
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bing-Huei Chen | + 2168 word(s) | 2168 | 2021-05-11 11:29:25 | | | |
| 2 | Camila Xu | Meta information modification | 2168 | 2021-05-13 05:41:30 | | |
Video Upload Options
Carotenoids are colored natural pigments belonging to a large family of C40 skeleton with eight isoprene molecules.
1. Introduction
Carotenoids are colored natural pigments belonging to a large family of C40 skeleton with eight isoprene molecules [1]. They are classified into xanthophylls and carotenes with the former such as lutein, β-cryptoxanthin and astaxanthin containing one or more oxygen atoms, while the latter such as α- carotene and β-carotene, lycopene and phytoene consisting of hydrogen and carbon atoms [2]. Carotenoid-rich foods have received great attention in human health due to their physiological functions such as antioxidant and anti-cancer as well as the ability to prevent chronic diseases such as age-associated macular degeneration and cardiovascular disease [3][4]. It has been well demonstrated that the functional properties of carotenoids were associated with their chemical structure i.e., the number of conjugated double bonds and the presence of different kinds of end-groups. However, these structural properties are also responsible for the carotenoid’s instability to light, high temperature, oxygen and metal ions, resulting in high susceptibility to oxidation and low bioavailability [3].
Given the multiple health benefits of carotenoids, they are widely used as a natural colorant and antioxidant in both pharmaceutical and food industries for prolonging shelf-life in dairy, meat, confectionary and beverage products [2]. However, carotenoids may undergo loss in functional properties during food processing owing to their instability and interaction with other food ingredients. Also the presence of digestive enzymes and some other nutrients in vivo as well as pH can alter carotenoid stability [4]. Consequently, it is vital to develop novel techniques to prevent carotenoid degradation for enhancement of bioavailability and bioactivity. Over the past decade, micro- and/or nano-encapsulation have emerged as imperative techniques for formulating food-based carotenoid carriers with improved physicochemical property and release behavior, as well as for prolonging blood circulation and efficient cellular uptake [2][5]. Comparatively, the microencapsulation technique fails to produce nanoparticles that are capable of penetrating into deeper portions of specific organs and tissues, resulting in poor bioavailability and bioactivity in vivo [6][7]. Thus, the transformation from microencapsulation to nanoencapsulation plays a pivotal role in reducing particles to nanosize by employing either top-down or bottom-up methods [8].
Recent advancements in the field of nanoscience and nanotechnology have enabled preparation of nanoscale functional compounds by encapsulating into a wide variety of nanostructures including nanoemulsions (NEs), nanoliposomes (NLs), nanocapsules (NCs), nanofibers (NFs), nanoparticles (NPs), solid lipid nanoparticles (SLNPs), nanostructured lipid carriers (NLCs) and supercritical fluid-based nanoparticles [4][5][9]. Based on the bibliometric search conducted in the Web of Science database (version 5.34) using keywords such as carotenoid nanoemulsion, carotenoid nanoparticle, carotenoid nanoencapsulation, carotenoid nanoliposome, carotenoid liposome, carotenoid micelle and carotenoid dispersion, the number of articles published from 2015-2020 was 441, of which the publications from 2019–2020 was higher (229) in terms of publication rate compared to that published between 2015–2018 (212). This trend is in line with the previous bibliometric studies [10][11]. Moreover, of the top 10 countries listed on research publications in carotenoid nanoencapsulation (2015–2020), China showed the highest publication output (23.7%) followed by USA (16.3%), Brazil (14.5%), Iran (14.5%), India (13.6%), while the other five countries including Saudi Arabia, Romania, Turkey, Pakistan and Italy, accounting for 17.4%, with an overall contribution from Asia, Europe and Americas being 60, 20 and 20%, respectively (Figure 1). Further analysis on difference in research characteristics among the top five highly-contributed countries during 2015–2020 showed that China (30) dominated with studies related to preparation and stability evaluation of nanocarotenoids, followed by Iran (20), India (19), USA (17) and Brazil (13). Likewise, most nanocarotenoid studies dealing with in vitro gastrointestinal release and bioavailability were from China (20), followed by USA (19), Iran (18), India (12) and Brazil (10). Many studies have also focused on fortification of nanoencpasulated carotenoids in a wide range of functional foods in dairy, bakery, and confectionary industries over last five years, with the top five countries accounting for 2.8–9.3% of total nanocarotenoid publications. For in vivo studies, although there was less publications by top 5 countries (5–22), a significant research output is apparent. Notably, China remained on the top with 22 publications dealing with determination of bioavailability and bioaccessibility, followed by USA (14), Iran/India (10 each) and Brazil (5). This highlights the need for many in vivo studies for proof-of-concept, functional validation, utility and clinical relevance of nanocarotenoids, which can be attained through promoting collaborative researches among institutes and countries for translation of nanocarotenoids into a botanic drug.
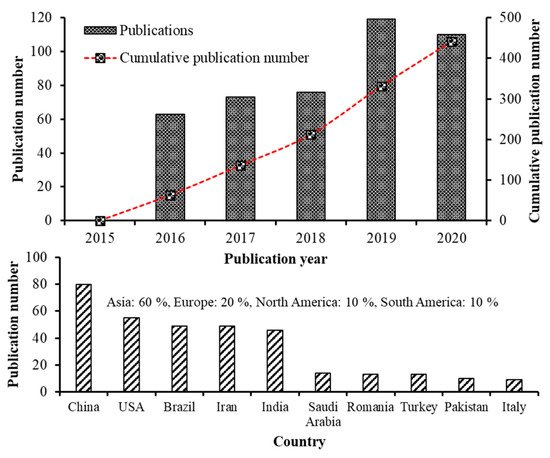
Figure 1. Research on carotenoid nanoemulsions over the last 5 years. The number of publications and global distribution. Source: Web of Science™.
Reported studies on nanocarotenoids were mainly dealing with formulation of nanosized carotenoid carriers by nanotechniques, characterization and stability evaluation as well as determination of in vitro release behavior, bioaccessibility, bioavailability and biological activity [5].
2. Carotenoid Biosynthesis and Stability-Overview
Carotenoids, a class of isoprenoids, are formed by the C5 building units of isopentenyl diphosphate and dimethylallyl diphosphate, obtained separately by two different pathways including the mevalonate (MVA) and the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathways [12]. The isopentenyl diphosphate undergoes isomerization to yield dimethylallyl diphosphate, which further condenses with another molecule of isopentenyl diphosphate to yield C20 geranylgeranyl pyrophosphate. Then the two molecules of geranylgeranyl pyrophosphate combine with each other to yield the first carotenoid molecule phytoene (C40) and sequential incorporation of double bonds at alternate positions of phytoene, resulting in formation of phytofluene, ζ-carotene, neurosporene and lycopene (Figure 2) [13]. Through branched cyclization of lycopene, carotenoids with one β-ring and one ε-ring (e.g., α-carotene and lutein) and two β-rings (β-carotene, zeaxanthin and antheraxanthin) are produced. Further advancement of carotenoid synthesis occurred through attachment of oxygen moieties to hydrocarbon carotenoids such as α-carotene and β-carotene for formation of xanthophylls (Figure 2) [13].
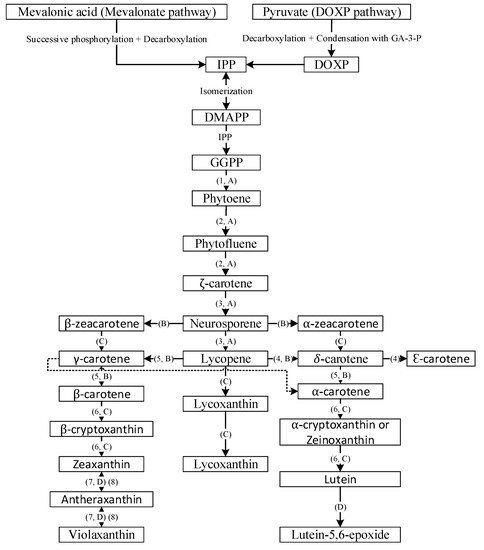
Figure 2. General overview of carotenoid biosynthesis pathway. DOXP = 1-deoxy-D-xylulose, GA-3-P = glyceraldehyde 3-phosphate, IPP = isopentyl diphosphate, DMAPP = dimethylallyl diphosphate and GGPP = geranylgeranyl pyrophosphate; 1 = phytoene synthase, 2 = phytoene desaturase, 3 = ζ-carotene desaturase, 4 = lycopene e-cyclase, 5 = lycopene β-cyclase; 6 = β-carotene hydroxylase, 7 = zeaxanthin epoxidase and 8 = violaxanthin de-epoxidase; A = desaturation, B = cyclization, C = hydroxylation and D = epoxidation.
The presence of long-chain conjugated double bonds in carotenoids makes them highly susceptible to degradation under acid, light and high temperature conditions [3]. For instance, carotenoids were shown to degrade at a faster rate in the presence of light through generation of singlet oxygen that eventually binds with the hydrocarbon chain in carotenoids leading to degradation [14]. More recently, two theories have been proposed for oxidative degradation of carotenoids, namely random and central cleavage theories, with oxidation occurring randomly at different sites or at the central bond of a carotenoid molecule, respectively [15]. In a study dealing with thermal degradation of lutein and β-carotene, Giménez, et al. [16] reported a progressive increase in degradation following a rise in heating temperature from 30–90 °C. Also, β-carotene could undergo degradation to form epoxides and carbonyl compounds (apocarotenals) via a free radical reduction mechanism [13]. Thus, owing to the instability of carotenoids caused by multiple factors, it is important to develop appropriate strategies for preventing degradation, prolonging shelf-life and enhancing bioavailability of carotenoids.
3. Conventional Microencapsulation vs. Nanoencapsulation
Microencapsulation of unstable and water-insoluble bioactive compounds such as carotenoids involves trapping them within a special coating material for preparation of micron-sized particles with a mean size ranging from 1 to 500 µm. Micronized spherical particles are capable of controlling both loading and releasing of bioactive compounds [17]. The conventional microencapsulation process can be broadly classified into three categories depending on how microparticles are prepared including chemical, physicochemical and physicomechanical processes [18][19]. For example, Polyakov and Kispert [20] reviewed a carotenoid inclusion complex (e.g., β-carotene enriched inclusion complex) with polysaccharides including arabinogalactan, cyclodextrin and glycyrrhizin, and demonstrated increased stability and bioavailability compared to free carotenoids, while García, et al. [21] reported an enhanced thermal stability (up to 100 °C) of spherical microcapsules produced from carotenoid-rich mango, banana and tamarillo powders by spray-drying with maltodextrin. Likewise, several studies have demonstrated the ability of microencapsulated carotenoids to improve physicochemical characteristics, storage stability and bioavailability for further development into value-added functional foods [22][23][24][25][26]. Several microencapsulation techniques used for enhancement of carotenoid stability and bioavailability have been reviewed by Soukoulis and Bohn [2].
Although the microencapsulation techniques are efficient, the recent clean labelling trends have prevented the use of dairy, lactose, sugar, sodium, gluten, fats and carbohydrate as coating material, thus further limiting the choice of suitable encapsulation materials [27]. In addition, the most commonly used encapsulant maltodextrin possesses low emulsifying ability thereby reducing the encapsulation efficiency (EE) [28]. More recently, Sun, et al. [6] pointed out that the average size of microcapsules is a critical parameter which can significantly affect the physicochemical characteristics, stability, sensory property, bioavailability and release behavior. Also, micron-sized particles have many drawbacks such as nontargeting of specific organs, tissues and cells as well as instability, poor aqueous solubility and low bioavailability in human body [7]. Therefore, it is necessary to decrease the size of encapsulated material to sub-micron (0.10–10 μm) and nano size (<0.10 μm).
Due to the increasing prevalence rate of chronic diseases, the emerging challenges in delivering functional compounds to target tissues, organs and cells, as well as instability, poor aqueous solubility and bioavailability, and low release and absorption in vivo could not be overcome by microencapsulation techniques [7]. Recent developments in the field of nanotechnology have provided some excellent means to reduce particle size through top-down (high energy method) or bottom up (self-assembly) processes [29]. Such reduction in particle size has been shown to enhance the stability, targeting ability, bioavailability and release properties [30]. Most importantly, the reduction in particle size enables penetration into deeper portions of cells or tissues resulting in high bioavailability [31]. In the following sections, we have reviewed the research articles published within the last five years on nanoencapsulation of various carotenoid compounds by using different preparation techniques. These studies demonstrated the impact of nanoencapsulation to improve physicochemical property, bioavailability, controlled release and bioactivity. Table 1 and Table 2 summarize various nanosystems used for encapsulation of carotenoids and highlight their advantages as well as disadvantages, respectively.
Table 1. Nanosystems for encapsulation of carotenoids 1.
| Nanosystem | Carotenoids | Particle Size (nm) | EE (%) | Zeta Potential (mV) | Storage Stability (Days) | References |
|---|---|---|---|---|---|---|
| Nanoemulsions | β-carotene | 218 | NA | 40 | 21 at 37 °C | [32] |
| 143.7 | −38.2 | 30 at 25 °C | [33] | |||
| Microbial carotenoids | 142.1 | NA | 30 at 25 °C | [34] | ||
| Carotenoids | 290 to 350 | −53.4 to −58.8 | 21 at 25 °C | [35] | ||
| β-carotene | 198.4 to 315.6 | −29.9 to −38.5 | 90 at 4, 25, and 37 °C | [36] | ||
| Carotenoids | <200 | −30 to −45 | 35 at 25 °C | [37] | ||
| Lycopene | 145.1 to 161.9 | −19.7 to −20.7 | 1 at 25 °C | [38] | ||
| 200.1 to 287.1 | 61 to 89.1 | 20 to 45 | 42 at 4, 25, and 37 °C | [39] | ||
| Polymeric/biopolymeric NPs | Carotenoids | 153 | 83.7 | NA | NA | [40] |
| 84.4 | >96 | −41.3 to −43.6 | 60 at 41 °C | [41] | ||
| β-carotene | 77.8 to 371.8 | 98.7 to 99.1 | −37.8 to −29.9 | NA | [42] | |
| β-carotene | 70.4 | 97.4 | NA | NA | [43] | |
| Lycopene | 152 | 89 | 58.3 | NA | [44] | |
| ~ 200 | >95 | −36 | 210 at 5 °C | [45] | ||
| 193 | NA | −11.5 | 14 at 25 °C | [46] | ||
| Lutein | <250 | 74.5 | −27.2 | NA | [47] | |
| Lutein | 240 to 340 | ~91.9 | NA | NA | [48] | |
| Crocetin | 288 to 584 | 59.6 to 97.2 | NA | NA | [49] | |
| Fucoxanthin | 200 to 500 | 47 to 90 | 30 to 50 | 6 at 37 °C | [50] | |
| Nanoliposomes/liposomes | Carotenoids | 70 to100 | 75 | −5.3 | NA | [51] |
| β-carotene | 162.8 to 365.8 | ~98 | 64.5 to 42.6 | 70 at 4 °C | [52] | |
| Astaxanthin | 80.6 | 97.6 | 31.8 | 15 at 4 and 25 °C | [53] | |
| 60 to 80 | 97.4 | NA | NA | [54] | ||
| Lutein | 264.8 to 367.1 | 91.8 to 92.9 | −34.3 to −27.9 | NA | [55] | |
| SLNPs and NLCs | β-carotene SLNPs | 200 to 400 | 53.4 to 68.3 | −6.1 to −9.3 | 90 at 5, 25, and 40 °C | [56] |
| <220 | NA | 20 to 30 | 10 at 25 °C | [57] | ||
| 120 | NA | −30 | 56 at 25 °C | [58] | ||
| Lycopene SLNPs | 125 to 166 | 86.6 to 98.4 | NA | 60 at 4 °C | [59] | |
| Lycopene NLCs | 157 to 166 | > 99 | −74.2 to −74.6 | 120 at 4, 30, and 40 °C | [8] | |
| 121.9 | 84.50 | −29 | 90 at 25 °C | [60] | ||
| Supercritical fluid-based NPs | Astaxanthin | 150 to 175 | NA | NA | NA | [61] |
| 266 | 84 | NA | NA | [62] | ||
| Metal/metal oxide-based NPs and hybrid nanocomposites | Carotenoids | 20 to 140 | NA | NA | NA | [63] |
| Lycopene | 3 to 5 | −48.5 | 90 at 4 and 25 °C | [64] | ||
| 20.8 | −25.3 | NA | [65] |
1 EE = encapsulation efficiency, NPs = nanoparticles, SLNPs = solid lipid nanoparticles, NA = data not available and NLCs = nanostructured lipid carriers.
Table 2. The advantages and disadvantages of nanosystems for encapsulation of carotenoids 1.
|
Nanosystem |
Advantages and Disadvantages |
References |
|---|---|---|
|
Nanoemulsions |
Advantages
Disadvantages
|
|
|
Polymeric/biopolymeric NPs |
Advantages
Disadvantages
|
|
|
Nanoliposomes/liposomes |
Advantages
Disadvantages
|
|
|
SLNPs |
Advantages
Disadvantages
|
|
|
NLCs |
Advantages
Disadvantages
|
|
|
Supercritical fluid-based NPs |
Advantages
Disadvantages
|
|
|
Metal/metal oxide-based NPs and hybrid nanocomposites |
Advantages
Disadvantages
|
1 EE = encapsulation efficiency, NPs = nanoparticles, SLNPs = solid lipid nanoparticles and NLCs = nanostructured lipid carriers.
References
- Caballero, B.; Finglas, P.M.; Toldrá, F. Carotenoids: Physiology. In Encyclopedia of Food and Health; Caballero, B.; Finglas, P.M.; Toldrá, F. Academic Press: Oxford, UK, 2016; pp. 670–675.
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for enhancing the chemical stability and bioavailability of carotenoids. Crit. Rev. Food Sci. Nutri. 2018, 58, 1–36.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-the way of bioavailability improvement. Molecules 2019, 24, 3947.
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Cont. Rel. 2019, 298, 38–67.
- Dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860.
- Sun, X.; Cameron, R.G.; Manthey, J.A.; Hunter, W.B.; Bai, J. Microencapsulation of tangeretin in a citrus pectin mixture matrix. Foods 2020, 9, 1200.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71.
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formulations for topical administration. Int. J. Pharm. 2015, 478, 726–735.
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Interface Sci. 2020, 275, 102048.
- Usman, M.; Ho, Y.-S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Environ. Manag. 2020, 270, 110886.
- Andreo-Martínez, P.; Oliva, J.; Giménez-Castillo, J.J.; Motas, M.; Quesada-Medina, J.; Cámara, M.Á. Science production of pesticide residues in honey research: A descriptive bibliometric study. Environ. Toxicol. Pharmacol. 2020, 79, 103413.
- Zhang, C. Biosynthesis of carotenoids and apocarotenoids by microorganisms and their industrial potential. In Progress in Carotenoid Research; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 85–105.
- Inbaraj, B.S.; Chen, B.H. Carotenoids in tomato plants. In Tomatoes and Tomato Products-Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2008; pp. 133–164.
- Da Silva, M.M.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Rutz, J.K.; Flores Cantillano, R.F.; Nora, L.; Rios, A.d.O. Thermal and ultraviolet–visible light stability kinetics of co-nanoencapsulated carotenoids. Food Bioprod. Process. 2017, 105, 86–94.
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038.
- Giménez, P.J.; Fernández-López, J.A.; Angosto, J.M.; Obón, J.M. Comparative thermal degradation patterns of natural yellow colorants used in foods. Plant Foods Hum. Nutr. 2015, 70, 380–387.
- Do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and its uses in food science and technology: A review. In Microencapsulation—Processes, Technologies and Industrial Applications; Salaün, F., Ed.; IntechOpen Limited: London, UK, 2019; pp. 1–18.
- Krajišnik, D.; Čalija, B.; Cekić, N. Polymeric microparticles and inorganic micro/nanoparticulate drug carriers: An overview and pharmaceutical application. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs; Čalija, B., Ed.; Academic Press: Boston, MA, USA, 2017; Chapter 15; pp. 31–67.
- Fu, F.; Hu, L. Temperature sensitive colour-changed composites. In Advanced High Strength Natural Fibre Composites in Construction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 15; pp. 405–423.
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohy. Poly. 2015, 128, 207–219.
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterisation of carotenoid-rich microencapsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709.
- Ding, Z.; Tao, T.; Yin, X.; Prakash, S.; Wang, X.; Zhao, Y.; Han, J.; Wang, Z. Improved encapsulation efficiency and storage stability of spray dried microencapsulated lutein with carbohydrates combinations as encapsulating material. LWT 2020, 124, 109139.
- Feng, Z.-Z.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. Astaxanthin from Phaffia rhodozyma: Microencapsulation with carboxymethyl cellulose sodium and microcrystalline cellulose and effects of microencapsulated astaxanthin on yogurt properties. LWT 2018, 96, 152–160.
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81.
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crops Prod. 2018, 111, 846–855.
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24.
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportunities, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382.
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571.
- Rashidinejad, A.; Jafari, S.M. Nanoencapsulation of bioactive food ingredients. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 8; pp. 279–344.
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An overview of nanotechnology in food science: Preparative methods, practical applications, and safety. J. Chem. 2018, 2018, 5427978.
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Perception and overview. Front. Microbiol. 2017, 8, 1501.
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of thermal and UV-light stability of β-carotene-loaded nanoemulsions by water-soluble chitosan coating. Int. J. Biol. Macromol. 2020, 165, 1156–1163.
- Barman, K.; Chowdhury, D.; Baruah, P.K. Development of β-carotene loaded nanoemulsion using the industrial waste of orange (Citrus reticulate) peel to improve in vitro bioaccessibility of carotenoids and use as natural food colorant. J. Food Process Preserv. 2020, 44, e14429.
- Mansur, M.C.P.P.R.; Campos, C.; Vermelho, A.B.; Nobrega, J.; da Cunha Boldrini, L.; Balottin, L.; Lage, C.; Rosado, A.S.; Ricci-Júnior, E.; dos Santos, E.P. Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: Efficacy and safety study. Arab. J. Chem. 2020, 13, 6741–6752.
- Gasa-Falcon, A.; Arranz, E.; Odriozola-Serrano, I.; Martín-Belloso, O.; Giblin, L. Delivery of β-carotene to the in vitro intestinal barrier using nanoemulsions with lecithin or sodium caseinate as emulsifiers. LWT 2021, 135, 110059.
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical stability of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237.
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bioaccessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470.
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66.
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemulsions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920.
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT 2015, 63, 100–107.
- Bezerra, P.Q.M.; Matos, M.F.R.d.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405.
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40.
- Rostamabadi, H.; Sadeghi Mahoonak, A.; Allafchian, A.; Ghorbani, M. Fabrication of β-carotene loaded glucuronoxylan-based nanostructures through electrohydrodynamic processing. Int. J. Biol. Macromol. 2019, 139, 773–784.
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124.
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ε-caprolactone lipid-core nanocapsules loaded with lycopene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548.
- Dos Santos, P.P.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Costa, T.H.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Development of lycopene-loaded lipid-core nanocapsules: Physicochemical characterization and stability study. J. Nano Res. 2015, 17, 107.
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-loaded, biotin-decorated polymeric nanoparticles enhance lutein uptake in retinal cells. Pharmaceutics 2020, 12, 798.
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449.
- Hafezi Ghahestani, Z.; Alebooye Langroodi, F.; Mokhtarzadeh, A.; Ramezani, M.; Hashemi, M. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 955–960.
- Ravi, H.; Baskaran, V. Biodegradable chitosan-glycolipid hybrid nanogels: A novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015, 43, 717–725.
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784.
- Hassane Hamadou, A.; Huang, W.-C.; Xue, C.; Mao, X. Comparison of β-carotene loaded marine and egg phospholipids nanoliposomes. J. Food Eng. 2020, 283, 110055.
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA J. Food 2018, 16, 607–618.
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822.
- Jiao, Y.; Li, D.; Liu, C.; Chang, Y.; Song, J.; Xiao, Y.J.R.A. Polypeptide–decorated nanoliposomes as novel delivery systems for lutein. RSC Adv. 2018, 8, 31372–31381.
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS Pharm. Sci. Tech. 2019, 20, 100.
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969.
- Schjoerring-Thyssen, J.; Olsen, K.; Koehler, K.; Jouenne, E.; Rousseau, D.; Andersen, M.L. Morphology and structure of solid lipid nanoparticles loaded with high concentrations of β-carotene. J. Agric. Food Chem. 2019, 67, 12273–12282.
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and physicochemical characterization of lycopene-loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241.
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based nanoformulation of lycopene improves oral delivery: Formulation optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencap. 2017, 34, 416–429.
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51.
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin encapsulation in ethyl cellulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release profile and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136.
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264.
- Huang, R.F.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846.
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green synthesis of gold nanoparticles using sumac aqueous extract and their antioxidant activity. J. Mat. Res. 2017, 20, 264–270.
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics 2019, 11, 629.
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Environ. Chem. Let. 2020, 18, 1659–1683.
- Seabra, A.B.; Durán, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975.




