Calcium carbonate (CaCO3)-based materials have received notable attention for biomedical applications owing to their safety and beneficial characteristics, such as pH sensitivity, carbon dioxide (CO2) gas generation, and antacid properties. Herein, to additionally incorporate antioxidant and anti-inflammatory functions, we prepared tannylated CaCO3 (TA-CaCO3) materials using a simple reaction between tannic acid (TA), calcium (Ca2+), and carbonate (CO32−) ions. TA-CaCO3 synthesized at a molar ratio of 1:75 (TA:calcium chloride (CaCl2)/sodium carbonate (Na2CO3)) showed 3–6 μm particles, comprising small nanoparticles in a size range of 17–41 nm. The TA-CaCO3 materials could efficiently neutralize the acid solution and scavenge free radicals.
- calcium carbonate (CaCO3)
- tannic acid (TA)
- antacid
- anti-inflammation
- ROS scavenging activity
- chondrocytes
1. Introduction
Inorganic minerals have been widely used in drug delivery systems for biomedical applications [1]. Calcium carbonate (CaCO
3), an inorganic biomineral, has been used as an antacid agent. It can be orally administered as a tablet, chewable tablet, capsule, or liquid. Furthermore, it has been used for the controlled and sustained delivery of chemical drugs [2][3][4], photosensitizers [5], and proteins [6][7] because of its biocompatibility and slow biodegradation [8]. CaCO
), an inorganic biomineral, has been used as an antacid agent. It can be orally administered as a tablet, chewable tablet, capsule, or liquid. Furthermore, it has been used for the controlled and sustained delivery of chemical drugs [2,3,4], photosensitizers [5], and proteins [6,7] because of its biocompatibility and slow biodegradation [8]. CaCO
3 is stable at physiological pH, but can be dissociated under acidic conditions [9][10]. Owing to their pH sensitivity, CaCO
is stable at physiological pH, but can be dissociated under acidic conditions [9,10]. Owing to their pH sensitivity, CaCO
3-based delivery systems concentrate drugs into targeted cancer tissues within the acidic tumor microenvironment (TME) [4][5][10]. In addition, these systems react with protons (H
-based delivery systems concentrate drugs into targeted cancer tissues within the acidic tumor microenvironment (TME) [4,5,10]. In addition, these systems react with protons (H
+) to neutralize the acid [11][12]. CaCO
) to neutralize the acid [11,12]. CaCO
3
can generate carbon dioxide (CO
2) in the acidic TME, and this gas-generating property has extended its application as an ultrasound contrast agent for cancer imaging [5][10]. These previous studies have revealed the pH sensitivity, CO
) in the acidic TME, and this gas-generating property has extended its application as an ultrasound contrast agent for cancer imaging [5,10]. These previous studies have revealed the pH sensitivity, CO
2
gas generation, and antacid properties of CaCO
3
materials.
Polyphenol tannic acid (TA) is composed of five digalloyl ester groups that have been linked to a central glucose molecule, and exhibits various biological properties, such as antibacterial, anti-inflammatory, antioxidant, and anticancer activities [13][14]. Previous studies have revealed the TA-mediated scavenging of free radicals, leading to the inhibition of lipid oxidation and radical-mediated DNA damage [13][15][16]. Given their ability to undergo multiple interactions with various biomacromolecules (i.e., nucleic acids, peptides, proteins, and polysaccharides) through electrostatic and hydrogen bonding and/or hydrophobic interactions [17][18][19][20][21], TA–macromolecular complexes can be easily produced and used as surface modifiers on organic and inorganic substrates [22][23]. Thus, the TA-modified polymeric hydrogels and scaffolds greatly enhance anti-inflammatory effects in vitro or protect cells under reactive oxygen species (ROS) environments [23][24]. Moreover, as TA can coordinate with metal ions, it can be used to synthesize inorganic Ag and Au nanomaterials [25]. Our group recently prepared oxygen-generating calcium peroxide using TA through coordination between the catechol moieties of TA and calcium ions [26].
Polyphenol tannic acid (TA) is composed of five digalloyl ester groups that have been linked to a central glucose molecule, and exhibits various biological properties, such as antibacterial, anti-inflammatory, antioxidant, and anticancer activities [13,14]. Previous studies have revealed the TA-mediated scavenging of free radicals, leading to the inhibition of lipid oxidation and radical-mediated DNA damage [13,15,16]. Given their ability to undergo multiple interactions with various biomacromolecules (i.e., nucleic acids, peptides, proteins, and polysaccharides) through electrostatic and hydrogen bonding and/or hydrophobic interactions [17,18,19,20,21], TA–macromolecular complexes can be easily produced and used as surface modifiers on organic and inorganic substrates [22,23]. Thus, the TA-modified polymeric hydrogels and scaffolds greatly enhance anti-inflammatory effects in vitro or protect cells under reactive oxygen species (ROS) environments [23,24]. Moreover, as TA can coordinate with metal ions, it can be used to synthesize inorganic Ag and Au nanomaterials [25]. Our group recently prepared oxygen-generating calcium peroxide using TA through coordination between the catechol moieties of TA and calcium ions [26].
Based on these previous reports, we propose that the introduction of TA into CaCO
3
materials can endow them with anti-inflammatory and antioxidant functions. As CaCO
3
materials exert no anti-inflammatory or antioxidant functions, herein we fabricated tannylated CaCO
3
(termed as TA-CaCO
3
) materials via the coordination of TA to calcium (Ca
2+
) ions and further nucleation of CaCO
3
using carbonate ions (CO
32−
). We performed their physicochemical characterization and evaluated their antacid and antioxidant effects using colorimetric methods. In addition, we validated their in vitro antioxidant and anti-inflammatory properties in chondrocytes under inflammatory and ROS conditions.
2. Preparation of TA-CaCO
3
Materials
TA-CaCO3
materials were synthesized by combining equimolar amounts of Ca2+
and CO32−
at different concentrations with a fixed molar concentration of TA under constant stirring, as depicted inFigure 1. Given the tendency of TA to coordinate with metal ions [25][27][28], TA interacts with Ca
. Given the tendency of TA to coordinate with metal ions [25,27,28], TA interacts with Ca2+
ions and facilitates the nucleation of CaCO3
to form TA-CaCO3
nanoparticles, which then agglomerate into microparticles due to the interactions between TA-CaCO3
nanoparticles.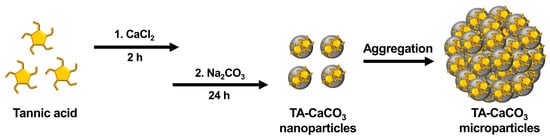

Figure 1.
3
2
2
3
3. Characterization of TA-CaCO
3
Materials
The morphologies of the synthesized TA-CaCO3
materials were examined using scanning electron microscopy (SEM). As shown in , at 25 molar ratios of calcium chloride (CaCl2
)/sodium carbonate (Na2
CO3
), aggregates comprising small TA-CaCO3
particles were predominantly observed, and very small amounts of micron-sized spherical TA-CaCO3
particles were formed. More spherical TA-CaCO3
microparticles were gradually observed as the molar ratio of CaCl2
/Na2
CO3
was increased to 75. Interestingly, although spherical TA-CaCO3
microparticles were still observed when the molar ratios of TA and CaCl2
/Na2
CO3
were 100 or 150, more irregular and broken TA-CaCO3
particles were detected. These results suggested that small TA-CaCO3
particles were formed at lower molar ratios of CaCl2
/Na2
CO3
and that spherical and broken TA-CaCO3
particles were more common at higher molar ratios of TA and CaCl2
/Na2
CO3
. Based on SEM images, 1:75 TA-CaCO3
particles were selected for further experiments because they produced more spherical TA-CaCO3
microparticles than other TA-CaCO3
particles. Next, we investigated the particle size of the 1:75 TA-CaCO3
materials and found it to range from approximately 3 to 6 μm (a and ). Interestingly, the magnified SEM images revealed the presence of small particles on the surface of the 1:75 TA-CaCO3
materials. These individual small particles ranged from 17 to 41 nm in size, and were approximately 26.18 ± 4.6 nm in diameter and spherical in shape (b). SEM images revealed that the micron-sized TA-CaCO3
materials consisted of small TA-CaCO3
nanoparticles, probably owing to agglomeration following interactions between individual small nanoparticles.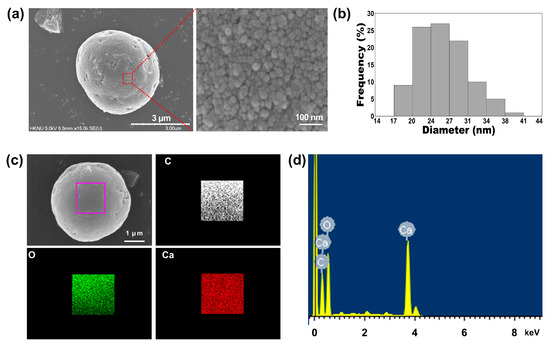
The preparation of TA-CaCO
The preparation of TA-CaCO3
materials was confirmed using an SEM coupled with energy-dispersive (SEM-EDS) X-ray spectroscopy, inductively coupled plasma optical emission spectrometry (ICP-OES), Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD) patterns, and X-ray photoelectron spectroscopy (XPS). The EDS mapping and spectrum data revealed the presence of carbon, oxygen, and calcium on the surface of TA-CaCO3
materials (c,d). Consistent with a previous report [29], the EDS spectrum showed the peaks of Kα (0.277 eV) corresponding to carbon, Kα (0.523 eV) corresponding to oxygen, and Kα (3.691 eV) and Lα (0.341 eV) corresponding to calcium. ICP-OES analysis showed that 0.1 mg of 1:75 TA-CaCO3
contained 18.6 μg of TA and 81.4 μg of CaCO3
. The FT-IR spectrum of commercial CaCO3
showed the characteristic vibrations of carbonate ions (at 1805, 1410, 1090, and 874 cm−1
) (Figure 3), as previously reported [30][31]. The preparation of TA-CaCO
), as previously reported [30,31]. The preparation of TA-CaCO3
materials was confirmed by the presence of the main asymmetric vibrations at 1460 and 1410 cm−1
. However, the symmetric vibration at 725 cm−1
disappeared, implying that TA-CaCO3
has an amorphous structure. Meanwhile, TA-CaCO3
showed characteristic peaks at 3300–3600 cm−1
(O-H stretching), 1445 cm−1
(C-C stretching of benzene ring and methylene; C-O stretching of phenolic), and 755 cm−1
(C-H torsion of benzene ring) [32], indicative of the presence of TA on the material.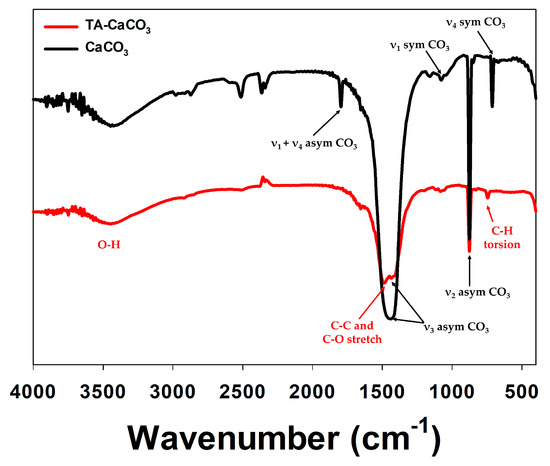
Next, commercial CaCO
Next, commercial CaCO3
and synthesized TA-CaCO3
crystal phases were identified via XRD analysis (a). CaCO3
exhibited the characteristic peaks, such as the plane of the calcite at 29.3° (104), and the calcite crystal faces at 23.02° (012), 35.9° (110), 39.4° (113), and 43.1° (202), respectively. Meanwhile, the diffraction peaks of TA-CaCO3 were observed at 24.8° (110), 27.08° (112), 32.7° (114), 43.8° (300), 49.1° (304), and 50.08° (118), respectively, and these diffraction peaks were consistent with the vaterite crystal faces [33][34]. Based on XRD data, we think that the prepared TA-CaCO
were observed at 24.8° (110), 27.08° (112), 32.7° (114), 43.8° (300), 49.1° (304), and 50.08° (118), respectively, and these diffraction peaks were consistent with the vaterite crystal faces [33,34]. Based on XRD data, we think that the prepared TA-CaCO3
materials are the vaterite form of calcium carbonate.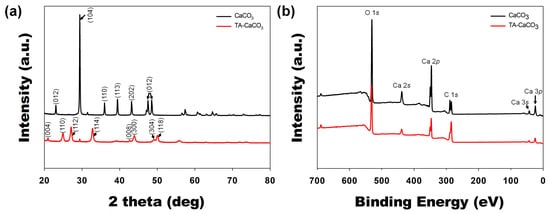
XPS data revealed that CaCO
XPS data revealed that CaCO3
and TA-CaCO3
showed Ca, O, and C signals (b). The binding energy peaks of these two materials appeared O1s at 531 eV, Ca2s at 441 eV, two Ca2p at 351 and 347.2 eV, and two C1s peaks at 289.3 eV (CO3
in the CaCO3
surface) and 284.6 eV (adventitious carbon peak), respectively. These data demonstrated the successful synthesis of TA-CaCO3
, and the synthesized TA-CaCO3
materials are vaterite calcium carbonate.4. Antacid Effects of TA-CaCO
3
CaCO3 exists as a stable crystalline solid at physiological pH, but can be dissociated into ionic species at or below weakly acidic pH [5][9]. Under acidic pH, CaCO
exists as a stable crystalline solid at physiological pH, but can be dissociated into ionic species at or below weakly acidic pH [5,9]. Under acidic pH, CaCO3
neutralizes acids by reacting with the proton (H+) [11][12], and it has been used as an acid neutralizer [35].
) [11,12], and it has been used as an acid neutralizer [35]. To verify the antacid effects of TA-CaCO3
materials, commercial CaCO3
and TA-CaCO3 were dispersed in phosphate-buffered saline (PBS; physiological pH = 7.4) and simulated gastric fluid (SGF, pH 1.5) containing bromothymol blue (BTB). The color and absorption changes of BTB were monitored before and after the reaction, because BTB is a useful acid/base indicator to distinguish the acidity, neutrality, and alkalinity of an aqueous solution [36][37]. As shown in
were dispersed in phosphate-buffered saline (PBS; physiological pH = 7.4) and simulated gastric fluid (SGF, pH 1.5) containing bromothymol blue (BTB). The color and absorption changes of BTB were monitored before and after the reaction, because BTB is a useful acid/base indicator to distinguish the acidity, neutrality, and alkalinity of an aqueous solution [36,37]. As shown in a and , the aqueous BTB solution without commercial CaCO3
and TA-CaCO3
exhibited a blue color at pH = 7.4 and turned to a yellowish color in the presence of SGF (pH = 1.5). CaCO3
and TA-CaCO3
showed a deep blue color of BTB at pH = 7.4, indicating the slight pH increases of the solution following the degradation of CaCO3
and TA-CaCO3
, even at pH = 7.4. In contrast, the yellowish BTB solution at pH = 1.5 turned to a bluish green color after the reaction with CaCO3
and TA-CaCO3
, indicating that the pH of the solution increased to a nearly neutral pH (approximately 7). Consistent with these color changes, the λmax
shift of BTB occurred from 615 nm at pH = 7.4 to 433 nm under SGF (pH = 1.5) (b). However, the absorptions of both CaCO3
and TA-CaCO3
groups increased at 615 nm, while the λmax
of BTB at pH = 1.5 was blue-shifted from 433 nm to 403 nm, implying the pH increases of the solutions after reacting with two kinds of CaCO3
materials. Meanwhile, TA-CaCO3
showed significantly higher absorbance below 400 nm at both pH = 1.5 and pH = 7.4, indicating the presence of TA in the solutions. Furthermore, TA-CaCO3
had a lower absorbance than commercial CaCO3
at 615 nm. This is attributed to the fact that TA-CaCO3
contained a lower amount of CaCO3
than commercial CaCO3
.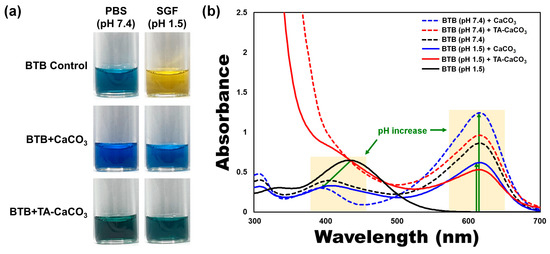

Figure 5.
3
a
b
3
3
5. Antioxidant Effects of TA-CaCO
3
The antioxidant effects of TA-CaCO3
were determined using the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) [38]. As shown in , the DPPH solution showed a deep violet color, with an absorbance at approximately 520 nm, owing to the delocalization of the spare electron over the molecule [38]. CaCO3
-treated DPPH solution also had a deep violet color and an absorption band at around 520 nm, indicating that CaCO3
had no antioxidant effect. In contrast, TA-CaCO3
-treated DPPH solution turned yellowish in color and lost its absorbance at 520 nm. This loss of violet color might be attributed to the presence of TA molecules within TA-CaCO3 because TA molecules have an effective radical-scavenging activity, as its hydroxyl groups easily reduce the free radicals of DPPH [39][40]. These data imply that TA-CaCO
because TA molecules have an effective radical-scavenging activity, as its hydroxyl groups easily reduce the free radicals of DPPH [39,40]. These data imply that TA-CaCO3
materials have antioxidant properties and effective ROS-scavenging activity.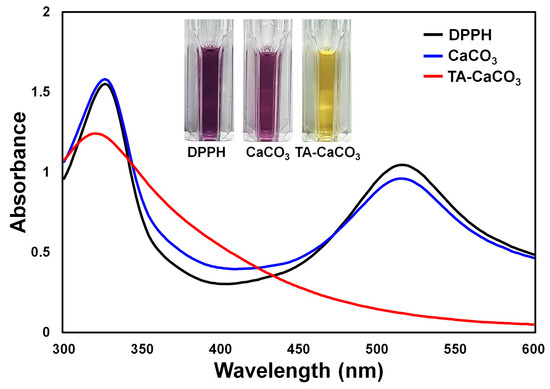

Figure 6.
3
3
3
3
3
6. Conclusions
In the present study, we prepared TA-CaCO
3
materials by reacting TA with CaCl
2
and Na
2
CO
3
, which led to the interaction between TA and Ca
2+
ions, followed by nucleation of CaCO
3
. Micron-sized 1:75 TA-CaCO
3
materials (ranging from 3 to 6 μm) comprised small nanoparticles in a size range of 17–41 nm. TA-CaCO
3
materials could effectively neutralize the SGF solution and scavenge free radicals. In addition, these particles significantly suppressed the mRNA expression of pro-inflammatory cytokines and mediators and scavenged intracellular ROS in cells. Their anti-inflammatory and antioxidant activities protected chondrocytes from ROS. These results suggest that TA-CaCO
3
materials have excellent antacid, antioxidant, and anti-inflammatory properties. Importantly, TA molecules can undergo multiple interactions with nucleic acids, peptides, proteins, and polysaccharides. Furthermore, due to the molecular adsorption of CaCO
3
materials, CaCO
3
-based materials can improve the incorporation efficacy of drugs. Thus, using TA-CaCO
3
materials, we will develop dual drug delivery systems that can ferry both a chemical drug and protein drug, and then apply them to treat inflammatory cells or diseases.
