σ-hole is a region of depleted electronic density located on the outer side of a covalently-bonded atom, situated roughly along an extension of the bond axis
- molecular electrostatic potential
- halogen bond
- electronic density
- chalcogen bond
- pnicogen bond
- tetrel bond
- triel bond
- π-hole
1. Introduction
The concept of the σ-hole, introduced to a wide audience in 2005 at a conference in Prague by Tim Clark [1], influenced a way of thinking about noncovalent interactions that prevails to this day. Early experimental findings [2][3][4][5][2,3,4,5] of unusual halogen·halogen contacts were explained in part by the anisotropic distribution of electronic density around a halogen atom when linked to an electron-withdrawing group. Already in 1992, it had been learned that electronegative atoms from Groups 14–17 have regions of positive molecular electrostatic potential (MEP) on their outer surfaces, along an extension of a covalent bond, which may attract an incoming Lewis base [6]. This observation led to the further computational studies which resulted in formulation of the σ-hole idea [7] which was further generalized in later works [7][8][9][10][11][12][13][14][15][16][17][7,8,9,10,11,12,13,14,15,16,17]. These ideas concerning the halogen bond were successfully adapted to atoms from other families of the periodic table, which were later grouped into the category of σ-hole bonds. This general sort of noncovalent bond has been subdivided by the specific family from which the bridging atom is drawn, i.e., chalcogen [18][19][20][21][22][23][18,19,20,21,22,23], pnicogen (pnictogen) [24][25][26][27][28][24,25,26,27,28], or tetrel bonds [29][30][31][29,30,31]. The former, along with the halogen bond, has been formally recognized and detailed in recent IUPAC recommendations [32][33][32,33].
Quite the opposite from representing exotic or unusual contacts, these bonds make important contributions to numerous fields of chemistry and biology. As examples, understanding the forces behind crystal engineering and supramolecular chemistry benefits from a knowledge of σ-hole interactions due to their directionality, strength, and self-organization properties which promote formation of adducts in the solid state [34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. The importance of σ-hole bonding has also been verified in the context of anion recognition processes [53][54][55][56][57][58][59][60][61][53,54,55,56,57,58,59,60,61], materials chemistry [62][63][64][65][66][67][68][69][70][71][72][62,63,64,65,66,67,68,69,70,71,72], or biochemistry [73][74][75][76][77][78][79][80][81][73,74,75,76,77,78,79,80,81]. An early work connecting σ-hole bonds with crucial concepts in chemistry occurred when Grabowski recognized that tetrel bonds can be thought of as a preliminary stage of the very important SN2 reaction [82].
As ideas concerning the σ-hole were proliferating, it was recognized that density depletion is not necessarily limited only to the extensions of covalent bonds. Depletions can also occur above planar groups as well, as for example above a carbonyl or phenyl group. Linear systems such as HCN can also suffer from low density off the molecular axis. Because of their location and association with π-electronic systems, these regions of density depletion and positive electrostatic potential have come to be called π-holes. As one specific example, tricoordinated triel atoms typically occur at the center of a planar triangle, with a π-hole located above the central triel atom [13][83][84][85][86][87][13,83,84,85,86,87], and the resulting triel bond [88][89][90][91][92][93][94][95][88,89,90,91,92,93,94,95] falls into the category of a π-hole bond. The π-hole situated above the C atom of a carbonyl group offers another common example, whose presence was manifested in early work of Burgi and Dunitz [96]. Protein structures can also involve participation of π-hole bonds [97][98][97,98], as is also true of self-assembling systems [99]. As work has progressed, there has been recognition of π-holes as providing a means by which an aerogen bond (involving rare gas atoms) can form [100][101][102][103][104][100,101,102,103,104]. Other newly introduced types of σ/π- hole directed interactions are alkali and alkaline earth bond (e.g., beryllium bond, magnesium bond) in which atoms of 1st and 2nd groups contribute [105][106][107][108][109][105,106,107,108,109] or regium and spodium bonds which employ transition metals from 11th (regium [110][111][112][113][114][115][110,111,112,113,114,115]) or 12th (spodium [105][116][117][118][119][105,116,117,118,119]) groups of the periodic table. The full range of these sorts of bonds, along with their designations, is summarized in Figure 1.
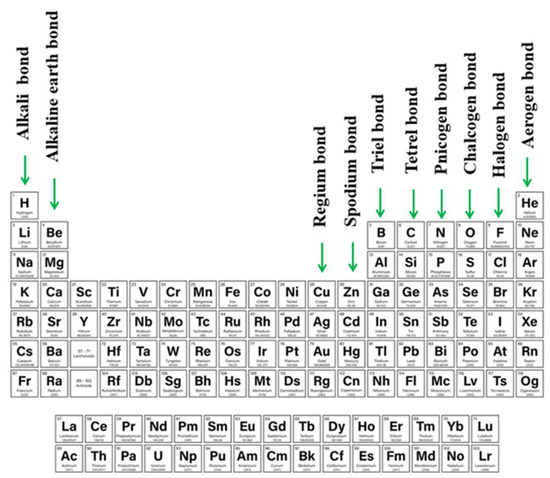
There have been a number of earlier reviews addressing the issue of σ-hole and π-hole bonds [13][49][51][52][81][84][105][120][121][13,49,51,52,81,84,105,120,121]. However, little attention has been devoted to situations where both hole types are present on a single molecule, and the competition between the two for a nucleophile. Indeed, it is also of intense interest to examine the result when both of these bonds are present at the same time. As has already been explored, the tunability of single σ or π-holes enables the construction of interesting assemblies with desired properties [122][123][124][122,123,124]. The possibilities multiply when both types of bonds are present and influence one another.
2. Origin of π-Holes
It should be reiterated that the causality is as follows: depletion of electron density causes a rise in the electrostatic potential [125]. In fact, sometimes a hole is labeled as such even though the potential is not positive, just less negative than its surroundings. For practical reasons, the magnitude of a hole is typically measured on the 0.001 au electron isodensity surface and is quantified by the VS,max parameter developed for this purpose [12][126][12,126]. The electron density can be accessed not only by quantum calculations but also experimentally by diffraction methods [127].
It has been shown that the intensity of a σ-hole can be adjusted by changing the polarizability of the central atom and the electron-withdrawing power of its substituents [13][84][125][13,84,127], and the same considerations apply to π-holes as well [126][128]. However, it must be borne in mind that the strength of a given interaction is not a function solely of electrostatic considerations. Polarization and dispersion forces are important attractive forces as well [13][17][127][13,17,129,130,131].
