
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wiktor Zierkiewicz | + 860 word(s) | 860 | 2021-04-25 11:06:23 | | | |
| 2 | Catherine Yang | + 4 word(s) | 864 | 2021-05-11 10:27:11 | | |
Video Upload Options
σ-hole is a region of depleted electronic density located on the outer side of a covalently-bonded atom, situated roughly along an extension of the bond axis
1. Introduction
The concept of the σ-hole, introduced to a wide audience in 2005 at a conference in Prague by Tim Clark [1], influenced a way of thinking about noncovalent interactions that prevails to this day. Early experimental findings [2][3][4][5] of unusual halogen·halogen contacts were explained in part by the anisotropic distribution of electronic density around a halogen atom when linked to an electron-withdrawing group. Already in 1992, it had been learned that electronegative atoms from Groups 14–17 have regions of positive molecular electrostatic potential (MEP) on their outer surfaces, along an extension of a covalent bond, which may attract an incoming Lewis base [6]. This observation led to the further computational studies which resulted in formulation of the σ-hole idea [7] which was further generalized in later works [7][8][9][10][11][12][13][14][15][16][17]. These ideas concerning the halogen bond were successfully adapted to atoms from other families of the periodic table, which were later grouped into the category of σ-hole bonds. This general sort of noncovalent bond has been subdivided by the specific family from which the bridging atom is drawn, i.e., chalcogen [18][19][20][21][22][23], pnicogen (pnictogen) [24][25][26][27][28], or tetrel bonds [29][30][31]. The former, along with the halogen bond, has been formally recognized and detailed in recent IUPAC recommendations [32][33].
Quite the opposite from representing exotic or unusual contacts, these bonds make important contributions to numerous fields of chemistry and biology. As examples, understanding the forces behind crystal engineering and supramolecular chemistry benefits from a knowledge of σ-hole interactions due to their directionality, strength, and self-organization properties which promote formation of adducts in the solid state [34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52]. The importance of σ-hole bonding has also been verified in the context of anion recognition processes [53][54][55][56][57][58][59][60][61], materials chemistry [62][63][64][65][66][67][68][69][70][71][72], or biochemistry [73][74][75][76][77][78][79][80][81]. An early work connecting σ-hole bonds with crucial concepts in chemistry occurred when Grabowski recognized that tetrel bonds can be thought of as a preliminary stage of the very important SN2 reaction [82].
As ideas concerning the σ-hole were proliferating, it was recognized that density depletion is not necessarily limited only to the extensions of covalent bonds. Depletions can also occur above planar groups as well, as for example above a carbonyl or phenyl group. Linear systems such as HCN can also suffer from low density off the molecular axis. Because of their location and association with π-electronic systems, these regions of density depletion and positive electrostatic potential have come to be called π-holes. As one specific example, tricoordinated triel atoms typically occur at the center of a planar triangle, with a π-hole located above the central triel atom [13][83][84][85][86][87], and the resulting triel bond [88][89][90][91][92][93][94][95] falls into the category of a π-hole bond. The π-hole situated above the C atom of a carbonyl group offers another common example, whose presence was manifested in early work of Burgi and Dunitz [96]. Protein structures can also involve participation of π-hole bonds [97][98], as is also true of self-assembling systems [99]. As work has progressed, there has been recognition of π-holes as providing a means by which an aerogen bond (involving rare gas atoms) can form [100][101][102][103][104]. Other newly introduced types of σ/π- hole directed interactions are alkali and alkaline earth bond (e.g., beryllium bond, magnesium bond) in which atoms of 1st and 2nd groups contribute [105][106][107][108][109] or regium and spodium bonds which employ transition metals from 11th (regium [110][111][112][113][114][115]) or 12th (spodium [105][116][117][118][119]) groups of the periodic table. The full range of these sorts of bonds, along with their designations, is summarized in Figure 1.
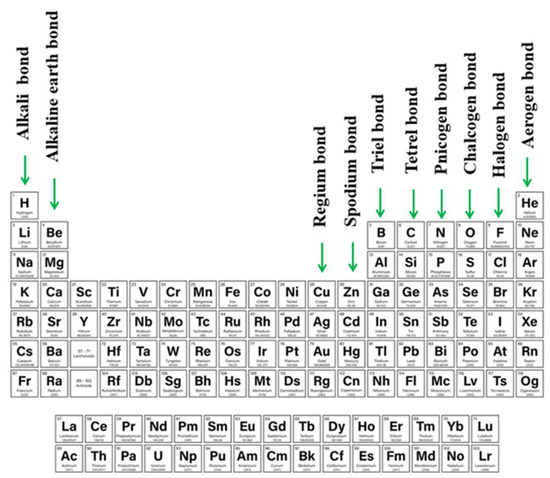
There have been a number of earlier reviews addressing the issue of σ-hole and π-hole bonds [13][49][51][52][81][84][105][120][121]. However, little attention has been devoted to situations where both hole types are present on a single molecule, and the competition between the two for a nucleophile. Indeed, it is also of intense interest to examine the result when both of these bonds are present at the same time. As has already been explored, the tunability of single σ or π-holes enables the construction of interesting assemblies with desired properties [122][123][124]. The possibilities multiply when both types of bonds are present and influence one another.
2. Origin of π-Holes
It should be reiterated that the causality is as follows: depletion of electron density causes a rise in the electrostatic potential [125]. In fact, sometimes a hole is labeled as such even though the potential is not positive, just less negative than its surroundings. For practical reasons, the magnitude of a hole is typically measured on the 0.001 au electron isodensity surface and is quantified by the VS,max parameter developed for this purpose [12][126]. The electron density can be accessed not only by quantum calculations but also experimentally by diffraction methods [127].
It has been shown that the intensity of a σ-hole can be adjusted by changing the polarizability of the central atom and the electron-withdrawing power of its substituents [13][84][125], and the same considerations apply to π-holes as well [126]. However, it must be borne in mind that the strength of a given interaction is not a function solely of electrostatic considerations. Polarization and dispersion forces are important attractive forces as well [13][17][127].
References
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole: Proceedings of “Modeling interactions in biomolecules II”, Prague, September 5th–9th, 2005. J. Mol. Model. 2007, 13, 291–296.
- Guthrie, F. On the Iodide of Iodammonium. J. Chem. Soc. 1863, 16, 239–244.
- Remsen, I.; Norris, J.F. Action of the halogens on the methyl-amines. Am. Chem. J. 1896, 18, 90–96.
- Hope, H.; McCullough, J.D. The crystal structure of the molecular complex of iodine with tetrahydroselenophene, C4H8Se.I2. Acta Cryst. 1964, 17, 712–718.
- Olie, K.; Mijlhoff, F.C. The crystal structure of POBr3 and intermolecular bonding. Acta Cryst. Sect. B 1969, 25, 974–977.
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. 1992, 44, 57–64.
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. Sigma-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038.
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.G.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311.
- Politzer, P.; Murray, J.S.; Concha, M.C. Sigma-hole bonding between like atoms; a fallacy of atomic charges. J. Mol. Model. 2008, 14, 659–665.
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729.
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757.
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wires Comput. Mol. Sci. 2011, 1, 153–163.
- Murray, J.S.; Politzer, P. Interaction and polarization energy relationships in σ-hole and π-hole bonding. Crystals 2020, 10, 76.
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294.
- Politzer, P.; Murray, J.S.; Clark, T. sigma-Hole Bonding: A Physical Interpretation. Top Curr. Chem. 2015, 358, 19–42.
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The sigma-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178.
- Politzer, P.; Murray, J.S. Electrostatics and Polarization in sigma- and pi-Hole Noncovalent Interactions: An Overview. ChemPhysChem 2020, 21, 579–588.
- Azofra, L.M.; Alkorta, I.; Scheiner, S. Chalcogen bonds in complexes of SOXY (X, Y = F, Cl) with nitrogen bases. J. Phys. Chem. A 2015, 119, 535–541.
- Vincent De Paul, N.N.; Scheiner, S. Chalcogen bonding between tetravalent SF4 and amines. J. Phys. Chem. A 2014, 118, 10849–10856.
- Alikhani, E.; Fuster, F.; Madebene, B.; Grabowski, S.J. Topological reaction sites-very strong chalcogen bonds. Phys. Chem. Chem. Phys. 2014, 16, 2430–2442.
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2009, 113, 8132–8135.
- de Azevedo Santos, L.; Ramalho, T.C.; Hamlin, T.A.; Bickelhaupt, F.M. Chalcogen bonds: Hierarchical ab initio benchmark and density functional theory performance study. J. Comput. Chem. 2021, 42, 688–698.
- de Azevedo Santos, L.; van der Lubbe, S.C.C.; Hamlin, T.A.; Ramalho, T.C.; Matthias Bickelhaupt, F. A Quantitative Molecular Orbital Perspective of the Chalcogen Bond. ChemistryOpen 2021.
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Pnicogen Bonded Complexes of PO2X (X = F, Cl) with Nitrogen Bases. J. Phys. Chem. A 2013, 117, 10497–10503.
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Properties of Complexes H2C=(X)P:PXH2, for X = F, Cl, OH, CN, NC, CCH, H, CH3, and BH2: P center dot center dot center dot P Pnicogen Bonding at sigma-Holes and pi-Holes. J. Phys. Chem. A 2013, 117, 11592–11604.
- Scheiner, S. Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620.
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288.
- Guan, L.Y.; Mo, Y.R. Electron Transfer in Pnicogen Bonds. J. Phys. Chem. A 2014, 118, 8911–8921.
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321.
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487.
- Marin-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656.
- Desiraju, G.R.; Shing Ho, P.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713.
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892.
- Mahmoudi, G.; Afkhami, F.A.; Zangrando, E.; Kaminsky, W.; Frontera, A.; Safin, D.A. A supramolecular 3D structure constructed from a new metal chelate self-assembled from Sn(NCS)(2) and phenyl(pyridin-2-yl)methylenepicolinohydrazide. J. Mol. Struct. 2021, 1224, 129188.
- Zhang, J.H.; Su, Z.Z.; Luo, J.X.; Zhao, Y.; Wang, H.G.; Ying, S.M. Synthesis, structure, and characterization of a mixed amines thiogermanate [NH4](2)[NH2(CH3)(2)](2)Ge2S6. Polyhedron 2020, 182, 114486.
- Zelenkov, L.E.; Ivanov, D.M.; Sadykov, E.K.; Bokach, N.A.; Galmes, B.; Frontera, A.; Kukushkin, V.Y. Semicoordination Bond Breaking and Halogen Bond Making Change the Supramolecular Architecture of Metal-Containing Aggregates. Cryst. Growth Des. 2020, 20, 6956–6965.
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Veerakumarasivam, A.; Page, A.J.; Tiekink, E.R.T.; Ravoof, T.B.S.A. Homoleptic tin(IV) compounds containing tridentate ONS dithiocarbazate Schiff bases: Synthesis, X-ray crystallography, DFT and cytotoxicity studies. J. Mol. Struct. 2020, 1205, 127635.
- Walton, I.; Chen, C.; Rimsza, J.M.; Nenoff, T.M.; Walton, K.S. Enhanced Sulfur Dioxide Adsorption in UiO-66 Through Crystal Engineering and Chalcogen Bonding. Cryst. Growth Des. 2020, 20, 6139–6146.
- Venosova, B.; Koziskova, J.; Kozisek, J.; Herich, P.; Luspai, K.; Petricek, V.; Hartung, J.; Muller, M.; Hubschle, C.B.; van Smaalen, S.; et al. Charge density of 4-methyl-3-[(tetrahydro-2H-pyran-2-yl)oxy]thiazole-2(3H)-thione. A comprehensive multipole refinement, maximum entropy method and density functional theory study. Acta Cryst. B 2020, 76, 450–468.
- Villasenor-Granados, T.O.; Montes-Tolentino, P.; Rodriguez-Lopez, G.; Sanchez-Ruiz, S.A.; Flores-Parra, A. Structural analysis of tris(5-methyl- [1,3,5]-dithiazinan-2-yl)stibine, its reactions with chalcogens. Intramolecular chalcogen-bonding interactions. J. Mol. Struct. 2020, 1200, 127050.
- Kumar, V.; Xu, Y.J.; Bryce, D.L. Double Chalcogen Bonds: Crystal Engineering Stratagems via Diffraction and Multinuclear Solid-State Magnetic Resonance Spectroscopy. Chem. Eur. J. 2020, 26, 3275–3286.
- Fourmigue, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084.
- Michalczyk, M.; Malik, M.; Zierkiewicz, W.; Scheiner, S. Experimental and Theoretical Studies of Dimers Stabilized by Two Chalcogen Bonds in the Presence of a N···N Pnicogen Bond. J. Phys. Chem. A 2021, 125, 657–668.
- Kinzhalov, M.A.; Popova, E.A.; Petrov, M.L.; Khoroshilova, O.V.; Mahmudov, K.T.; Pombeiro, A.J.L. Pnicogen and chalcogen bonds in cyclometalated iridium(III) complexes. Inorg. Chim. Acta 2018, 477, 31–33.
- Zapata, F.; Gonzalez, L.; Bastida, A.; Bautista, D.; Caballero, A. Formation of self-assembled supramolecular polymers by anti-electrostatic anion-anion and halogen bonding interactions. Chem. Commun. 2020, 56, 7084–7087.
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmes, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a double sigma-hole donor: The dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations. Org. Chem. Front. 2020, 7, 2230–2242.
- Scilabra, P.; Terraneo, G.; Daolio, A.; Baggioli, A.; Famulari, A.; Leroy, C.; Bryce, D.L.; Resnati, G. 4,4 ‘-Dipyridyl Dioxide center dot SbF3 Cocrystal: Pnictogen Bond Prevails over Halogen and Hydrogen Bonds in Driving Self-Assembly. Cryst. Growth Des. 2020, 20, 916–922.
- Nascimento, M.A.; Heyer, E.; Less, R.J.; Pask, C.M.; Arauzo, A.; Campo, J.; Rawson, J.M. An Investigation of Halogen Bonding as a Structure-Directing Interaction in Dithiadiazolyl Radicals. Cryst. Growth Des. 2020, 20, 4313–4324.
- Pramanik, S.; Chopra, D. Unravelling the Importance of H bonds, σ–hole and π–hole-Directed Intermolecular Interactions in Nature. J. Indian I Sci. 2020, 100, 43–59.
- Orlova, A.P.; Jasien, P.G. Halogen bonding in self-assembling systems: A comparison of intra- and interchain binding energies. Comput. Chem. 2018, 1139, 63–69.
- Tiekink, E.R.T. A Survey of Supramolecular Aggregation Based on Main Group Element Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature. Crystals 2020, 10, 503.
- Seth, S.K.; Bauzá, A.; Frontera, A. Chapter 9 Quantitative Analysis of Weak Non-covalent σ-Hole and π-Hole Interactions. In Monogr Supramol Chem; The Royal Society of Chemistry: London, UK, 2019; pp. 285–333.
- Taylor, M.S. Anion recognition based on halogen, chalcogen, pnictogen and tetrel bonding. Coord. Chem. Rev. 2020, 413, 213270.
- Navarro-Garcia, E.; Galmes, B.; Velasco, M.D.; Frontera, A.; Caballero, A. Anion Recognition by Neutral Chalcogen Bonding Receptors: Experimental and Theoretical Investigations. Chem. Eur. J. 2020, 26, 4706–4713.
- Borissov, A.; Marques, I.; Lim, J.Y.C.; Felix, V.; Smith, M.D.; Beer, P.D. Anion Recognition in Water by Charge-Neutral Halogen and Chalcogen Bonding Foldamer Receptors. J. Am. Chem. Soc. 2019, 141, 4119–4129.
- Semenov, N.A.; Gorbunov, D.E.; Shakhova, M.V.; Salnikov, G.E.; Bagryanskaya, I.Y.; Korolev, V.V.; Beckmann, J.; Gritsan, N.P.; Zibarev, A.V. Donor-Acceptor Complexes between 1,2,5-Chalcogenadiazoles (Te, Se, S) and the Pseudohalides CN- and XCN- (X = O, S, Se, Te). Chem. Eur. J. 2018, 24, 12983–12991.
- Naseer, M.M.; Hussain, M.; Bauza, A.; Lo, K.M.; Frontera, A. Intramolecular Noncovalent Carbon Bonding Interaction Stabilizes the cis Conformation in Acylhydrazones. Chempluschem 2018, 83, 881–885.
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem-Us 2018, 4, 731–783.
- Liu, Y.-Z.; Yuan, K.; Liu, L.; Yuan, Z.; Zhu, Y.-C. Anion Recognition Based on a Combination of Double-Dentate Hydrogen Bond and Double-Side Anion−π Noncovalent Interactions. J. Phys. Chem. A 2017, 121, 892–900.
- Lim, J.Y.C.; Marques, I.; Thompson, A.L.; Christensen, K.E.; Felix, V.; Beer, P.D. Chalcogen Bonding Macrocycles and [2]Rotaxanes for Anion Recognition. J. Am. Chem. Soc. 2017, 139, 3122–3133.
- Scheiner, S. Tetrel Bonding as a Vehicle for Strong and Selective Anion Binding. Molecules 2018, 23, 1147.
- Kociok-Kohn, G.; Mahon, M.F.; Molloy, K.C.; Price, G.J.; Prior, T.J.; Smith, D.R.G. Biomimetic polyorganosiloxanes: Model compounds for new materials. Dalton Trans. 2014, 43, 7734–7746.
- Politzer, P.; Murray, J.S.; Concha, M.C. Halogen bonding and the design of new materials: Organic bromides, chlorides and perhaps even fluorides as donors. J. Mol. Model. 2007, 13, 643–650.
- Radovic, L.R. Probing the ‘elephant’: On the essential difference between graphenes and polycyclic aromatic hydrocarbons. Carbon 2021, 171, 798–805.
- Maruthapandi, M.; Eswaran, L.; Cohen, R.; Perkas, N.; Luong, J.H.T.; Gedanken, A. Silica-Supported Nitrogen-Enriched Porous Benzimidazole-Linked and Triazine-Based Polymers for the Adsorption of CO2. Langmuir 2020, 36, 4280–4288.
- Kar, I.; Chatterjee, J.; Harnagea, L.; Kushnirenko, Y.; Fedorov, A.V.; Shrivastava, D.; Buchner, B.; Mahadevan, P.; Thirupathaiah, S. Metal-chalcogen bond-length induced electronic phase transition from semiconductor to topological semimetal in ZrX2 (X = Se and Te). Phys. Rev. B 2020, 101, 165122.
- Isaeva, A.; Ruck, M. Crystal Chemistry and Bonding Patterns of Bismuth-Based Topological Insulators. Inorg. Chem. 2020, 59, 3437–3451.
- Ho, P.C.; Wang, J.Z.; Meloni, F.; Vargas-Baca, I. Chalcogen bonding in materials chemistry. Coord. Chem. Rev. 2020, 422, 213464.
- Daniels, C.L.; Knobeloch, M.; Yox, P.; Adamson, M.A.S.; Chen, Y.H.; Dorn, R.W.; Wu, H.; Zhou, G.Q.; Fan, H.J.; Rossini, A.J.; et al. Intermetallic Nanocatalysts from Heterobimetallic Group 10-14 Pyridine-2-thiolate Precursors. Organometallics 2020, 39, 1092–1104.
- Zhang, Y.; Wang, W.Z.; Wang, Y.B. Tetrel bonding on graphene. Comput. Chem. 2019, 1147, 8–12.
- Sung, S.H.; Schnitzer, N.; Brown, L.; Park, J.; Hovden, R. Stacking, strain, and twist in 2D materials quantified by 3D electron diffraction. Phys. Rev. Mater. 2019, 3, 064003.
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138.
- Pina, M.D.N.; Frontera, A.; Bauza, A. Quantifying Intramolecular Halogen Bonds in Nucleic Acids: A Combined Protein Data Bank and Theoretical Study. Acs Chem. Biol. 2020, 15, 1942–1948.
- Lundemba, A.S.; Bibelayi, D.D.; Wood, P.A.; Pradon, J.; Yav, Z.G. sigma-Hole interactions in small-molecule compounds containing divalent sulfur groups R-1-S-R-2. Acta Cryst. B 2020, 76, 707–718.
- Scheiner, S. Differential Binding of Tetrel-Bonding Bipodal Receptors to Monatomic and Polyatomic Anions. Molecules 2019, 24, 227.
- Riel, A.M.S.; Huynh, H.T.; Jeannin, O.; Berryman, O.; Fourmigue, M. Organic Selenocyanates as Halide Receptors: From Chelation to One-Dimensional Systems. Cryst. Growth Des. 2019, 19, 1418–1425.
- Michalczyk, M.; Zierkiewicz, W.; Wysokinski, R.; Scheiner, S. Theoretical Studies of IR and NMR Spectral Changes Induced by Sigma-Hole Hydrogen, Halogen, Chalcogen, Pnicogen, and Tetrel Bonds in a Model Protein Environment. Molecules 2019, 24, 3329.
- Wei, Y.; Li, Q.; Scheiner, S. The pi-Tetrel Bond and its Influence on Hydrogen Bonding and Proton Transfer. Chemphyschem 2018, 19, 736–743.
- Trievel, R.C.; Scheiner, S. Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases. Molecules 2018, 23, 2965.
- Scheiner, S. Halogen Bonds Formed between Substituted Imidazoliums and N Bases of Varying N-Hybridization. Molecules 2017, 22, 1634.
- Montaña, Á.M. The σ and π Holes. The Halogen and Tetrel Bondings: Their Nature, Importance and Chemical, Biological and Medicinal Implications. Chemistryselect 2017, 2, 9094–9112.
- Grabowski, S.J. Tetrel bond-sigma-hole bond as a preliminary stage of the S(N)2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834.
- Angarov, V.; Kozuch, S. On the σ, π and δ hole interactions: A molecular orbital overview. New J. Chem. 2018, 42, 1413–1422.
- Bauza, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional sigma/-Hole Interactions. Chemphyschem 2015, 16, 2496–2517.
- Gao, L.; Zeng, Y.; Zhang, X.; Meng, L. Comparative studies on group III sigma-hole and pi-hole interactions. J. Comput. Chem. 2016, 37, 1321–1327.
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Directionality of pi-holes in nitro compounds. Chem. Commun. 2015, 51, 1491–1493.
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. sigma-Holes, pi-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548.
- Grabowski, S.J. The Nature of Triel Bonds, a Case of B and Al Centres Bonded with Electron Rich Sites. Molecules 2020, 25, 2703.
- Grabowski, S.J. Triel bond and coordination of triel centres-Comparison with hydrogen bond interaction. Coord. Chem. Rev. 2020, 407, 213171.
- Xu, Z.F.; Li, Y. Triel bonds in RZH(2)center dot center dot center dot NH3: Hybridization, solvation, and substitution. J. Mol. Model. 2019, 25, 219.
- Jablonski, M. Hydride-Triel Bonds. J. Comput. Chem. 2018, 39, 1177–1191.
- Grabowski, S.J. Two faces of triel bonds in boron trihalide complexes. J. Comput. Chem. 2018, 39, 472–480.
- Esrafili, M.D.; Mousavian, P. The triel bond: A potential force for tuning anion-pi interactions. Mol. Phys. 2018, 116, 388–398.
- Grabowski, S.J. Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct. Chem. 2017, 28, 1163–1171.
- Grabowski, S.J. pi-Hole Bonds: Boron and Aluminum Lewis Acid Centers. Chemphyschem 2015, 16, 1470–1479.
- Burgi, H.B.; Dunitz, J.D.; Shefter, E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973, 95, 5065–5067.
- Harder, M.; Kuhn, B.; Diederich, F. Efficient Stacking on Protein Amide Fragments. Chemmedchem 2013, 8, 397–404.
- Bartlett, G.J.; Choudhary, A.; Raines, R.T.; Woolfson, D.N. n ->pi* interactions in proteins. Nat. Chem. Biol. 2010, 6, 615–620.
- Andleeb, H.; Khan, I.; Bauza, A.; Tahir, M.N.; Simpson, J.; Hameed, S.; Frontera, A. Synthesis and supramolecular self-assembly of thioxothiazolidinone derivatives driven by pi-bonding and diverse 7 hole interactions: A combined experimental and theoretical analysis. J. Mol. Struct. 2017, 1139, 209–221.
- Gomila, R.M.; Frontera, A. Covalent and Non-covalent Noble Gas Bonding Interactions in XeFn Derivatives (n = 2–6): A Combined Theoretical and ICSD Analysis. Front. Chem. 2020, 8, 395.
- Bauza, A.; Frontera, A. sigma/pi-Hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2019, 404, 213112.
- Esrafili, M.D.; Vessally, E. The strengthening effect of a hydrogen or lithium bond on the Z···N aerogen bond (Z = Ar, Kr and Xe): A comparative study. Mol. Phys. 2016, 114, 3265–3276.
- Bauza, A.; Frontera, A. pi-Hole aerogen bonding interactions. Phys. Chem. Chem. Phys. 2015, 17, 24748–24753.
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343.
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180.
- Hou, M.C.; Zhu, Y.F.; Li, Q.Z.; Scheiner, S. Tuning the Competition between Hydrogen and Tetrel Bonds by a Magnesium Bond. Chemphyschem 2020, 21, 212–219.
- Alkorta, I.; Legon, A.C. Non-Covalent Interactions Involving Alkaline-Earth Atoms and Lewis Bases B: An ab Initio Investigation of Beryllium and Magnesium Bonds, B center dot center dot center dot MR2 (M = Be or Mg, and R = H, F or CH3). Inorganics 2019, 7, 35.
- Grabowski, S.J. Magnesium Bonds: From Divalent Mg Centres to Trigonal and Tetrahedral Coordination. Chemistryselect 2018, 3, 3147–3154.
- Alikhani, M.E. Beryllium bonding: Insights from the sigma- and pi-hole analysis. J. Mol. Model. 2020, 26, 94.
- Zhang, Z.; Lu, T.; Ding, L.Y.; Wang, G.Y.; Wang, Z.X.; Zheng, B.S.; Liu, Y.; Ding, X.L. Cooperativity effects between regium-bonding and pnicogen-bonding interactions in ternary MF center dot center dot center dot PH3O center dot center dot center dot MF (M = Cu, Ag, Au): An ab initio study. Mol. Phys. 2020, 118, e1784478.
- Wang, R.J.; Wang, Z.; Yu, X.F.; Li, Q.Z. Synergistic and Diminutive Effects between Regium and Aerogen Bonds. Chemphyschem 2020, 21, 2426–2431.
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Rivalry between Regium and Hydrogen Bonds Established within Diatomic Coinage Molecules and Lewis Acids/Bases. Chemphyschem 2020, 21, 2557–2563.
- Alkorta, I.; Trujillo, C.; Sanchez-Sanz, G.; Elguero, J. Regium Bonds between Silver(I) Pyrazolates Dinuclear Complexes and Lewis Bases (N-2, OH2, NCH, SH2, NH3, PH3, CO and CNH). Crystals 2020, 10, 137.
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Understanding Regium Bonds and their Competition with Hydrogen Bonds in Au-2:HX Complexes. Chemphyschem 2019, 20, 1572–1580.
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Regium bonds between Mn clusters (M = Cu, Ag, Au and n = 2–6) and nucleophiles NH3 and HCN. Phys. Chem. Chem. Phys. 2018, 20, 22498–22509.
- Xia, T.; Li, D.; Cheng, L.J. Theoretical analysis of the spodium bonds in HgCl2 center dot center dot center dot L (L = ClR, SR2, and PR3) dimers. Chem. Phys. 2020, 539, 110978.
- Mahmoudi, G.; Masoudiasl, A.; Babashkina, M.G.; Frontera, A.; Doert, T.; White, J.M.; Zangrando, E.; Zubkov, F.I.; Safin, D.A. On the importance of pi-hole spodium bonding in tricoordinated Hg-II complexes. Dalton T 2020, 49, 17547–17551.
- Mahmoudi, G.; Lawrence, S.E.; Cisterna, J.; Cardenas, A.; Brito, I.; Frontera, A.; Safin, D.A. A new spodium bond driven coordination polymer constructed from mercury(ii) azide and 1,2-bis(pyridin-2-ylmethylene)hydrazine. New J. Chem. 2020, 44, 21100–21107.
- Bauze, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487.
- Frontera, A. σ- and π-Hole Interactions. Crystals 2020, 10, 721.
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601.
- Esrafili, M.D.; Mohammadirad, N. An ab initio study on tunability of σ-hole interactions in XHS:PH2Y and XH2P:SHY complexes (X = F, Cl, Br; Y = H, OH, OCH3, CH3, C2H5, and NH2). J. Mol. Model. 2015, 21, 2727.
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability II: The varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J. Mol. Model. 2013, 19, 4651–4659.
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability I: The effects of aromatic fluorine substitution on the strengths of halogen-bonding interactions involving chlorine, bromine, and iodine. J. Mol. Model. 2011, 17, 3309–3318.
- Politzer, P.; Murray, J.S. sigma-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212.
- Bulat, F.A.; Toro-Labbe, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691.
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. Wires Comput. Mol. Sci. 2017, 7, e13260.




