
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alexander Marcus Seifalian | + 8935 word(s) | 8935 | 2021-04-26 09:50:08 | | | |
| 2 | Rita Xu | -7514 word(s) | 1421 | 2021-05-08 09:00:26 | | | | |
| 3 | Alexander Marcus Seifalian | + 7514 word(s) | 8935 | 2021-05-08 16:26:24 | | | | |
| 4 | Barry Alexander | + 7514 word(s) | 8935 | 2021-05-08 17:37:28 | | |
Video Upload Options
Desirable carbon allotropes such as graphene oxide (GO) have entered the field with several biomedical applications, owing to their exceptional physicochemical and biological features, including extreme strength, found to be 200 times stronger than steel; remarkable light weight; large surface-to-volume ratio; chemical stability; unparalleled thermal and electrical conductivity; and enhanced cell adhesion, proliferation, and differentiation properties.
1. Introduction
Trauma, aging, and disease cause damage to tissue, which can result in loss of function. In the early stages, self-healing can be effective, but with substantial injury, the organ cannot restore itself sufficiently; thus, organ repair or replacement (autograft or allograft) is recommended, albeit with some challenges. The most critical challenges facing organ replacements are organ shortages, grafts, transplant rejection, high cost, inflammation, infection, and also death in some cases [1]. Alternatively, tissue engineering has attracted considerable attention due to its significant potential for tissue restoration. Tissue engineering is a combination of active molecules, cells, and also a scaffold that accurately mimics the extracellular matrix [2]. An optimal scaffold should be nontoxic, porous, provide mechanical strength and proper cell attachment, and, consequently, induce cell proliferation and differentiation [3].
Carbon-based nanomaterials have recently boomed in biomaterial applications. Graphene is an important new addition to these carbon family materials due to its unique properties. Researchers in tissue engineering have already extensively investigated graphene-containing structures, specifically for bone, neuronal, cardiac, skin, cartilage, and dental tissue regeneration, as highlighted in graphical abstract. Carbon-based nanomaterials have recently boomed in biomaterial applications. Graphene is an important new addition to these carbon family materials due to its unique properties. Researchers in tissue engineering have already extensively investigated graphene-containing structures, specifically for bone, neuronal, cardiac, skin, cartilage, and dental tissue regeneration, as highlighted in Figure 1.
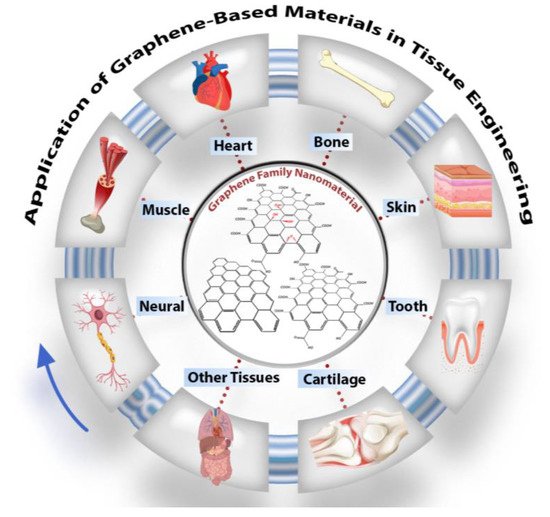
Figure 1. Schematic of graphene oxide nanomaterials and their application in tissue engineering, particularly in nerve, muscle, heart, skin, cartilage, dental, and other tissues.
In this review, graphene oxide (GO), reduced graphene oxide (rGO), and functionalized GO (FGO) are noted among the various allotropic forms of carbons due to their significant surface area, strength, light weight, chemical stability, enhanced cell adhesion, proliferation, differentiation, and application in the repair of tissue [4][5]. The basic structure of all carbon allotropes is graphene; it is a single sheet of sp2-hybridized carbon atoms arranged in a hexagonal lattice-like honeycomb. Valuable research has completely described the synthesis method, but in brief, the synthesis approach was divided into top-down and bottom-up strategies. In the top-down approach, graphite is treated by exfoliation (mechanical or electrochemical). The bottom-up approach is a developed approach, which contains epitaxial growth, chemical vapor deposition, and the dry ice method for graphene material production [6][7][8] (Figure 2).
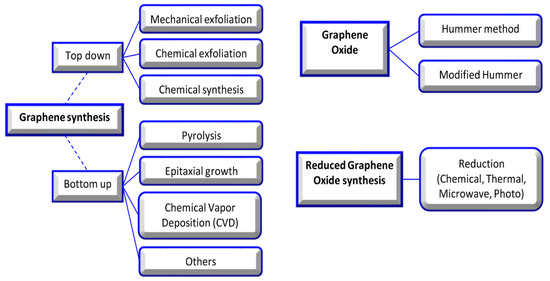
Figure 2. Graphene, graphene oxide, and reduced graphene oxide synthesis methods [9].
Two-dimensional (2D) graphene lattice structures have some shortcomings, such as an unstable chemical structure and limited active sites for interacting with other molecules or nanomaterials; hence, it has some incompatibility. The chemical modification of graphene and the production of GO resolves some of these problems. GO maintains graphene’s atomic configuration and only has carboxyl groups (-OOH) on the edge of its structure, as well as epoxy (-O) and hydroxyl (-OH) groups on the basal plane. By reducing the amount of oxygen in GO through thermal, chemical, or UV exposure processes, rGO is produced. Most often, the reduction of GO does not complete, and some oxygen groups remain because not all sp3 bonds could return to the sp2 structure [10]. rGO promotes the cell differentiation and mechanical properties of scaffolds, although there is no apparent effect on scaffold hydrophilicity [11]. Thus, the repair and replacement of organs using tissue engineering strategies have focused on GO, FGO, and rGO more than graphene. The aim of this research was to highlight the physicochemical and biological properties of GO-based materials (GOBMs) and critically review the literature over the last three years on the applications of these materials in the repair and development of human organs.
2. Graphene and Its Physicochemical Properties
The distinctive properties of graphene are derived from its particular crystal lattice structure. Within this, the bonding between each carbon atom is hybridized sp2 with the addition of π orbitals. In each unit cell of graphene, two π orbitals exist that are dispersed to form two π bonds, both of which could be known as bonding and antibonding [12]. This arranged lattice is a fundamental building block for all graphitic materials in various dimensions, namely (1) zero-dimensional (0D), e.g., carbon dot, fullerenes and nanodiamonds; (2) rolled one-dimensional (1D), e.g., carbon nanotubes; (3) two-dimensional (2D), e.g., graphene and GO; and (4) stacked three-dimensional (3D), e.g., graphite [13]. The graphene family structure also results in an exceptional surface-to-volume ratio, high intrinsic mobility, unparalleled thermal conductivity, and excellent electrical, optoelectronic, and mechanical properties that have paved the way due to being attractive technological tools [12][14]. Graphene is renowned as one of the most robust materials known to humans, and it is found to be 200 times stronger than steel [15]. In GO, hydrogen bonding forms between hydroxyl and epoxy groups and weak interactions with other groups. The existence of the carboxylic acid group offers a negative surface charge (hydrophilic section); therefore, GO has stability in different polar solutions (particularly water), while graphene is inclined to aggregation. Moreover, owing to free surface π electrons from unmodified graphene (hydrophobic section), GO has an amphiphilic structure that could act as a surfactant. Graphene is hydrophobic, and GO, in comparison with graphene, could be hydrophilic or hydrophobic depending on the chemical and functionalization of the surface chemistry. These characteristics make GO the most important derivative of graphene, which possesses an easy process and a high affinity to accommodate biomolecules. The enhancement of chemical reactivity and graphene stability in solution is intertwined with the presence of reactive oxygen functional groups. Disrupted sp2 reduces its mechanical, electrical, and thermal properties [12][14]. Although rGO has less oxygen content, hydrophilic functional groups, or surface charge, through the modification of noncovalent interactions (e.g., van der Waals interactions and π–π stacking), the physical adsorption of both polymers and small biomolecules onto its basal plane is enhanced remarkably [16].
3. Graphene Oxide and Its Biological Properties
It has been proven that most GO and derivatives are cytocompatible in vitro and in vivo. However, the physicochemical properties of 2D materials, such as structure, shape, size, surface functionality, concentration, and aggregation state have an essential impact on cellular behavior. Graphene, with its sharp edge properties, has the potential to cause cell damage during the penetration of cell membranes. Its aggregation can also lead to cytotoxicity. Graphene at the nanoscale, when <100 nm, results in cytotoxicity, inflammation, and even genotoxicity (due to facing less steric hindrance). In contrast, graphene with functionalized groups (i.e., GO, FGO such as the amine group, and rGO) is easily internalized by cells (especially in nano sizes), in addition to causing more irregular cell membrane perturbation [12][17]. GO and its derivatives have been ascertained to have specific antibacterial properties, which are also emphasized in tissue engineering applications [18]. The antibacterial activity of GO is related to various mechanisms, including membrane stress, oxidative stress, entrapment, the basal plane, and the photothermal effect. GO has sharp edges that damage the cell membrane, meaning it could, in turn, lead to bacterial cell mortality via the membrane stress mechanism. The structure of GO allows it to act as an electron acceptor; thus, in the vicinity of bacteria, the abstraction of electrons within the membrane occurs, compromising membrane integrity and killing bacterial cells (particularly P. aeruginosa and S. aureus). GO and rGO, owing to the existence of functionalized groups, can alter the partial pressure of intracellular oxygen, which results in oxidative damage that destroys the bacterial cell internal composition, particularly E. coli, through the deactivation of their proteins and lipids, which eventually leads to cell death. GO has illustrated synergistic effects with laser energy; hence, it has been used for photothermal therapy, directly enhancing its antibacterial activity [19]. Another fascinating property that GOBMs possess is antioxidant activity, and sp2 carbons play an essential role in scavenging radicals by radical adduct formation and electron transfer. Because of this characteristic, these biomaterials can effectively scavenge radicals and protect cells from high levels of oxidative stress [20]. Graphene, being nonbiodegradable (except FGO), presents serious concerns for potential toxicity, immune response, and environmental hazards [21]. It is reported that GO is susceptible to biodegradation by oxidative attack through hydrogen peroxide and horseradish peroxidase. Therefore, many attempts, such as the fabrication of nanocomposites, have been carried out to accelerate GO biodegradation, as the degradation rate of biomaterials (i.e., scaffold) must be compatible with the rate of tissues and organs [15][22][23].
References
- Dag Line, P. The Fundamental Challenges in Organ Transplantation. OBM Transpl. 2017, 1, 6.
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A.M. Can tissue engineering bring hope in the development of human tympanic membrane? Tissue Eng. Part B Rev. 2020, 1–62.
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495.
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1107, pp. 129–142.
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404.
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of nanocellulose/nanocarbon composites: Focus on biotechnology and medicine. Nanomaterials 2020, 10, 196.
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and Application Advancement of Organic-Functionalized Graphene-Based Materials: A Review. J. Sep. Sci. 2020, 43, 1544–1557.
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564.
- Lakshmanan, R.; Maulik, N. Graphene-based drug delivery systems in tissue engineering and nanomedicine. Can. J. Physiol. Pharmacol. 2018, 96, 869–878.
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921.
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337.
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D graphene-containing structures for tissue engineering. Mater. Today Chem. 2019, 14, 100199.
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317.
- Shang, L.; Qi, Y.; Lu, H.; Pei, H.; Li, Y.; Qu, L.; Wu, Z.; Zhang, W. Graphene and Graphene Oxide for Tissue Engineering and Regeneration. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185.
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843.
- Sayyar, S.; Officer, D.L.; Wallace, G.G. Fabrication of 3D structures from graphene-based biocomposites. J. Mater. Chem. B 2017, 5, 3462–3482.
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546.
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2020, 22, 250–263.
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737.
- Han, J.; Kim, Y.S.; Lim, M.-Y.; Kim, H.Y.; Kong, S.; Kang, M.; Choo, Y.W.; Jun, J.H.; Ryu, S.; Jeong, H.-Y.; et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano 2018, 12, 1959–1977.
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769.
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416.
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846.




