | Recommended dose for soybean | B. elkanii and B. japonicum | Drastic decrease in cell concentration after 7 days of exposure. Colony formation with smaller diameter | Rodrigues et al. [25] |
2.2. Compatibility with Insecticides
Many herbivorous insects feed on plants during their larval and adult stages, and/or some are important vectors of plant diseases. In both cases, insects may cause serious damages to crops. Insecticides, usually synthetic chemicals, acting as ovicidal, larvicidal, and adulticidal agents are used to prevent growth or kill insects [45]. Neonicotinoids, organophosphates, diamides, pyrethroids, and carbamates act on nerves and muscles; phosphides, cyanides, and carboxamides on respiration, and cyclic ketoenols and ecdysone are agonists that interfere with insect growth and development [46].
Rathjen et al. [42] evaluated the in vitro toxicity of an imidacloprid-based insecticide on R. leguminosarum bv. viciae (WSM1455), and Mesorhizobium ciceri (CC1192). The strains were applied on solid culture medium in Petri dishes and sterile filter discs containing different concentrations (0, 100, 200, and 300 µg discs−1) of the insecticide. The inhibition of R. leguminosarum growth was observed at all concentrations. In alfalfa (Medicago sativa L.), Fox et al. [22] reported that seed treatment with the insecticides methyl parathion, DDT and pentachlorophenol affected the symbiosis with Ensifer (syn. Sinorhizobium) meliloti. The insecticides not only reduced nodule number and dry weight but also nitrogenase activity in nodules and plant biomass production. Ahemad [23] also reported several negative effects with the application of pyriproxifene, at the recommended dose of 1300 μg kg−1 soil, in chickpeas (Cicer arietinum L.), peas (Pisum sativum L.), mung beans (Vigna radiata L. Wiclzek), and lentils (Lens esculentus, = Lens culinaris Medik) grown in pots that remained in an open field. Although the plants had not been inoculated, inferring that nodulation was related to indigenous rhizobia, pyriproxifer resulted in a 44% decrease in nodule number in peas, 14% in mung beans, and 5% in chickpeas and lentils, resulting in decreases in nodule dry weight compared with the controls not treated with insecticide. There was also a 17% decrease in the concentration of nitrogen in the roots of chickpeas, 15% in peas, 14% in mung beans, and 18% in lentils, and a 5% decrease in the protein contents in grains of chickpeas, 4% in mung beans, 3% in lentils, and 1% in peas, compared with the controls not treated with insecticide.
Insecticides also affect PGPB other than rhizobia. For example, Fernandes et al. [44] studied the effects of five insecticides (imidacloprid, fipronil, fenamethoxam, endosulfan, and carbofuran) indicated for sugarcane (Saccharum spp.) on the diazotrophic bacterium Herbaspirillum seropedicae. In vitro evaluations of cell growth after 33 h indicated that the insecticides that most interfered with bacterial growth were endosulfan and carbofuran. Madhaiyan et al. [43] evaluated the effect of different insecticides (monocrotophos, malathion, chlorpyriphos, diclorvos, lindane and endosulfan) on Gluconacetobacter diazotrophicus, another PGPB found in association with sugarcane. Except for malathion, all other insecticides reduced cell concentration, and lindane lysed the cells (Figure 2). In the same study, nitrogenase activity was fully inhibited by monocrotophos, dichlorvos, and lindane, 83.3% by chlorpyriphos, 80.9% by melation, and 33.4% by endosulfan. Concerning the synthesis of indoleacetic acid (IAA) and gibberellin A3 (GA3) by G. diazotrophicus, inhibition was observed with lindane, with decreases of 93.2% and 96.5% for IAA and GA3, respectively. The authors also described that the insecticides dichlorvos, chlorpyriphos, and lindane completely inhibited the solubilization of phosphate (P) and zinc (Zn) [43] (Figure 2).
2.3. Compatibility with Herbicides
Another class of important pesticides for agriculture are herbicides used for weed control. Herbicides have different degrees of specificity based on differences in biochemical pathways in certain plant groups. The mode of action of herbicides has specific degrees of toxicity, depending on the biochemical differences of the plants, and is generally related to the cell division process [47]. Souza and Guedes [47] gathered studies on the action of several herbicides on Allium cepa and Vicia faba plants used as bioindicators, and described how most of these studies reported that herbicides induced both a decrease in the mitotic index, and the occurrence of mitotic chromosomal aberrations.
Examples of herbicides applied worldwide include glyphosate, paraquat, and diuron. Among these, the most well-known is glyphosate; since its introduction in the 1970s, its use spread quickly, facilitated cropping, but also implied in the growing appearance of resistant weeds, resulting from a natural process of plant adaptation, and decreasing its efficacy [48][49][50]. An important alternative to minimize this problem, in addition to integration with other control methods, would be diversification in the use of herbicides, including others with different mechanisms of action [51].
Few studies have investigated the compatibility between herbicides and inoculants. Madhaiyan et al. [43] evaluated the effects of different herbicides (butachlor, alachlor, atrazine, and 2,4-D) in liquid culture medium on the growth and metabolism of G. diazotrophicus. Cell growth was impaired only in the presence of 2,4-D, but all herbicides reduced nitrogenase activity, the production of IAA and GA3, and the solubilization of P and Zn. The highest inhibition of nitrogenase activity (73.6% and 65.3%) was observed with alachlor and atrazine, respectively, while butachlor mostly affected production of IAA (53.3%), whereas 2.4-D mostly affected the production of GA3 (78.8%). Butachlor was also responsible for the strongest reduction in the solubilization of P and Zn. Therefore, although herbicides do not affect cell survival, they significantly affect metabolism of G. diazotrophicus [43].
In an assay performed under greenhouse conditions, Angelini et al. [52] evaluated the effects of imazetapir, imazapic, S-metachlor, diclosulam, and glyphosate on diazotrophic bacteria in the soil during the cultivation of peanuts (Arachis hypogaea L.). The seeds were sown and the herbicide was sprayed on the soil surface. All herbicides caused reduction in cell concentration in both free and symbiotic diazotrophic bacteria, and this negative impact was confirmed under field conditions even one year after the application. Nitrogenase activity also reduced due to herbicides, except for glyphosate [52] (Table 2).
Table 2. Effect of pesticides on the development of plants the seeds of which had been inoculated.
| Culture | Fungicide | Microorganism | local | Effect | Reference |
|---|---|---|---|---|---|
| Soybean (Glycine max) | Thiram f | B. japonicum | Greenhouse | Lower nodule number, nodule dry weight and nitrogenase activity | [21] |
| Benomyl f, Captan f, Carbendazin f, Carboxin f, Difenoconazole f, Thiabendazole f, Thiram, Tolylfluanid f | B. elkanii (strain SEMIA 5019) + B. japonicum (strain SEMIA 5079) | Greenhouse and field | Reduction in the number of nodules and in the total N in grains | [24] | |
| Carbendazin f + Thiram f; Carboxin f + Thiram f | B. elkanii (strains 5019 + 587), B. japonicum (strain 5079) + B. diazoefficiens (strain SEMIA 5080) | Field | Reduction of nodulation efficiency. Reduction of N content and grain yield to SEMIA 587 with Carbendazin + Thiram | [38] | |
| Carbendazin f + Thiram f | B. japonicum (strain 5079) + B. diazoefficiens (strain SEMIA 5080) | Field | Reduction in the number of pods per plant and grains per plant | [39] | |
| Mefenoxam f + Fludioxonil f | B. japonicum | Field | Reduced grain yield and protein and oleic acid content | [40] | |
| Pyraclostrobin f, thiophanato-methyl f and fipronil i | B. japonicum (strain 5079) and B. diazoefficiens (strain 5080) | Field | Less N accumulation in grains | [25] | |
| Alfafa (Medicago sativa) | Methyl parathion i, DDT i e pentachlorophenol i | Sinorhizobium meliloti, | Greenhouse | Reduction of nitrogenase activity, number of nodules and plant dry weight | [22] |
| Peanut (Arachis hypogaea). | Imazetapir h, Imazapic h, S-metachloro h, Dichlosulam h and Glyphosate h | Diazotrophic bacteria present in the soil | Greenhouse | Reduction of cell concentration of free and symbiotic diazotrophic bacteria | [52] |
| Field | Reduced cell concentration of free and symbiotic diazotrophic bacteria and reduced nitrogenase activity except for glyphosate | ||||
| Chickpeas (Cicer arietinum L.), pea (Pisum sativum L.), Mung beans (Vigna radiata L. Wiclzek) and lentil (Lens esculentus, = Lens culinaris Medik). | Pyriproxyfen i | Bacteria commonly present in the soil used | Pots in the field | Reduction in the number of nodules, in the dry weight of nodules, in the concentration of N in roots and in protein concentration in the grains | [23] |
| Rice (Oryza sativa) | Benthicarb h | Cyanobacteria naturally found in the rice paddy soil | Field | Decreased cell growth, nitrogenase activity and Naccumulation | [53] |
| Maize (Zea mays) | Pyraclostrobin f, thyophanato-methyl f and fipronil i | A. brasilense (strains Ab-V5 and Ab-V6) | Greenhouse | Fewer branch roots, root hair and shorter root hair length | [26] |
The ability of cyanobacteria to fix atmospheric nitrogen in flooded soils suitable for rice cultivation make this group an important ally in maintaining soil fertility and contributing to cereal yield. Thus, Dash et al. [53] evaluated the responses of cyanobacteria in rice plantation soil to the exposure of different agrochemicals, including the herbicide benthiocarb that was applied in one dose at the time of puddling. The herbicide decreased cell growth, which was even worse when combined with urea (used as a fertilizer). Benthiocarb reduced nitrogenase activity by between 13–27%, compared with the control without herbicide, and its combination with urea resulted in an even greater reduction in addition to a decrease in the nitrogen accumulation that reached 47% at 60 days.
Concerning the symbiosis between legumes and rhizobia, in general, herbicides have been considered less toxic than fungicides and insecticides [37], with glyphosate being the one with lower toxicity [54][55]. Although the negative effects of glyphosate on B. japonicum growth were reported by King et al. [56], the doses in the experiment were far higher than those recommended for field application. With the release of genetically modified (GM) genotypes tolerant of herbicides, studies on the compatibility with the GM genotypes and herbicides have begun. In soybean, which represents the most used herbicide-tolerant species, glyphosate-resistant (Roundup Ready) pairs of nearly isogenic cultivars were evaluated in six field sites in Brazil for three crop seasons. Although the transgenic trait negatively affected some BNF variables, these effects had no significant impact on soybean grain yield, and no consistent differences between glyphosate and conventional herbicide application were observed on BNF-associated parameters [57]. Similar results were reported in 20 field trials performed with soybean with the ahas transgene, imidazolinone, and conventional herbicides [58]. However, it is worth mentioning that with the increasing doses of the herbicides, BNF can be reduced, especially under abiotic stressing conditions, as shown for glyphosate in soybean [59][60]. Interestingly, it has been long shown that several members of the family Rhizobiaceae were able to degrade glyphosate [61], ability that has been few explored, but that it can contribute to decrease the toxicity effects. Indeed, the search for indigenous and engineered microorganisms, used as isolated microbial species or microbial consortia can result in the complete mineralization of herbicides such as atrazine [62].
2.4. Compatibility with Mixtures (Fungicides, Insecticides, and Herbicides)
Approximately 70% of the pesticides available in the market contain mixtures of two or more types of fungicides and insecticides [63] and are often combined with herbicides at the time of application, aiming to facilitate the combined control of pests, diseases, and weeds. However, the damage to microbial inoculants increases with the number of combined chemicals. As previously mentioned, Standak® Top, one of the most used for treatments of seeds in several countries, especially for soybean, is composed of two fungicides and one insecticide; it affects the survival of B. japonicum and, especially, B. elkanii cells, with a drastic decrease verified after 7 days of contact [25]. It is worth mentioning that pre-inoculated soybean seeds have been in contact with Standak® Top for up to 90 days, often resulting in zero recoveries of rhizobial cells from seeds [63].
Another interesting observation in the study by Rodrigues et al. [25] was the changes in colony morphology, smaller with the increase of the exposure to the pesticide. However, regular colonies were recovered after the bacteria were grown under optimal conditions, indicating an adaptive mechanism to the stressful conditions when exposed to the pesticides.
Santos et al. [26] evaluated the compatibility of Standak® Top with A. brasilense strains Ab-V5 and Ab-V6. First, differences were observed between strains, with lower tolerance of Ab-V5, so that after 24 h of exposure the recovery of viable cells dropped from 4.56 × 105 to 4.37 × 102 cells seed−1. In a greenhouse experiment with the combined strains, Standak® Top decreased the number of lateral roots and root hairs and resulted in shorter root hair length.
Pereira et al. [28] also reported the mortality of A. brasilense strains Ab-V5 and Ab-V6 in maize seeds treated with a mixture of fungicides and insecticides (metalaxyl-M + fludioxonil + thiamethoxam + abamectin). The cell survival rate after 12 h of inoculation of seeds treated with the pesticide was only 13.56%, whereas in untreated seeds it was 65.87%.
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019 Highlights. Available online: (accessed on 4 January 2021).
- Ameen, A.; Raza, S. Green Revolution: A Review. Int. J. Adv. Sci. Res. 2018, 3, 129–137.
- Armanda, D.T.; Guinée, J.B.; Tukker, A. The second green revolution: Innovative urban agriculture’s contribution to food security and sustainability—A review. Glob. Food Secur. 2019, 22, 13–24.
- Arora, N.K. Agricultural sustainability and food security. Environ. Sustain. 2018, 1, 217–219.
- Arora, N.K.; Fatima, T.; Mishra, I.; Verma, S. Microbe-based Inoculants: Role in Next Green Revolution. In Environmental Concerns and Sustainable Development; Metzler, J.B., Ed.; Springer Singapore: Singapore, 2019; Volume 1, pp. 191–246.
- Harwood, J. Could the adverse consequences of the green revolution have been foreseen? How experts responded to unwelcome evidence. Agroecol. Sustain. Food Syst. 2019, 44, 509–535.
- Singh, Z.; Kaur, J.; Kaur, R.; Hundal, S.S. Toxic Effects of Organochlorine Pesticides: A Review. Am. J. Biosci. 2016, 4, 11.
- Arora, N.K.; Tewari, S.; Singh, S.; Lal, N.; Maheshwari, D.K. PGPR for Protection of Plant Health Under Saline Conditions. In Bacteria in Agrobiology: Stress Management; Metzler, J.B., Ed.; Springer Singapore: Singapore, 2011; pp. 239–258.
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677.
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342.
- Hungria, M.; Nogueira, M.A. Microrganismos e a sustentabilidade de sistemas agrícolas de alta produtividade. In FertBio 2016; SBCS: Goiânia, Brazil, 2016; ISBN 978-85-86504-15-0.
- Hungria, M.; Nogueira, M.A.; Araujo, R.S. Inoculation of Brachiaria spp. with the plant growth-promoting bacterium Azospirillum brasilense: An environment-friendly component in the reclamation of degraded pastures in the tropics. Agric. Ecosyst. Environ. 2016, 221, 125–131.
- Llewellyn, D. Does Global Agriculture Need Another Green Revolution? Engineering 2018, 4, 449–451.
- Malusá, E.; Vassilev, N. A contribution to set a legal framework for biofertilisers. Appl. Microbiol. Biotechnol. 2014, 98, 6599–6607.
- Hungria, M.; Mendes, I.C. Nitrogen Fixation with Soybean: The Perfect Symbiosis? In Biological Nitrogen Fixation; Wiley: Hoboken, NJ, USA, 2015; pp. 1009–1024.
- Martin-Guay, M.-O.; Paquette, A.; Dupras, J.; Rivest, D. The new Green Revolution: Sustainable intensification of agriculture by intercropping. Sci. Total Environ. 2018, 615, 767–772.
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 1–22.
- FAOSTAT. 2019. Available online: (accessed on 15 March 2021).
- Fishel, F.M. Pest Management and Pesticides: A Historical Perspective. Agronomy Department, UF/IFAS Extension, 2016. Available online: (accessed on 16 November 2020).
- Hungria, M.; Nogueira, M.A.; Campos, L.J.M.; Menna, P.; Brandi, F.; Ramos, Y.G. Seed pre-inoculation with Bradyrhizobium as time-optimizing option for large-scale soybean cropping systems. Agron. J. 2020, 112, 5222–5236.
- Anupama, B.; Nidhi, S.; Kiran, S.; Bikrol, A.; Saxena, N.; Singh, K. Response of Glycine max in relation to nitrogen fixation as influenced by fungicide seed treatment. Afr. J. Biotechnol. 2005, 4, 667–671.
- Fox, J.E.; Gulledge, J.; Engelhaupt, E.; Burow, M.E.; McLachlan, J.A. Pesticides reduce symbiotic efficiency of nitrogen-fixing rhizobia and host plants. Proc. Natl. Acad. Sci. USA 2007, 104, 10282–10287.
- Ahemad, M. Growth suppression of legumes in pyriproxyfen stressed soils: A comparative study. Emir. J. Food Agric. 2014, 26, 66–72.
- Campo, R.J.; Araujo, R.S.; Hungria, M. Nitrogen fixation with the soybean crop in Brazil: Compatibility between seed treatment with fungicides and bradyrhizobial inoculants. Symbiosis 2009, 48, 154–163.
- Rodrigues, T.F.; Bender, F.R.; Sanzovo, A.W.S.; Ferreira, E.; Nogueira, M.A.; Hungria, M. Impact of pesticides in properties of Bradyrhizobium spp. and in the symbiotic performance with soybean. World J. Microbiol. Biotechnol. 2020, 36, 172.
- Santos, M.S.; Rondina, A.B.L.; Nogueira, M.A.; Hungria, M. Compatibility of Azospirillum brasilense with Pesticides Used for Treatment of Maize Seeds. Int. J. Microbiol. 2020, 2020, 1–8.
- Santos, M.S.; Rodrigues, T.F.; Ferreira, E.; Megias, M.; Nogueira, M.A.; Hungria, M. Method for Recovering and Counting Viable Cells from Maize Seeds Inoculated with Azospirillum brasilense. J. Pure Appl. Microbiol. 2020, 14, 195–204.
- Pereira, L.C.; De Carvalho, C.; Suzukawa, A.K.; Correia, L.V.; Pereira, R.C.; Dos Santos, R.F.; Braccini, A.L.; Osipi, E.A.F. Toxicity of seed-applied pesticides to Azospirillum spp.: An approach based on bacterial count in the maize rhizosphere. Seed Sci. Technol. 2020, 48, 241–246.
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of Pesticides on Environment; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2016; pp. 253–269.
- Abubakar, Y.; Tijjani, H.; Egbuna, C.; Adetunji, C.O.; Kala, S.; Kryeziu, T.L.; Ifemeje, J.C.; Patrick-Iwuanyanwu, K.C. Pesticides, History, and Classification. In Natural Remedies for Pest, Disease and Weed Control; Elsevier BV: Amsterdam, The Netherlands, 2020; pp. 29–42.
- Costa, L.G.; Galli, C.L.; Murphy, S.D. Toxicology of Pesticides: Experimental, Clinical and Regulatory Aspects, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1987.
- Garcia, A. Fungicidas I: Utilização no Controle Químico de Doenças e sua Ação contra os Fitopatógenos; Embrapa Rondônia: Porto Velho, Brazil, 1999; p. 34.
- Hungria, M.; Loureiro, M.F.; Mendes, I.C.; Campo, R.J.; Graham, P.H. Inoculant Preparation, Production and Application. In Nitrogen Fixation in Agriculture, Forestry, Ecology, and the Environment; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2005; pp. 223–253.
- Curley, R.L.; Burton, J.C. Compatibility of Rhizobium japonicum with Chemical Seed Protectants 1. Agron. J. 1975, 67, 807–808.
- Leterme, P.; Revellin, C.; Catroux, G. Effect of some fungicide seed treatments on the survival of Bradyrhizobium japonicum and on the nodulation and yield of soybean [Glycine max. (L) Merr.]. Biol. Fertil. Soils 1993, 16, 211–214.
- Cattelan, A.J.; Hungria, M. Nitrogen nutrition and inoculation. In Tropical Soybean, Improvement and Production; FAO, Plant Production and Protection Series, No. 27; Embrapa-CNPSo: Londrina, Brazil, 1994; pp. 201–215.
- Campo, R.J.; Hungria, M. Compatibilidade de Uso de Inoculantes e Fungicidas no Tratamento de Sementes de Soja; Embrapa Soja: Londrina, Brazil, 2000; p. 31.
- Zilli, J.É.; Ribeiro, K.G.; Campo, R.J.; Hungria, M. Influence of fungicide seed treatment on soybean nodulation and grain yield. Rev. Bras. Ciência Solo 2009, 33, 917–923.
- Gomes, Y.C.B.; Valadão, F.C.D.A.; Dalchiavon, F.C. Joint use of fungicides, insecticides and inoculants in the treatment of soybean seeds. Rev. Ceres 2017, 64, 258–265.
- Schulz, T.J.; Thelen, K.D. Soybean Seed Inoculant and Fungicidal Seed Treatment Effects on Soybean. Crop. Sci. 2008, 48, 1975–1983.
- Ahmed, T.H.M.; Elsheikh, E.A.E.; Mahdi, A.A. The in vitro compatibility of some Rhizobium and Bradyrhizobium strains with fungicides. Afr. Crop. Sci. Conf. Proc. 2007, 8, 1171–1178.
- Rathjen, J.; Ryder, M.; Riley, I.; Lai, T.; Denton, M. Impact of seed-applied pesticides on rhizobial survival and legume nodulation. J. Appl. Microbiol. 2020, 129, 389–399.
- Madhaiyan, M.; Poonguzhali, S.; Hári, K.; Saravanan, V.; Sa, T. Influence of pesticides on the growth rate and plant-growth promoting traits of Gluconacetobacter diazotrophicus. Pestic. Biochem. Physiol. 2006, 84, 143–154.
- Fernandes, M.F.; Procópio, S.D.O.; Teles, D.A.; Filho, J.G.D.S.; Filho, A.C.; Machado, T.N. Toxicidade de inseticidas utilizados na cultura da cana-de-açúcar à bactéria diazotrófica Herbaspirillum seropedicae. Rev. Ciências Agrar. Amaz. J. Agric. Environ. Sci. 2012, 55, 318–326.
- Insecticidal efficacy of lichens and their metabolites—A mini review. J. Appl. Pharm. Sci. 2018, 8, 159–164.
- Nauen, R.; Slater, R.; Sparks, T.C.; Elbert, A.; McCaffery, A. IRAC: Insecticide Resistance and Mode-of-action Classification of Insecticides. In Modern Crop Protection Compounds; Wiley: Weinheim, Germany, 2019; pp. 995–1012.
- De Souza, C.P.; Guedes, T.D.A.; Fontanetti, C.S. Evaluation of herbicides action on plant bioindicators by genetic biomarkers: A review. Environ. Monit. Assess. 2016, 188, 694.
- Martinez, D.A.; Loening, U.E.; Graham, M.C. Impacts of glyphosate-based herbicides on disease resistance and health of crops: A review. Environ. Sci. Eur. 2018, 30, 1–14.
- Barros, V.M.D.S.; Pedrosa, J.L.F.; Gonçalves, D.R.; De Medeiros, F.C.L.; Carvalho, G.R.; Gonçalves, A.H.; Teixeira, P.V.V.Q. Herbicides of biological origin: A review. J. Hortic. Sci. Biotechnol. 2020, 1–9.
- Wilms, W.; Woźniak-Karczewska, M.; Syguda, A.; Niemczak, M.; Ławniczak, Ł.; Pernak, J.; Rogers, R.D.; Chrzanowski, L. Herbicidal ionic liquids—A promising future for old herbicides? Review on synthesis, toxicity, biodegradation and efficacy studies. J. Agric. Food Chem. 2020, 68, 10456–10488.
- Adegas, F.S.; Vergas, L.; Gazziero, D.L.P.; Karam, D. Impacto Econômico da Resistência de Plantas Daninhas a Herbicidas no Brasil; Embrapa Soja: Londrina, Brazil, 2017; p. 12.
- Angelini, J.; Silvina, G.; Taurian, T.; Ibáñez, F.; Tonelli, M.L.; Valetti, L.; Anzuay, M.S.; Ludueña, L.; Muñoz, V.; Fabra, A. The effects of pesticides on bacterial nitrogen fixers in peanut-growing area. Arch. Microbiol. 2013, 195, 683–692.
- Dash, N.P.; Kumar, A.; Kaushik, M.S.; Abraham, G.; Singh, P.K. Agrochemicals influencing nitrogenase, biomass of N2-fixing cyanobacteria and yield of rice in wetland cultivation. Biocatal. Agric. Biotechnol. 2017, 9, 28–34.
- Procópio, S.O.; Santos, J.B.; Jacques, R.J.S.; Kasuya, M.C.M.; Silva, A.A.; Werlang, R.C. crescimento de estirpes de Bradyrhizobium sob influência dos herbicidas glyphosate potássico, fomesafen, imazethapyr e carfentrazone-ethyl. Rev. Ceres. 2004, 51, 179–188.
- Drouin, P.; Sellami, M.; Prévost, D.; Fortin, J.; Antoun, H. Tolerance to agricultural pesticides of strains belonging to four genera ofRhizobiaceae. J. Environ. Sci. Health Part B 2010, 45, 757–765.
- King, C.; Purcell, L.C.; Vories, E.D. Plant Growth and Nitrogenase Activity of Glyphosate-Tolerant Soybean in Response to Foliar Glyphosate Applications. Agron. J. 2001, 93, 179–186.
- Hungria, M.; Mendes, I.C.; Nakatani, A.S.; Dos Reis-Junior, F.B.; Morais, J.Z.; De Oliveira, M.C.N.; Fernandes, M.F. Effects of the glyphosate-resistance gene and herbicides on soybean: Field trials monitoring biological nitrogen fixation and yield. Field Crop. Res. 2014, 158, 43–54.
- Hungria, M.; Nakatani, A.S.; Souza, R.A.; Sei, F.B.; Chueire, L.M.D.O.; Arias, C.A. Impact of the ahas transgene for herbicides resistance on biological nitrogen fixation and yield of soybean. Transgenic Res. 2014, 24, 155–165.
- Zablotowicz, R.M.; Reddy, K.N. Nitrogenase activity, nitrogen content, and yield responses to glyphosate in glyphosate-resistant soybean. Crop. Prot. 2007, 26, 370–376.
- Zobiole, L.H.; Kremer, R.J.; Oliveira, R.S.; Constantin, J. Glyphosate affects chlorophyll, nodulation and nutrient accumulation of “second generation” glyphosate-resistant soybean (Glycine max L.). Pestic. Biochem. Physiol. 2011, 99, 53–60.
- Liu, C.-M.; McLean, P.A.; Sookdeo, C.C.; Cannon, F.C. Degradation of the Herbicide Glyphosate by Members of the Family Rhizobiaceae. Appl. Environ. Microbiol. 1991, 57, 1799–1804.
- Sene, L.; Converti, A.; Secchi, G.A.R.; Simão, R.D.C.G. New aspects on atrazine biodegradation. Braz. Arch. Biol. Technol. 2010, 53, 487–496.
- Hungria, M.; Nogueira, M.A. Tecnologias de inoculação da cultura da soja: Mitos, verdades e desafios. In Boletim de Pesquisa 2019/2020; Fundação MT: Rondonópolis, Brazil, 2019; pp. 50–62.




