Carotenoids are colored natural pigments belonging to a large family of C40 skeleton with eight isoprene molecules.
- nanoencapsulation
- carotenoid
- in vitro release
- antioxidant activity
1. Introduction
Carotenoids are colored natural pigments belonging to a large family of C40 skeleton with eight isoprene molecules [1]. They are classified into xanthophylls and carotenes with the former such as lutein, β-cryptoxanthin and astaxanthin containing one or more oxygen atoms, while the latter such as α- carotene and β-carotene, lycopene and phytoene consisting of hydrogen and carbon atoms [2]. Carotenoid-rich foods have received great attention in human health due to their physiological functions such as antioxidant and anti-cancer as well as the ability to prevent chronic diseases such as age-associated macular degeneration and cardiovascular disease [3,4]. It has been well demonstrated that the functional properties of carotenoids were associated with their chemical structure i.e., the number of conjugated double bonds and the presence of different kinds of end-groups. However, these structural properties are also responsible for the carotenoid’s instability to light, high temperature, oxygen and metal ions, resulting in high susceptibility to oxidation and low bioavailability [3].
Given the multiple health benefits of carotenoids, they are widely used as a natural colorant and antioxidant in both pharmaceutical and food industries for prolonging shelf-life in dairy, meat, confectionary and beverage products [2]. However, carotenoids may undergo loss in functional properties during food processing owing to their instability and interaction with other food ingredients. Also the presence of digestive enzymes and some other nutrients in vivo as well as pH can alter carotenoid stability [4]. Consequently, it is vital to develop novel techniques to prevent carotenoid degradation for enhancement of bioavailability and bioactivity. Over the past decade, micro- and/or nano-encapsulation have emerged as imperative techniques for formulating food-based carotenoid carriers with improved physicochemical property and release behavior, as well as for prolonging blood circulation and efficient cellular uptake [2,5]. Comparatively, the microencapsulation technique fails to produce nanoparticles that are capable of penetrating into deeper portions of specific organs and tissues, resulting in poor bioavailability and bioactivity in vivo [6,7]. Thus, the transformation from microencapsulation to nanoencapsulation plays a pivotal role in reducing particles to nanosize by employing either top-down or bottom-up methods [8].
Recent advancements in the field of nanoscience and nanotechnology have enabled preparation of nanoscale functional compounds by encapsulating into a wide variety of nanostructures including nanoemulsions (NEs), nanoliposomes (NLs), nanocapsules (NCs), nanofibers (NFs), nanoparticles (NPs), solid lipid nanoparticles (SLNPs), nanostructured lipid carriers (NLCs) and supercritical fluid-based nanoparticles [4,5,9]. Based on the bibliometric search conducted in the Web of Science database (version 5.34) using keywords such as carotenoid nanoemulsion, carotenoid nanoparticle, carotenoid nanoencapsulation, carotenoid nanoliposome, carotenoid liposome, carotenoid micelle and carotenoid dispersion, the number of articles published from 2015-2020 was 441, of which the publications from 2019–2020 was higher (229) in terms of publication rate compared to that published between 2015–2018 (212). This trend is in line with the previous bibliometric studies [10,11]. Moreover, of the top 10 countries listed on research publications in carotenoid nanoencapsulation (2015–2020), China showed the highest publication output (23.7%) followed by USA (16.3%), Brazil (14.5%), Iran (14.5%), India (13.6%), while the other five countries including Saudi Arabia, Romania, Turkey, Pakistan and Italy, accounting for 17.4%, with an overall contribution from Asia, Europe and Americas being 60, 20 and 20%, respectively (Figure 1). Further analysis on difference in research characteristics among the top five highly-contributed countries during 2015–2020 showed that China (30) dominated with studies related to preparation and stability evaluation of nanocarotenoids, followed by Iran (20), India (19), USA (17) and Brazil (13). Likewise, most nanocarotenoid studies dealing with in vitro gastrointestinal release and bioavailability were from China (20), followed by USA (19), Iran (18), India (12) and Brazil (10). Many studies have also focused on fortification of nanoencpasulated carotenoids in a wide range of functional foods in dairy, bakery, and confectionary industries over last five years, with the top five countries accounting for 2.8–9.3% of total nanocarotenoid publications. For in vivo studies, although there was less publications by top 5 countries (5–22), a significant research output is apparent. Notably, China remained on the top with 22 publications dealing with determination of bioavailability and bioaccessibility, followed by USA (14), Iran/India (10 each) and Brazil (5). This highlights the need for many in vivo studies for proof-of-concept, functional validation, utility and clinical relevance of nanocarotenoids, which can be attained through promoting collaborative researches among institutes and countries for translation of nanocarotenoids into a botanic drug.
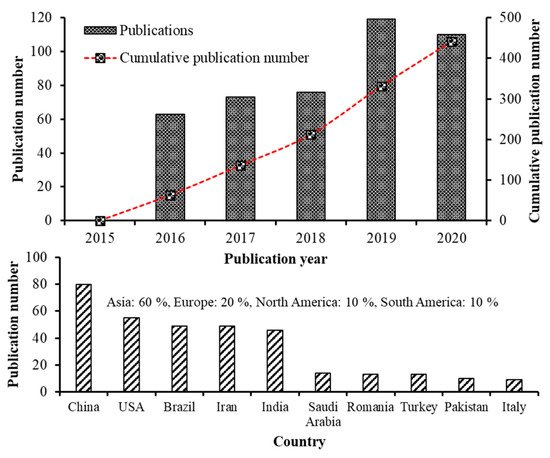
Reported studies on nanocarotenoids were mainly dealing with formulation of nanosized carotenoid carriers by nanotechniques, characterization and stability evaluation as well as determination of in vitro release behavior, bioaccessibility, bioavailability and biological activity [5].
2. Carotenoid Biosynthesis and Stability-Overview
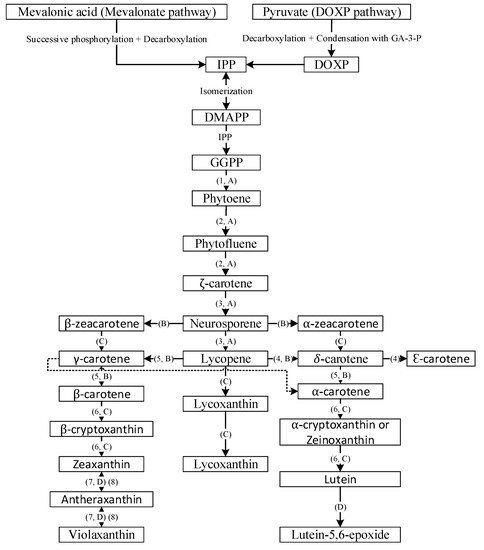
3. Conventional Microencapsulation vs. Nanoencapsulation
| Nanosystem | Carotenoids | Particle Size (nm) | EE (%) | Zeta Potential (mV) | Storage Stability (Days) | References |
|---|---|---|---|---|---|---|
| Nanoemulsions | β-carotene | 218 | NA | 40 | 21 at 37 °C | [32] |
| 143.7 | −38.2 | 30 at 25 °C | [33] | |||
| Microbial carotenoids | 142.1 | NA | 30 at 25 °C | [34] | ||
| Carotenoids | 290 to 350 | −53.4 to −58.8 | 21 at 25 °C | [35] | ||
| β-carotene | 198.4 to 315.6 | −29.9 to −38.5 | 90 at 4, 25, and 37 °C | [36] | ||
| Carotenoids | <200 | −30 to −45 | 35 at 25 °C | [37] | ||
| Lycopene | 145.1 to 161.9 | −19.7 to −20.7 | 1 at 25 °C | [38] | ||
| 200.1 to 287.1 | 61 to 89.1 | 20 to 45 | 42 at 4, 25, and 37 °C | [39] | ||
| Polymeric/biopolymeric NPs | Carotenoids | 153 | 83.7 | NA | NA | [40] |
| 84.4 | >96 | −41.3 to −43.6 | 60 at 41 °C | [41] | ||
| β-carotene | 77.8 to 371.8 | 98.7 to 99.1 | −37.8 to −29.9 | NA | [42] | |
| β-carotene | 70.4 | 97.4 | NA | NA | [43] | |
| Lycopene | 152 | 89 | 58.3 | NA | [44] | |
| ~ 200 | >95 | −36 | 210 at 5 °C | [45] | ||
| 193 | NA | −11.5 | 14 at 25 °C | [46] | ||
| Lutein | <250 | 74.5 | −27.2 | NA | [47] | |
| Lutein | 240 to 340 | ~91.9 | NA | NA | [48] | |
| Crocetin | 288 to 584 | 59.6 to 97.2 | NA | NA | [49] | |
| Fucoxanthin | 200 to 500 | 47 to 90 | 30 to 50 | 6 at 37 °C | [50] | |
| Nanoliposomes/liposomes | Carotenoids | 70 to100 | 75 | −5.3 | NA | [51] |
| β-carotene | 162.8 to 365.8 | ~98 | 64.5 to 42.6 | 70 at 4 °C | [52] | |
| Astaxanthin | 80.6 | 97.6 | 31.8 | 15 at 4 and 25 °C | [53] | |
| 60 to 80 | 97.4 | NA | NA | [54] | ||
| Lutein | 264.8 to 367.1 | 91.8 to 92.9 | −34.3 to −27.9 | NA | [55] | |
| SLNPs and NLCs | β-carotene SLNPs | 200 to 400 | 53.4 to 68.3 | −6.1 to −9.3 | 90 at 5, 25, and 40 °C | [56] |
| <220 | NA | 20 to 30 | 10 at 25 °C | [57] | ||
| 120 | NA | −30 | 56 at 25 °C | [58] | ||
| Lycopene SLNPs | 125 to 166 | 86.6 to 98.4 | NA | 60 at 4 °C | [59] | |
| Lycopene NLCs | 157 to 166 | > 99 | −74.2 to −74.6 | 120 at 4, 30, and 40 °C | [8] | |
| 121.9 | 84.50 | −29 | 90 at 25 °C | [60] | ||
| Supercritical fluid-based NPs | Astaxanthin | 150 to 175 | NA | NA | NA | [61] |
| 266 | 84 | NA | NA | [62] | ||
| Metal/metal oxide-based NPs and hybrid nanocomposites | Carotenoids | 20 to 140 | NA | NA | NA | [63] |
| Lycopene | 3 to 5 | −48.5 | 90 at 4 and 25 °C | [64] | ||
| 20.8 | −25.3 | NA | [65] |
Table 2. The advantages and disadvantages of nanosystems for encapsulation of carotenoids 1.
|
Nanosystem |
Advantages and Disadvantages |
References |
|---|---|---|
|
Nanoemulsions |
Advantages
Disadvantages
|
|
|
Polymeric/biopolymeric NPs |
Advantages
Disadvantages
|
|
|
Nanoliposomes/liposomes |
Advantages
Disadvantages
|
|
|
SLNPs |
Advantages
Disadvantages
|
|
|
NLCs |
Advantages
Disadvantages
|
|
|
Supercritical fluid-based NPs |
Advantages
Disadvantages
|
|
|
Metal/metal oxide-based NPs and hybrid nanocomposites |
Advantages
Disadvantages
|
1 EE = encapsulation efficiency, NPs = nanoparticles, SLNPs = solid lipid nanoparticles and NLCs = nanostructured lipid carriers.
This entry is adapted from the peer-reviewed paper 10.3390/antiox10050713
References
- Ellison, S.L. Carotenoids: Physiology. In Encyclopedia of Food and Health, Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Aca-demic Press: Oxford, UK, 2016; pp. 670–675.
- Soukoulis, C.; Bohn, T. A comprehensive overview on the micro- and nano-technological encapsulation advances for en-hancing the chemical stability and bioavailability of carotenoids. Cri. Rev. Food Sci. Nutri. 2018, 58, 1–36.
- Focsan, A.L.; Polyakov, N.E.; Kispert, L.D. Supramolecular carotenoid complexes of enhanced solubility and stability-the way of bioavailability improvement. Molecules 2019, 24, 3947.
- Rostamabadi, H.; Falsafi, S.R.; Jafari, S.M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Cont. Rel. 2019, 298, 38–67.
- Dos Santos, P.P.; Andrade, L.d.A.; Flôres, S.H.; Rios, A.D.O. Nanoencapsulation of carotenoids: A focus on different delivery systems and evaluation parameters. J. Food Sci. Technol. 2018, 55, 3851–3860.
- Sun, X.; Cameron, R.G.; Manthey, J.A.; Hunter, W.B.; Bai, J. Microencapsulation of tangeretin in a citrus pectin mixture ma-trix. Foods 2020, 9, 1200.
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobi-otechnol. 2018, 16, 71–71.
- Okonogi, S.; Riangjanapatee, P. Physicochemical characterization of lycopene-loaded nanostructured lipid carrier formula-tions for topical administration. Int. J. Pharm. 2015, 478, 726–735.
- Rehman, A.; Tong, Q.; Jafari, S.M.; Assadpour, E.; Shehzad, Q.; Aadil, R.M.; Iqbal, M.W.; Rashed, M.M.A.; Mushtaq, B.S.; Ashraf, W. Carotenoid-loaded nanocarriers: A comprehensive review. Adv. Colloid Inter. Sci. 2020, 275, 102048.
- Usman, M.; Ho, Y.-S. A bibliometric study of the Fenton oxidation for soil and water remediation. J. Env. Manag. 2020, 270, 110886.
- Andreo-Martínez, P.; Oliva, J.; Giménez-Castillo, J.J.; Motas, M.; Quesada-Medina, J.; Cámara, M.Á. Science production of pesticide residues in honey research: A descriptive bibliometric study. Env. Toxicol. Pharmacol. 2020, 79, 103413.
- Zhang, C. Biosynthesis of carotenoids and apocarotenoids by microorganisms and their industrial potential. In Progress in Carotenoid Research; BoD—Books on Demand: Norderstedt, Germany, 2018; pp. 85–105.
- Inbaraj, B.S.; Chen, B.H. Carotenoids in tomato plants. In Tomatoes and Tomato Products-Nutritional, Medicinal and Therapeutic Properties; CRC Press: Boca Raton, FL, USA, 2008; pp. 133–164.
- Da Silva, M.M.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Rutz, J.K.; Flores Cantillano, R.F.; Nora, L.; Rios, A.d.O. Thermal and ultraviolet–visible light stability kinetics of co-nanoencapsulated carotenoids. Food Bioprod. Proces. 2017, 105, 86–94.
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038.
- Giménez, P.J.; Fernández-López, J.A.; Angosto, J.M.; Obón, J.M. Comparative thermal degradation patterns of natural yellow colorants used in foods. Plant Foods Hum. Nutr. 2015, 70, 380–387.
- Do Amaral, P.H.R.; Andrade, P.L.; de Conto, L.C. Microencapsulation and its uses in food science and technology: A review. In Microencapsulation—Processes, Technologies and Industrial Applications; Salaün, F., Ed.; IntechOpen Limited: London, UK, 2019; pp. 1–18.
- Krajišnik, D.; Čalija, B.; Cekić, N. Polymeric microparticles and inorganic micro/nanoparticulate drug carriers: An overview and pharmaceutical application. In Microsized and Nanosized Carriers for Nonsteroidal Anti-Inflammatory Drugs; Čalija, B., Ed.; Academic Press: Boston, MA, USA, 2017; Chapter 2, pp. 31–67.
- Fu, F.; Hu, L. Temperature sensitive colour-changed composites. In Advanced High Strength Natural Fibre Composites in Con-struction; Fan, M., Fu, F., Eds.; Woodhead Publishing: Cambridge, UK, 2017; Chapter 15, pp. 405–423.
- Polyakov, N.E.; Kispert, L.D. Water soluble biocompatible vesicles based on polysaccharides and oligosaccharides inclusion complexes for carotenoid delivery. Carbohy. Poly. 2015, 128, 207–219.
- García, J.M.; Giuffrida, D.; Dugo, P.; Mondello, L.; Osorio, C. Development and characterisation of carotenoid-rich microen-capsulates from tropical fruit by-products and yellow tamarillo (Solanum betaceum Cav.). Powder Technol. 2018, 339, 702–709.
- Ding, Z.; Tao, T.; Yin, X.; Prakash, S.; Wang, X.; Zhao, Y.; Han, J.; Wang, Z. Improved encapsulation efficiency and storage stability of spray dried microencapsulated lutein with carbohydrates combinations as encapsulating material. LWT 2020, 124, 109139.
- Feng, Z.-Z.; Li, M.-Y.; Wang, Y.-T.; Zhu, M.-J. Astaxanthin from Phaffia rhodozyma: Microencapsulation with carboxymethyl cellulose sodium and microcrystalline cellulose and effects of microencapsulated astaxanthin on yogurt properties. LWT 2018, 96, 152–160.
- Zhou, Q.; Yang, L.; Xu, J.; Qiao, X.; Li, Z.; Wang, Y.; Xue, C. Evaluation of the physicochemical stability and digestibility of microencapsulated esterified astaxanthins using in vitro and in vivo models. Food Chem. 2018, 260, 73–81.
- Tupuna, D.S.; Paese, K.; Guterres, S.S.; Jablonski, A.; Flôres, S.H.; Rios, A.d.O. Encapsulation efficiency and thermal stability of norbixin microencapsulated by spray-drying using different combinations of wall materials. Ind. Crops Prod. 2018, 111, 846–855.
- Ursache, F.M.; Andronoiu, D.G.; Ghinea, I.O.; Barbu, V.; Ioniţă, E.; Cotârleţ, M.; Dumitraşcu, L.; Botez, E.; Râpeanu, G.; Stănciuc, N. Valorizations of carotenoids from sea buckthorn extract by microencapsulation and formulation of value-added food products. J. Food Eng. 2018, 219, 16–24.
- Arenas-Jal, M.; Suñé-Negre, J.M.; García-Montoya, E. An overview of microencapsulation in the food industry: Opportuni-ties, challenges, and innovations. Eur. Food Res. Technol. 2020, 246, 1371–1382.
- Corrêa-Filho, L.C.; Moldão-Martins, M.; Alves, V.D. Advances in the application of microcapsules as carriers of functional compounds for food products. Appl. Sci. 2019, 9, 571.
- Rashidinejad, A.; Jafari, S.M. Nanoencapsulation of bioactive food ingredients. In Handbook of Food Nanotechnology; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; Chapter 8, pp. 279–344.
- Yu, H.; Park, J.-Y.; Kwon, C.W.; Hong, S.-C.; Park, K.-M.; Chang, P.-S. An overview of nanotechnology in food science: Pre-parative methods, practical applications, and safety. J. Chem. 2018, 2018, 5427978.
- Singh, T.; Shukla, S.; Kumar, P.; Wahla, V.; Bajpai, V.K.; Rather, I.A. Application of nanotechnology in food science: Percep-tion and overview. Front. Microbiol. 2017, 8, 1501.
- Baek, E.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Improvement of thermal and UV-light stability of β-carotene-loaded nanoemul-sions by water-soluble chitosan coating. Int. J. Biolog. Macromol. 2020, 165, 1156–1163.
- Barman, K.; Chowdhury, D.; Baruah, P.K. Development of β-carotene loaded nanoemulsion using the industrial waste of orange (Citrus reticulate) peel to improve in vitro bioaccessibility of carotenoids and use as natural food colorant. J. Food Pro-cess Preserv. 2020, 44, e14429.
- Mansur, M.C.P.P.R.; Campos, C.; Vermelho, A.B.; Nobrega, J.; da Cunha Boldrini, L.; Balottin, L.; Lage, C.; Rosado, A.S.; Ricci-Júnior, E.; dos Santos, E.P. Photoprotective nanoemulsions containing microbial carotenoids and buriti oil: Efficacy and safety study. Arab. J. Chem. 2020, 13, 6741–6752.
- Gasa-Falcon, A.; Arranz, E.; Odriozola-Serrano, I.; Martín-Belloso, O.; Giblin, L. Delivery of β-carotene to the in vitro intesti-nal barrier using nanoemulsions with lecithin or sodium caseinate as emulsifiers. LWT 2021, 135, 110059.
- Borba, C.M.; Tavares, M.N.; Macedo, L.P.; Araújo, G.S.; Furlong, E.B.; Dora, C.L.; Burkert, J.F.M. Physical and chemical sta-bility of β-carotene nanoemulsions during storage and thermal process. Food Res. Int. 2019, 121, 229–237.
- Sotomayor-Gerding, D.; Oomah, B.D.; Acevedo, F.; Morales, E.; Bustamante, M.; Shene, C.; Rubilar, M. High carotenoid bio-accessibility through linseed oil nanoemulsions with enhanced physical and oxidative stability. Food Chem. 2016, 199, 463–470.
- Li, D.; Li, L.; Xiao, N.; Li, M.; Xie, X. Physical properties of oil-in-water nanoemulsions stabilized by OSA-modified starch for the encapsulation of lycopene. Colloids Surf. A Physicochem. Eng. Asp. 2018, 552, 59–66.
- Zhao, C.; Wei, L.; Yin, B.; Liu, F.; Li, J.; Liu, X.; Wang, J.; Wang, Y. Encapsulation of lycopene within oil-in-water nanoemul-sions using lactoferrin: Impact of carrier oils on physicochemical stability and bioaccessibility. Int. J. Biol. Macromol. 2020, 153, 912–920.
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly (dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiro-ba fruit (Campomanesia xanthocarpa O. Berg) study. LWT 2015, 63, 100–107.
- Bezerra, P.Q.M.; Matos, M.F.R.d.; Ramos, I.G.; Magalhães-Guedes, K.T.; Druzian, J.I.; Costa, J.A.V.; Nunes, I.L. Innovative functional nanodispersion: Combination of carotenoid from Spirulina and yellow passion fruit albedo. Food Chem. 2019, 285, 397–405.
- Yi, J.; Lam, T.I.; Yokoyama, W.; Cheng, L.W.; Zhong, F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocoll. 2015, 43, 31–40.
- Rostamabadi, H.; Sadeghi Mahoonak, A.; Allafchian, A.; Ghorbani, M. Fabrication of β-carotene loaded glucuronoxy-lan-based nanostructures through electrohydrodynamic processing. Int. J. Biol. Macromol. 2019, 139, 773–784.
- Li, W.; Yalcin, M.; Lin, Q.; Ardawi, M.-S.M.; Mousa, S.A. Self-assembly of green tea catechin derivatives in nanoparticles for oral lycopene delivery. J. Control. Release 2017, 248, 117–124.
- Vasconcelos, A.G.; Valim, M.O.; Amorim, A.G.N.; do Amaral, C.P.; de Almeida, M.P.; Borges, T.K.S.; Socodato, R.; Portugal, C.C.; Brand, G.D.; Mattos, J.S.C.; et al. Cytotoxic activity of poly-ɛ-caprolactone lipid-core nanocapsules loaded with lyco-pene-rich extract from red guava (Psidium guajava L.) on breast cancer cells. Food Res. Int. 2020, 136, 109548.
- Dos Santos, P.P.; Paese, K.; Guterres, S.S.; Pohlmann, A.R.; Costa, T.H.; Jablonski, A.; Flôres, S.H.; Rios, A.D.O. Development of lycopene-loaded lipid-core nanocapsules: Physicochemical characterization and stability study. J. Nano Res. 2015, 17, 107.
- Bolla, P.K.; Gote, V.; Singh, M.; Patel, M.; Clark, B.A.; Renukuntla, J. Lutein-loaded, biotin-decorated polymeric nanoparticles enhance lutein uptake in retinal cells. Pharmaceutics 2020, 12, 798.
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of lutein-loaded PVA/sodium alginate nanofibers and investigation of its release behavior. Pharmaceutics 2019, 11, 449.
- Hafezi Ghahestani, Z.; Alebooye Langroodi, F.; Mokhtarzadeh, A.; Ramezani, M.; Hashemi, M. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif. Cells Nanomed. Biotechnol. 2017, 45, 955–960.
- Ravi, H.; Baskaran, V. Biodegradable chitosan-glycolipid hybrid nanogels: A novel approach to encapsulate fucoxanthin for improved stability and bioavailability. Food Hydrocoll. 2015, 43, 717–725.
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784.
- Hassane Hamadou, A.; Huang, W.-C.; Xue, C.; Mao, X. Comparison of β-carotene loaded marine and egg phospholipids nanoliposomes. J. Food Eng. 2020, 283, 110055.
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of astaxanthin-loaded liposomes: Characterization, storage stability and antioxidant activity. CyTA J. Food 2018, 16, 607–618, doi:10.1080/19476337.2018.1437080.
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and struc-ture. Molecules 2018, 23, 2822.
- Jiao, Y.; Li, D.; Liu, C.; Chang, Y.; Song, J.; Xiao, Y.J.R.A. Polypeptide–decorated nanoliposomes as novel delivery systems for lutein. RSC Adv. 2018, 8, 31372–31381.
- Jain, A.; Sharma, G.; Thakur, K.; Raza, K.; Shivhare, U.S.; Ghoshal, G.; Katare, O.P. Beta-carotene-encapsulated solid lipid nanoparticles (BC-SLNs) as promising vehicle for cancer: An investigative assessment. AAPS Pharm. Sci. Tech. 2019, 20, 100.
- Mehrad, B.; Ravanfar, R.; Licker, J.; Regenstein, J.M.; Abbaspourrad, A. Enhancing the physicochemical stability of β-carotene solid lipid nanoparticle (SLNP) using whey protein isolate. Food Res. Int. 2018, 105, 962–969.
- Schjoerring-Thyssen, J.; Olsen, K.; Koehler, K.; Jouenne, E.; Rousseau, D.; Andersen, M.L. Morphology and structure of solid lipid nanoparticles loaded with high concentrations of β-carotene. J. Agric. Food Chem. 2019, 67, 12273–12282.
- Nazemiyeh, E.; Eskandani, M.; Sheikhloie, H.; Nazemiyeh, H. Formulation and physicochemical characterization of lyco-pene-loaded solid lipid nanoparticles. Adv. Pharm. Bull. 2016, 6, 235–241.
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based nanoformulation of lycopene improves oral delivery: Formula-tion optimization, ex vivo assessment and its efficacy against breast cancer. J. Microencap. 2017, 34, 416–429.
- Kaga, K.; Honda, M.; Adachi, T.; Honjo, M.; Kanda, H.; Goto, M. Nanoparticle formation of PVP/astaxanthin inclusion complex by solution-enhanced dispersion by supercritical fluids (SEDS): Effect of PVP and astaxanthin Z-isomer content. J. Supercrit. Fluids 2018, 136, 44–51.
- Tirado, D.F.; Palazzo, I.; Scognamiglio, M.; Calvo, L.; Della Porta, G.; Reverchon, E. Astaxanthin encapsulation in ethyl cel-lulose carriers by continuous supercritical emulsions extraction: A study on particle size, encapsulation efficiency, release pro-file and antioxidant activity. J. Supercrit. Fluids 2019, 150, 128–136.
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of pro-teasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264.
- Huang, R.F.; Wei, Y.J.; Inbaraj, B.S.; Chen, B.H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nano-particles and lycopene. Int. J. Nanomed. 2015, 10, 2823–2846.
- Shabestarian, H.; Homayouni-Tabrizi, M.; Soltani, M.; Namvar, F.; Azizi, S.; Mohamad, R.; Shabestarian, H. Green synthesis of gold nanoparticles using sumac aqueous extract and their antioxidant activity. J. Mat. Res. 2017, 20, 264–270.
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical fluid technology as a green alternative during the preparation of drug delivery systems. Pharmaceutics 2019, 11, 629.
- Sengul, A.B.; Asmatulu, E. Toxicity of metal and metal oxide nanoparticles: A review. Env. Chem. Let. 2020, 18, 1659–1683.
- Seabra, A.B.; Durán, N. Nanotoxicology of metal oxide nanoparticles. Metals 2015, 5, 934–975.
