
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kyeongsoon Park | + 2002 word(s) | 2002 | 2021-05-12 04:53:01 | | | |
| 2 | Vivi Li | + 2002 word(s) | 2002 | 2021-05-12 05:20:50 | | |
Video Upload Options
Calcium carbonate (CaCO3)-based materials have received notable attention for biomedical applications owing to their safety and beneficial characteristics, such as pH sensitivity, carbon dioxide (CO2) gas generation, and antacid properties. Herein, to additionally incorporate antioxidant and anti-inflammatory functions, we prepared tannylated CaCO3 (TA-CaCO3) materials using a simple reaction between tannic acid (TA), calcium (Ca2+), and carbonate (CO32−) ions. TA-CaCO3 synthesized at a molar ratio of 1:75 (TA:calcium chloride (CaCl2)/sodium carbonate (Na2CO3)) showed 3–6 μm particles, comprising small nanoparticles in a size range of 17–41 nm. The TA-CaCO3 materials could efficiently neutralize the acid solution and scavenge free radicals.
1. Introduction
Inorganic minerals have been widely used in drug delivery systems for biomedical applications [1]. Calcium carbonate (CaCO3), an inorganic biomineral, has been used as an antacid agent. It can be orally administered as a tablet, chewable tablet, capsule, or liquid. Furthermore, it has been used for the controlled and sustained delivery of chemical drugs [2][3][4], photosensitizers [5], and proteins [6][7] because of its biocompatibility and slow biodegradation [8]. CaCO3 is stable at physiological pH, but can be dissociated under acidic conditions [9][10]. Owing to their pH sensitivity, CaCO3-based delivery systems concentrate drugs into targeted cancer tissues within the acidic tumor microenvironment (TME) [4][5][10]. In addition, these systems react with protons (H+) to neutralize the acid [11][12]. CaCO3 can generate carbon dioxide (CO2) in the acidic TME, and this gas-generating property has extended its application as an ultrasound contrast agent for cancer imaging [5][10]. These previous studies have revealed the pH sensitivity, CO2 gas generation, and antacid properties of CaCO3 materials.
Polyphenol tannic acid (TA) is composed of five digalloyl ester groups that have been linked to a central glucose molecule, and exhibits various biological properties, such as antibacterial, anti-inflammatory, antioxidant, and anticancer activities [13][14]. Previous studies have revealed the TA-mediated scavenging of free radicals, leading to the inhibition of lipid oxidation and radical-mediated DNA damage [13][15][16]. Given their ability to undergo multiple interactions with various biomacromolecules (i.e., nucleic acids, peptides, proteins, and polysaccharides) through electrostatic and hydrogen bonding and/or hydrophobic interactions [17][18][19][20][21], TA–macromolecular complexes can be easily produced and used as surface modifiers on organic and inorganic substrates [22][23]. Thus, the TA-modified polymeric hydrogels and scaffolds greatly enhance anti-inflammatory effects in vitro or protect cells under reactive oxygen species (ROS) environments [23][24]. Moreover, as TA can coordinate with metal ions, it can be used to synthesize inorganic Ag and Au nanomaterials [25]. Our group recently prepared oxygen-generating calcium peroxide using TA through coordination between the catechol moieties of TA and calcium ions [26].
Based on these previous reports, we propose that the introduction of TA into CaCO3 materials can endow them with anti-inflammatory and antioxidant functions. As CaCO3 materials exert no anti-inflammatory or antioxidant functions, herein we fabricated tannylated CaCO3 (termed as TA-CaCO3) materials via the coordination of TA to calcium (Ca2+) ions and further nucleation of CaCO3 using carbonate ions (CO32−). We performed their physicochemical characterization and evaluated their antacid and antioxidant effects using colorimetric methods. In addition, we validated their in vitro antioxidant and anti-inflammatory properties in chondrocytes under inflammatory and ROS conditions.
2. Preparation of TA-CaCO3 Materials
TA-CaCO3 materials were synthesized by combining equimolar amounts of Ca2+ and CO32− at different concentrations with a fixed molar concentration of TA under constant stirring, as depicted in Figure 1. Given the tendency of TA to coordinate with metal ions [25][27][28], TA interacts with Ca2+ ions and facilitates the nucleation of CaCO3 to form TA-CaCO3 nanoparticles, which then agglomerate into microparticles due to the interactions between TA-CaCO3 nanoparticles.
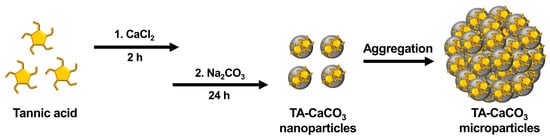
Figure 1. Schematic illustration of the synthesis of TA-CaCO3 materials. TA was sequentially reacted with CaCl2 for 2 h and Na2CO3 for an additional 24 h in pure water (pH = 7.0).
3. Characterization of TA-CaCO3 Materials
The morphologies of the synthesized TA-CaCO3 materials were examined using scanning electron microscopy (SEM). As shown in Figure S1, at 25 molar ratios of calcium chloride (CaCl2)/sodium carbonate (Na2CO3), aggregates comprising small TA-CaCO3 particles were predominantly observed, and very small amounts of micron-sized spherical TA-CaCO3 particles were formed. More spherical TA-CaCO3 microparticles were gradually observed as the molar ratio of CaCl2/Na2CO3 was increased to 75. Interestingly, although spherical TA-CaCO3 microparticles were still observed when the molar ratios of TA and CaCl2/Na2CO3 were 100 or 150, more irregular and broken TA-CaCO3 particles were detected. These results suggested that small TA-CaCO3 particles were formed at lower molar ratios of CaCl2/Na2CO3 and that spherical and broken TA-CaCO3 particles were more common at higher molar ratios of TA and CaCl2/Na2CO3. Based on SEM images, 1:75 TA-CaCO3 particles were selected for further experiments because they produced more spherical TA-CaCO3 microparticles than other TA-CaCO3 particles.
Next, we investigated the particle size of the 1:75 TA-CaCO3 materials and found it to range from approximately 3 to 6 μm (Figure 2a and Figure S1). Interestingly, the magnified SEM images revealed the presence of small particles on the surface of the 1:75 TA-CaCO3 materials. These individual small particles ranged from 17 to 41 nm in size, and were approximately 26.18 ± 4.6 nm in diameter and spherical in shape (Figure 2b). SEM images revealed that the micron-sized TA-CaCO3 materials consisted of small TA-CaCO3 nanoparticles, probably owing to agglomeration following interactions between individual small nanoparticles.
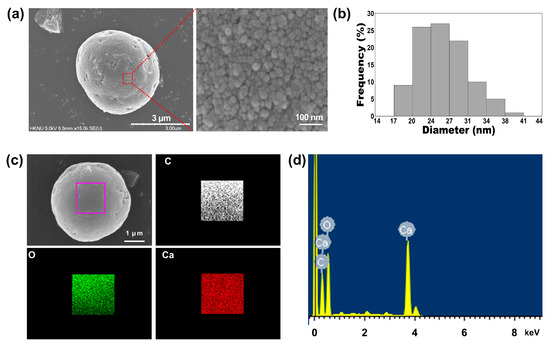
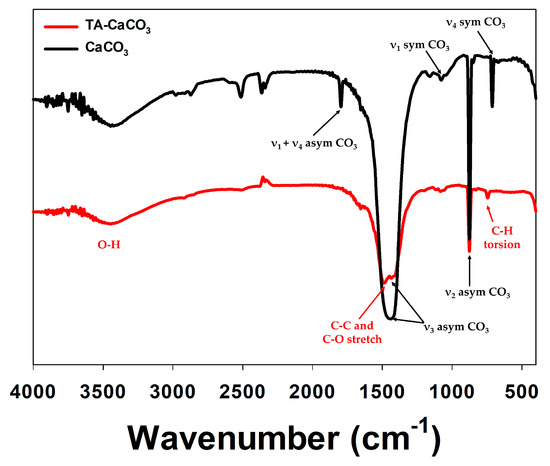
The preparation of TA-CaCO3 materials was confirmed using an SEM coupled with energy-dispersive (SEM-EDS) X-ray spectroscopy, inductively coupled plasma optical emission spectrometry (ICP-OES), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) patterns, and X-ray photoelectron spectroscopy (XPS). The EDS mapping and spectrum data revealed the presence of carbon, oxygen, and calcium on the surface of TA-CaCO3 materials (Figure 2c,d). Consistent with a previous report [29], the EDS spectrum showed the peaks of Kα (0.277 eV) corresponding to carbon, Kα (0.523 eV) corresponding to oxygen, and Kα (3.691 eV) and Lα (0.341 eV) corresponding to calcium. ICP-OES analysis showed that 0.1 mg of 1:75 TA-CaCO3 contained 18.6 μg of TA and 81.4 μg of CaCO3. The FT-IR spectrum of commercial CaCO3 showed the characteristic vibrations of carbonate ions (at 1805, 1410, 1090, and 874 cm−1) (Figure 3), as previously reported [30][31]. The preparation of TA-CaCO3 materials was confirmed by the presence of the main asymmetric vibrations at 1460 and 1410 cm−1. However, the symmetric vibration at 725 cm−1 disappeared, implying that TA-CaCO3 has an amorphous structure. Meanwhile, TA-CaCO3 showed characteristic peaks at 3300–3600 cm−1 (O-H stretching), 1445 cm−1 (C-C stretching of benzene ring and methylene; C-O stretching of phenolic), and 755 cm−1 (C-H torsion of benzene ring) [32], indicative of the presence of TA on the material.
Next, commercial CaCO3 and synthesized TA-CaCO3 crystal phases were identified via XRD analysis (Figure 4a). CaCO3 exhibited the characteristic peaks, such as the plane of the calcite at 29.3° (104), and the calcite crystal faces at 23.02° (012), 35.9° (110), 39.4° (113), and 43.1° (202), respectively. Meanwhile, the diffraction peaks of TA-CaCO3 were observed at 24.8° (110), 27.08° (112), 32.7° (114), 43.8° (300), 49.1° (304), and 50.08° (118), respectively, and these diffraction peaks were consistent with the vaterite crystal faces [33][34]. Based on XRD data, we think that the prepared TA-CaCO3 materials are the vaterite form of calcium carbonate.
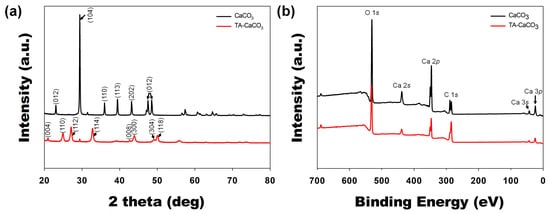
XPS data revealed that CaCO3 and TA-CaCO3 showed Ca, O, and C signals (Figure 4b). The binding energy peaks of these two materials appeared O1s at 531 eV, Ca2s at 441 eV, two Ca2p at 351 and 347.2 eV, and two C1s peaks at 289.3 eV (CO3 in the CaCO3 surface) and 284.6 eV (adventitious carbon peak), respectively. These data demonstrated the successful synthesis of TA-CaCO3, and the synthesized TA-CaCO3 materials are vaterite calcium carbonate.
4. Antacid Effects of TA-CaCO3
CaCO3 exists as a stable crystalline solid at physiological pH, but can be dissociated into ionic species at or below weakly acidic pH [5][9]. Under acidic pH, CaCO3 neutralizes acids by reacting with the proton (H+) [11][12], and it has been used as an acid neutralizer [35].
To verify the antacid effects of TA-CaCO3 materials, commercial CaCO3 and TA-CaCO3 were dispersed in phosphate-buffered saline (PBS; physiological pH = 7.4) and simulated gastric fluid (SGF, pH 1.5) containing bromothymol blue (BTB). The color and absorption changes of BTB were monitored before and after the reaction, because BTB is a useful acid/base indicator to distinguish the acidity, neutrality, and alkalinity of an aqueous solution [36][37]. As shown in Figure 5a and Figure S2, the aqueous BTB solution without commercial CaCO3 and TA-CaCO3 exhibited a blue color at pH = 7.4 and turned to a yellowish color in the presence of SGF (pH = 1.5). CaCO3 and TA-CaCO3 showed a deep blue color of BTB at pH = 7.4, indicating the slight pH increases of the solution following the degradation of CaCO3 and TA-CaCO3, even at pH = 7.4. In contrast, the yellowish BTB solution at pH = 1.5 turned to a bluish green color after the reaction with CaCO3 and TA-CaCO3, indicating that the pH of the solution increased to a nearly neutral pH (approximately 7). Consistent with these color changes, the λmax shift of BTB occurred from 615 nm at pH = 7.4 to 433 nm under SGF (pH = 1.5) (Figure 5b). However, the absorptions of both CaCO3 and TA-CaCO3 groups increased at 615 nm, while the λmax of BTB at pH = 1.5 was blue-shifted from 433 nm to 403 nm, implying the pH increases of the solutions after reacting with two kinds of CaCO3 materials. Meanwhile, TA-CaCO3 showed significantly higher absorbance below 400 nm at both pH = 1.5 and pH = 7.4, indicating the presence of TA in the solutions. Furthermore, TA-CaCO3 had a lower absorbance than commercial CaCO3 at 615 nm. This is attributed to the fact that TA-CaCO3 contained a lower amount of CaCO3 than commercial CaCO3.
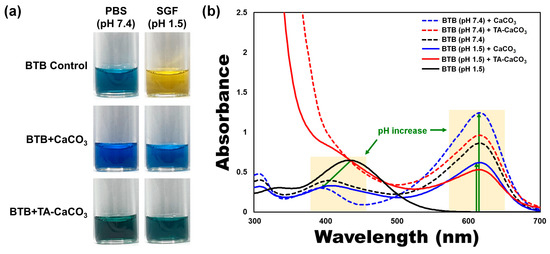
Figure 5. Antacid effects of TA-CaCO3 using the colorimetric bromothymol blue (BTB) method. (a) Color changes and (b) UV/vis spectra of BTB under PBS (pH = 7.4) and simulated gastric fluid (SGF, pH = 1.5) after treatment with commercial CaCO3 and 1:75 TA-CaCO3. Green-colored arrows indicate a pH increase.
5. Antioxidant Effects of TA-CaCO3
The antioxidant effects of TA-CaCO3 were determined using the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [38]. As shown in Figure 6, the DPPH solution showed a deep violet color, with an absorbance at approximately 520 nm, owing to the delocalization of the spare electron over the molecule [38]. CaCO3-treated DPPH solution also had a deep violet color and an absorption band at around 520 nm, indicating that CaCO3 had no antioxidant effect. In contrast, TA-CaCO3-treated DPPH solution turned yellowish in color and lost its absorbance at 520 nm. This loss of violet color might be attributed to the presence of TA molecules within TA-CaCO3 because TA molecules have an effective radical-scavenging activity, as its hydroxyl groups easily reduce the free radicals of DPPH [39][40]. These data imply that TA-CaCO3 materials have antioxidant properties and effective ROS-scavenging activity.
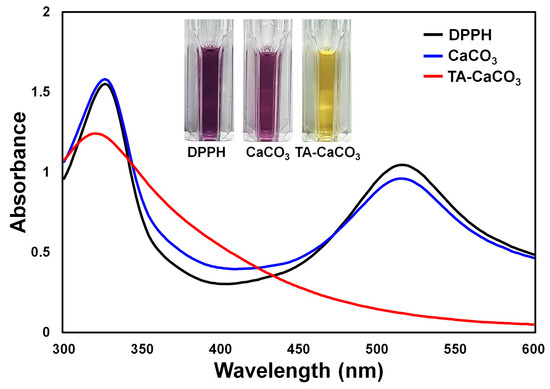
Figure 6. Antioxidant effects of TA-CaCO3 determined using the colorimetric DPPH method. Changes in the color and UV/vis spectra of DPPH after treatment with commercial CaCO3 and 1:75 TA-CaCO3. Inset: Photos of the DPPH solution treated with commercial CaCO3 and 1:75 TA-CaCO3.
6. Conclusions
In the present study, we prepared TA-CaCO3 materials by reacting TA with CaCl2 and Na2CO3, which led to the interaction between TA and Ca2+ ions, followed by nucleation of CaCO3. Micron-sized 1:75 TA-CaCO3 materials (ranging from 3 to 6 μm) comprised small nanoparticles in a size range of 17–41 nm. TA-CaCO3 materials could effectively neutralize the SGF solution and scavenge free radicals. In addition, these particles significantly suppressed the mRNA expression of pro-inflammatory cytokines and mediators and scavenged intracellular ROS in cells. Their anti-inflammatory and antioxidant activities protected chondrocytes from ROS. These results suggest that TA-CaCO3 materials have excellent antacid, antioxidant, and anti-inflammatory properties. Importantly, TA molecules can undergo multiple interactions with nucleic acids, peptides, proteins, and polysaccharides. Furthermore, due to the molecular adsorption of CaCO3 materials, CaCO3-based materials can improve the incorporation efficacy of drugs. Thus, using TA-CaCO3 materials, we will develop dual drug delivery systems that can ferry both a chemical drug and protein drug, and then apply them to treat inflammatory cells or diseases.
References
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374.
- Wei, W.; Ma, G.H.; Hu, G.; Yu, D.; McLeish, T.; Su, Z.G.; Shen, Z.Y. Preparation of hierarchical hollow CaCO3 particles and the application as anticancer drug carrier. J. Am. Chem. Soc. 2008, 130, 15808–15810.
- Peng, C.; Zhao, Q.; Gao, C. Sustained delivery of doxorubicin by porous CaCO3 and chitosan/alginate multilayers-coated CaCO3 microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 132–139.
- Zhu, Y.; Yang, Z.; Dong, Z.; Gong, Y.; Hao, Y.; Tian, L.; Yang, X.; Liu, Z.; Feng, L. CaCO3-Assisted Preparation of pH-Responsive Immune-Modulating Nanoparticles for Augmented Chemo-Immunotherapy. Nano-Micro Lett. 2021, 13, 1–18.
- Park, D.J.; Min, K.H.; Lee, H.J.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Lee, S.C. Photosensitizer-loaded bubble-generating mineralized nanoparticles for ultrasound imaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 1219–1227.
- Koo, A.N.; Min, K.H.; Lee, H.J.; Jegal, J.H.; Lee, J.W.; Lee, S.C. Calcium Carbonate Mineralized Nanoparticles as an Intracellular Transporter of Cytochrome c for Cancer Therapy. Chem. Asian J. 2015, 10, 2380–2387.
- Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 2018, 122, 96–103.
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874.
- Ogomi, D.; Serizawa, T.; Akashi, M. Controlled release based on the dissolution of a calcium carbonate layer deposited on hydrogels. J. Control. Release 2005, 103, 315–323.
- Min, K.H.; Min, H.S.; Lee, H.J.; Park, D.J.; Yhee, J.Y.; Kim, K.; Kwon, I.C.; Jeong, S.Y.; Silvestre, O.F.; Chen, X.; et al. pH-controlled gas-generating mineralized nanoparticles: A theranostic agent for ultrasound imaging and therapy of cancers. ACS Nano 2015, 9, 134–145.
- Rodriguez-Stanley, S.; Ahmed, T.; Zubaidi, S.; Riley, S.; Akbarali, H.I.; Mellow, M.H.; Miner, P.B. Calcium carbonate antacids alter esophageal motility in heartburn sufferers. Dig. Dis. Sci. 2004, 49, 1862–1867.
- Raliya, R.; Som, A.; Shetty, N.; Reed, N.; Achilefu, S.; Biswas, P. Nano-antacids enhance pH neutralization beyond their bulk counterparts: Synthesis and characterization. RSC Adv. 2016, 6, 54331–54335.
- Labieniec, M.; Gabryelak, T. Oxidatively modified proteins and DNA in digestive gland cells of the fresh-water mussel Unio tumidus in the presence of tannic acid and its derivatives. Mutat. Res. Toxicol. Environ. Mutagen. 2006, 603, 48–55.
- Turgut Cosan, D.; Saydam, F.; Ozbayer, C.; Doganer, F.; Soyocak, A.; Gunes, H.V.; Degirmenci, I.; Kurt, H.; Ustuner, M.C.; Bal, C. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in L-NNA-induced hypertensive rats. Cytotechnology 2015, 67, 97–105.
- Lopes, G.K.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta Gen. Subj. 1999, 1472, 142–152.
- Andrade, R.G., Jr.; Dalvi, L.T.; Silva, J.M., Jr.; Lopes, G.K.; Alonso, A.; Hermes-Lima, M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch. Biochem. Biophys. 2005, 437, 1–9.
- Shukla, A.; Fang, J.C.; Puranam, S.; Jensen, F.R.; Hammond, P.T. Hemostatic multilayer coatings. Adv. Mater. 2012, 24, 492–496.
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278.
- Abouelmagd, S.A.; Meng, F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303.
- Sahiner, N.; Sagbas, S.; Aktas, N.; Silan, C. Inherently antioxidant and antimicrobial tannic acid release from poly(tannic acid) nanoparticles with controllable degradability. Colloids Surf. B Biointerfaces 2016, 142, 334–343.
- Shin, M.; Lee, H.A.; Lee, M.; Shin, Y.; Song, J.J.; Kang, S.W.; Nam, D.H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317.
- Hong, S.; Yeom, J.; Song, I.T.; Kang, S.M.; Lee, H.; Lee, H. Pyrogallol 2-Aminoethane: A Plant Flavonoid-Inspired Molecule for Material-Independent Surface Chemistry. Adv. Mater. Interfaces 2014, 1, 1400113.
- Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. Int. J. Mol. Sci. 2018, 19, 3602.
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521.
- Aromal, S.A.; Philip, D. Facile one-pot synthesis of gold nanoparticles using tannic acid and its application in catalysis. Phys. E Low Dimens. Syst. Nanostructures 2012, 44, 1692–1696.
- Park, J.S.; Song, Y.J.; Lim, Y.G.; Park, K. Facile Fabrication of Oxygen-Releasing Tannylated Calcium Peroxide Nanoparticles. Materials 2020, 13, 3864.
- Lopes, L.C.S.; Brito, L.M.; Bezerra, T.T.; Gomes, K.N.; Carvalho, F.A.A.; Chaves, M.H.; Cantanhede, W. Silver and gold nanoparticles from tannic acid: Synthesis, characterization and evaluation of antileishmanial and cytotoxic activities. An. Acad. Bras. Cienc. 2018, 90, 2679–2689.
- Abulateefeh, S.R.; Taha, M.O. Enhanced drug encapsulation and extended release profiles of calcium-alginate nanoparticles by using tannic acid as a bridging cross-linking agent. J. Microencapsul. 2015, 32, 96–105.
- Gokhe, U.B.; Koparkar, K.A.; Omanwar, S.K. Synthesis and fluorescence properties of Ca2SiO4:Dy3+ phosphor for solid state lighting application. J. Mater. Sci. Mater. Electron. 2016, 27, 9286–9290.
- Andersen, F.A.; Brečević, L. Infrared spectra of amorphous and crystalline calcium carbonate. Acta Chem. Scand. 1991, 45, 1018–1024.
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271.
- Cakar, S.; Ozacar, M. Fe-tannic acid complex dye as photo sensitizer for different morphological ZnO based DSSCs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 163, 79–88.
- Wojtas, M.; Wołcyrz, M.; Ożyhar, A.; Dobryszycki, P. Phosphorylation of Intrinsically Disordered Starmaker Protein Increases Its Ability To Control the Formation of Calcium Carbonate Crystals. Cryst. Growth Des. 2012, 12, 158–168.
- Dong, W.; Tu, C.; Tao, W.; Zhou, Y.; Tong, G.; Zheng, Y.; Li, Y.; Yan, D. Influence of the Mole Ratio of the Interacting to the Stabilizing Portion (RI/S) in Hyperbranched Polymers on CaCO3 Crystallization: Synthesis of Highly Monodisperse Microspheres. Cryst. Growth Des. 2012, 12, 4053–4059.
- Torne, S.; Sheela, A.; Sarada, N.C. Investigation of the Role of the Alkalizing Agent in Sodium Alginate Liquid Anti-reflux Suspension. Curr. Drug Ther. 2020, 15, 53–60.
- Puschett, J.B.; Rao, B.S.; Karandikar, B.M.; Matyjaszewski, K. Indicator characteristics of bromothymol blue derivatives. Talanta 1991, 38, 335–338.
- De Meyer, T.; Hemelsoet, K.; Van der Schueren, L.; Pauwels, E.; De Clerck, K.; Van Speybroeck, V. Investigating the halochromic properties of azo dyes in an aqueous environment by using a combined experimental and theoretical approach. Chem. Eur. J. 2012, 18, 8120–8129.
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422.
- Cook, N.C.; Samman, S. Flavonoids—Chemistry, metabolism, cardioprotective effects, and dietary sources. J. Nutr. Biochem. 1996, 7, 66–76.
- Ozcelik, B.; Lee, J.H.; Min, D.B. Effects of Light, Oxygen, and pH on the Absorbance of 2,2-Diphenyl-1-picrylhydrazyl. J. Food Sci. 2003, 68, 487–490.




