Desirable carbon allotropes such as graphene oxide (GO) have entered the field with several biomedical applications, owing to their exceptional physicochemical and biological features, including extreme strength, found to be 200 times stronger than steel; remarkable light weight; large surface-to-volume ratio; chemical stability; unparalleled thermal and electrical conductivity; and enhanced cell adhesion, proliferation, and differentiation properties.
- graphene
- graphene oxide
- functionalization
- interface
- stem cells
- cell adhesion
- carbon
- human organs
- 3D scaffold
Review
Graphene Oxide: Opportunities and Challenges in Biomedicine
Pariya Zare, 1,†, Mina Aleemardani, 2,†, Amelia Seifalian, 3,4, Zohreh Bagher, 5,* and Alexander M. Seifalian 6,*
1 Department of Chemical Engineering, University of Tehran, Tehran 1417466191, Iran; paria.zare95@gmail.com
2 Biomaterials and Tissue Engineering Group, Department of Materials Science and Engineering,
Kroto Research Institute, The University of Sheffield, Sheffield S3 7HQ, UK; maleemardani1@sheffield.ac.uk
3 Watford General Hospital, Watford WD18 0HB, UK
4 University College London, London WC1E 6BT, UK; a.seifalian@nhs.net
5 ENT and Head and Neck Research Centre and Department, Hazrat Rasoul Akram Hospital, The Five Senses Health Institute, Iran University of Medical Sciences, Tehran 1445413131, Iran
6 Nanotechnology and Regenerative Medicine Commercialisation Centre (NanoRegMed Ltd.),
London BioScience Innovation Centre, London NW1 0NH, UK
* Correspondence: baharebagher@gmail.com (Z.B.); a.seifalian@gmail.com (A.M.S.); Tel: 44-(0)-2076911122 (A.M.S.)
† These authors contributed equally to this work.
Abstract: Desirable carbon allotropes such as graphene oxide (GO) have entered the field with several biomedical applications, owing to their exceptional physicochemical and biological features, including extreme strength, found to be 200 times stronger than steel; remarkable light weight; large surface-to-volume ratio; chemical stability; unparalleled thermal and electrical conductivity; and enhanced cell adhesion, proliferation, and differentiation properties. The presence of functional groups on graphene oxide (GO) enhances further interactions with other molecules. Therefore, recent studies have focused on GO-based materials (GOBMs) rather than graphene. The aim of this research was to highlight the physicochemical and biological properties of GOBMs, especially their significance to biomedical applications. The latest studies of GOBMs in biomedical applications are critically reviewed, and in vitro and preclinical studies are assessed. Furthermore, the challenges likely to be faced and prospective future potential are addressed. GOBMs, a high potential emerging material, will dominate the materials of choice in the repair and development of human organs and medical devices. There is already great interest among academics as well as in pharmaceutical and biomedical industries.
Keywords: graphene; graphene oxide; functionalization; interface; stem cells; cell adhesion; carbon; human organs; 3D scaffold
1. Introduction
Trauma, aging, and disease cause damage to tissue, which can result in loss of function. In the early stages, self-healing can be effective, but with substantial injury, the organ cannot restore itself sufficiently; thus, organ repair or replacement (autograft or allograft) is recommended, albeit with some challenges. The most critical challenges facing organ replacements are organ shortages, grafts, transplant rejection, high cost, inflammation, infection, and also death in some cases [1]. Alternatively, tissue engineering has attracted considerable attention due to its significant potential for tissue restoration. Tissue engineering is a combination of active molecules, cells, and also a scaffold that accurately mimics the extracellular matrix [2]. An optimal scaffold should be nontoxic, porous, provide mechanical strength and proper cell attachment, and, consequently, induce cell proliferation and differentiation [3].
Carbon-based nanomaterials have recently boomed in biomaterial applications. Graphene is an important new addition to these carbon family materials due to its unique properties. Researchers in tissue engineering have already extensively investigated graphene-containing structures, specifically for bone, neuronal, cardiac, skin, cartilage, and dental tissue regeneration, as highlighted in graphical abstract. Carbon-based nanomaterials have recently boomed in biomaterial applications. Graphene is an important new addition to these carbon family materials due to its unique properties. Researchers in tissue engineering have already extensively investigated graphene-containing structures, specifically for bone, neuronal, cardiac, skin, cartilage, and dental tissue regeneration, as highlighted in Figure 1.
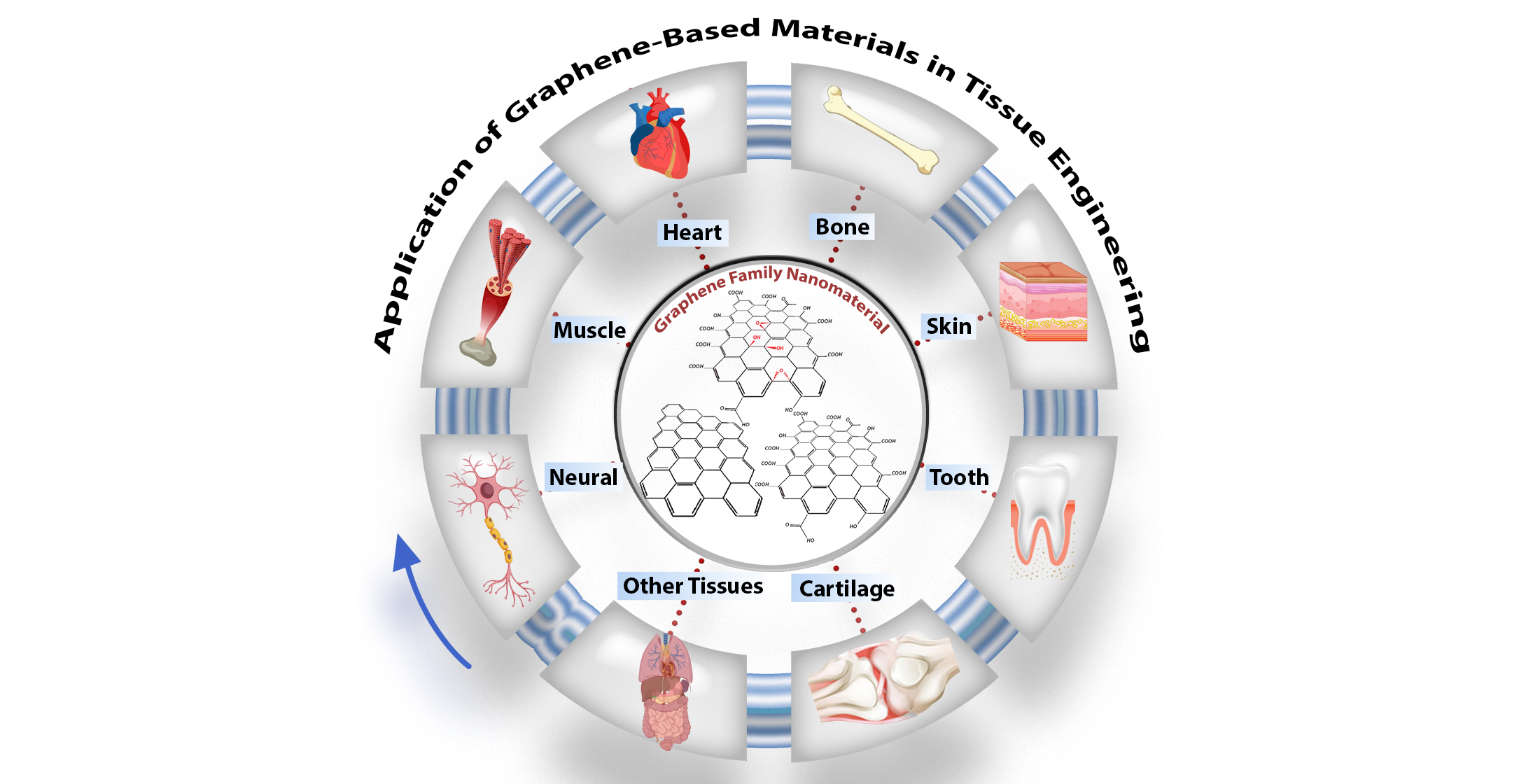
Figure 1. Schematic of graphene oxide nanomaterials and their application in tissue engineering, particularly in nerve, muscle, heart, skin, cartilage, dental, and other tissues.
In this review, graphene oxide (GO), reduced graphene oxide (rGO), and functionalized GO (FGO) are noted among the various allotropic forms of carbons due to their significant surface area, strength, light weight, chemical stability, enhanced cell adhesion, proliferation, differentiation, and application in the repair of tissue [4,5]. The basic structure of all carbon allotropes is graphene; it is a single sheet of sp2-hybridized carbon atoms arranged in a hexagonal lattice-like honeycomb. Valuable research has completely described the synthesis method, but in brief, the synthesis approach was divided into top-down and bottom-up strategies. In the top-down approach, graphite is treated by exfoliation (mechanical or electrochemical). The bottom-up approach is a developed approach, which contains epitaxial growth, chemical vapor deposition, and the dry ice method for graphene material production [6–8] (Figure 2).
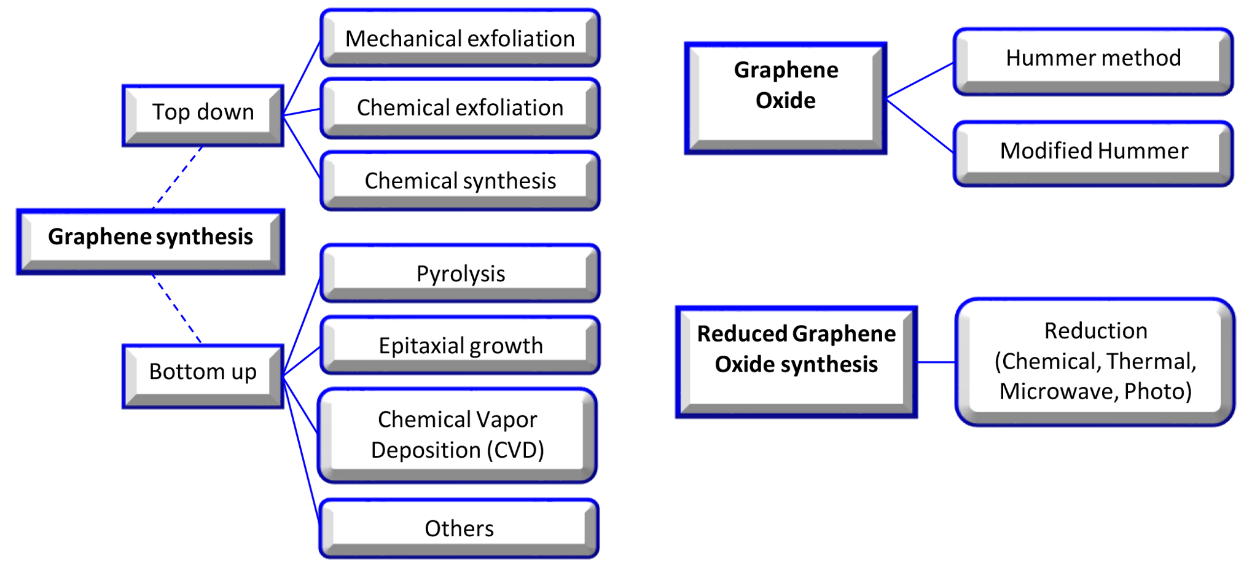
Figure 2. Graphene, graphene oxide, and reduced graphene oxide synthesis methods [9].
Two-dimensional (2D) graphene lattice structures have some shortcomings, such as an unstable chemical structure and limited active sites for interacting with other molecules or nanomaterials; hence, it has some incompatibility. The chemical modification of graphene and the production of GO resolves some of these problems. GO maintains graphene’s atomic configuration and only has carboxyl groups (-OOH) on the edge of its structure, as well as epoxy (-O) and hydroxyl (-OH) groups on the basal plane. By reducing the amount of oxygen in GO through thermal, chemical, or UV exposure processes, rGO is produced. Most often, the reduction of GO does not complete, and some oxygen groups remain because not all sp3 bonds could return to the sp2 structure [10]. rGO promotes the cell differentiation and mechanical properties of scaffolds, although there is no apparent effect on scaffold hydrophilicity [11]. Thus, the repair and replacement of organs using tissue engineering strategies have focused on GO, FGO, and rGO more than graphene. The aim of this research was to highlight the physicochemical and biological properties of GO-based materials (GOBMs) and critically review the literature over the last three years on the applications of these materials in the repair and development of human organs.
2. Graphene and Its Physicochemical Properties
The distinctive properties of graphene are derived from its particular crystal lattice structure. Within this, the bonding between each carbon atom is hybridized sp2 with the addition of π orbitals. In each unit cell of graphene, two π orbitals exist that are dispersed to form two π bonds, both of which could be known as bonding and antibonding [12]. This arranged lattice is a fundamental building block for all graphitic materials in various dimensions, namely (1) zero-dimensional (0D), e.g., carbon dot, fullerenes and nanodiamonds; (2) rolled one-dimensional (1D), e.g., carbon nanotubes; (3) two-dimensional (2D), e.g., graphene and GO; and (4) stacked three-dimensional (3D), e.g., graphite [13]. The graphene family structure also results in an exceptional surface-to-volume ratio, high intrinsic mobility, unparalleled thermal conductivity, and excellent electrical, optoelectronic, and mechanical properties that have paved the way due to being attractive technological tools [12,14]. Graphene is renowned as one of the most robust materials known to humans, and it is found to be 200 times stronger than steel [15]. In GO, hydrogen bonding forms between hydroxyl and epoxy groups and weak interactions with other groups. The existence of the carboxylic acid group offers a negative surface charge (hydrophilic section); therefore, GO has stability in different polar solutions (particularly water), while graphene is inclined to aggregation. Moreover, owing to free surface π electrons from unmodified graphene (hydrophobic section), GO has an amphiphilic structure that could act as a surfactant. Graphene is hydrophobic, and GO, in comparison with graphene, could be hydrophilic or hydrophobic depending on the chemical and functionalization of the surface chemistry. These characteristics make GO the most important derivative of graphene, which possesses an easy process and a high affinity to accommodate biomolecules. The enhancement of chemical reactivity and graphene stability in solution is intertwined with the presence of reactive oxygen functional groups. Disrupted sp2 reduces its mechanical, electrical, and thermal properties [12,14]. Although rGO has less oxygen content, hydrophilic functional groups, or surface charge, through the modification of noncovalent interactions (e.g., van der Waals interactions and π–π stacking), the physical adsorption of both polymers and small biomolecules onto its basal plane is enhanced remarkably [16].
3. Graphene Oxide and Its Biological Properties
It has been proven that most GO and derivatives are cytocompatible in vitro and in vivo. However, the physicochemical properties of 2D materials, such as structure, shape, size, surface functionality, concentration, and aggregation state have an essential impact on cellular behavior. Graphene, with its sharp edge properties, has the potential to cause cell damage during the penetration of cell membranes. Its aggregation can also lead to cytotoxicity. Graphene at the nanoscale, when <100 nm, results in cytotoxicity, inflammation, and even genotoxicity (due to facing less steric hindrance). In contrast, graphene with functionalized groups (i.e., GO, FGO such as the amine group, and rGO) is easily internalized by cells (especially in nano sizes), in addition to causing more irregular cell membrane perturbation [12,17]. GO and its derivatives have been ascertained to have specific antibacterial properties, which are also emphasized in tissue engineering applications [18]. The antibacterial activity of GO is related to various mechanisms, including membrane stress, oxidative stress, entrapment, the basal plane, and the photothermal effect. GO has sharp edges that damage the cell membrane, meaning it could, in turn, lead to bacterial cell mortality via the membrane stress mechanism. The structure of GO allows it to act as an electron acceptor; thus, in the vicinity of bacteria, the abstraction of electrons within the membrane occurs, compromising membrane integrity and killing bacterial cells (particularly P. aeruginosa and S. aureus). GO and rGO, owing to the existence of functionalized groups, can alter the partial pressure of intracellular oxygen, which results in oxidative damage that destroys the bacterial cell internal composition, particularly E. coli, through the deactivation of their proteins and lipids, which eventually leads to cell death. GO has illustrated synergistic effects with laser energy; hence, it has been used for photothermal therapy, directly enhancing its antibacterial activity [19]. Another fascinating property that GOBMs possess is antioxidant activity, and sp2 carbons play an essential role in scavenging radicals by radical adduct formation and electron transfer. Because of this characteristic, these biomaterials can effectively scavenge radicals and protect cells from high levels of oxidative stress [20]. Graphene, being nonbiodegradable (except FGO), presents serious concerns for potential toxicity, immune response, and environmental hazards [21]. It is reported that GO is susceptible to biodegradation by oxidative attack through hydrogen peroxide and horseradish peroxidase. Therefore, many attempts, such as the fabrication of nanocomposites, have been carried out to accelerate GO biodegradation, as the degradation rate of biomaterials (i.e., scaffold) must be compatible with the rate of tissues and organs [15,22,23].
4. Development of Tissues and Organs Using Graphene-Based Materials
GO and its derivatives have been added to other biomaterials either as filler to enhance their properties or as a conjugate during synthesis. The latter makes the nanocomposite materials much stronger. Below are examples of the applications of graphene derivatives in the repair and replacement of human organs (Table 1).
Table 1. Summary of preclinical studies of graphene-oxide-based materials in tissue engineering from 2017.
|
Organs |
Materials |
Animal |
Cells/Stem Cells |
Experiment |
Outcome |
Year [Ref] Country |
|
Cartilage |
Gelatin (G) and GO |
Rats |
Human chondrosarcoma cell, rat BMSCs, and rat chondrocyte cells |
Preparing nano-GO (NGO) solution => hydrogel crosslinking => three groups: non-crosslinked hydrogel (NGO (U)), crosslinked hydrogel (NGO(T)), control group (G) |
NGO(T) vs. NGO(U): ↑ mechanical properties. No significant cytotoxicity After implantation (8 weeks): fibrous tissue repair for U and Complete repair for T |
2020 [24] Taiwan |
|
Nervous system |
GO, antheraea pernyi silk fibroin (ApF) and PLCL |
Rats |
Schwann and PC12 cells |
Coating GO on ApF/PLCL nanofibers => GO reduction => applying ES => preparing the AP/RGO nerve guidance conduit |
↑ CPAM and ↑ MPs. GO: ↑ focal adhesion kinase expression of PC12 cells. ↑ Repair in animal model’s sciatic nerve |
2019 [25] China |
|
Muscle |
GO, rGO, polyacry- lamide (PAAm) |
Mice |
C2C12 myoblasts |
Incorporating GO into PAAm (GO-PAAm) => micropatterning of GO-PAAm with femtosecond laser ablation (FLA) => production of micropatterned conductive r(GO/PAAm) |
Micropatterned: ↑ differentiation and myoblast alignment r(GO/PAAm) vs. GO/PAAm: ↓ impedance values PD50/r(GO/PAAm) (optimum): ↑ tissue compatibility and ES => ↑ myogenesis. |
2019 [26] Korea |
|
Heart |
GO, rGO and alginate |
Rats with MI |
Human mesenchymal stem cells |
GO/Ag blend => hMSCs encapsulation => electrospraying and then crosslinking => GO/Ag microgels => reductive treatment => r(GO/Ag) |
rGO vs. GO: ↑ CPAM, ↑ antioxidant activity, and ↓ Oxidative stress hMSCs-CMs vs. CMs: ↑ cell viability and cardiac maturation => expressing cardiac markers |
2019 [27] Korea |
|
Bone |
GO and poly(ɛ-caprolactone) |
Rat |
MC3T3 preosteoblastic cells |
Synthesizing GO => PCL/GO pellets => melt blending => 3D printing |
↑ Protein absorbent and ↑ CPAM ↓ Immunogenicity. Treating a rat calvaria critical size defect => well-organized tissue deposition and bone remodeling. |
2019 [28] UK |
|
Skin |
Polydopamine (P), rGO (pGO), chitosan (CS), and silk fibroin (SF) (pGO-CS/SF) |
Rats with a full-thickness skin defect |
RAW 2467 cells and C2C12 myoblast cells |
Dispersing pGO into CS/SF mixture=> dual-crosslinking by poly(ethylene glycol) diglycidyl ether (PEGDE) and glutaraldehyde (GA) => freeze-drying => pGO-CS/SF scaffold |
↑ CPAM and ↑ mechanical properties ↑ Antioxidant activity => reduce cellular oxidation. Well-connected electric pathway ↑ Wound healing and ↓ oxidative stress and inflammatory responses |
2019 [29] China |
|
Nervous system |
Single (SG) and multilayered (GM) graphene PCL, RGD, polydopamine |
Rats |
Schwann cells |
Fabricating nanoscaffolds => seeding Schwann cells => implanting the 3D scaffold in sciatic nerve defect models. |
↑ CPAM and ↑ neural cell expression |
2018 [30] China |
|
Heart |
GO, gold nanoparticles (AuNPs) (GO-AuNPs), chitosan (CS) |
Rats with MI |
Rat smooth muscle cells, mouse fibroblasts, and human iPSC-CMs |
GO => embedding with AuNPs by thermal-reduction => GO-AuNPs => CS solution addition and freeze-drying => CS-GO-Au scaffolds |
↑ Electrical conductivity (at 0.5% w/v GO- AuNPs). ↑ CPAM, no immune response ↑ QRS interval (by ↑ conduction velocity and ↑ contractility). ↑ Connexin43 (Cx43) ↑ Electrical conduction and ventricular function |
2018 [31] Canada |
|
Dental |
GO, chitosan (CS), hydroxyapatite (HA) and Titanium (Ti) |
Rats |
Bone marrow stromal cells (BMSCs) |
Coating GO/CS/HA on Ti substrates by electrophoretic deposition (EPD) |
↑ CPAM and ↑ osseointegration in vivo |
2018 [32] China |
|
Dental |
GO and Collagen |
Dog |
Mouse osteoblastic MC3T3-E1 cells |
Coating Ti on the 3D collagen scaffold => evaluation of bone augmentation on the rat cranial bone => assessing the periodontal healing of class II furcation defects |
↓ Cytotoxicity. GO: cellular ingrowth behavior and angiogenesis => ↑ rat bone augmentation ↑ Periodontal attachment |
2018 [33] Japan |
|
Nervous system |
GO, rGO, and Gelatin |
Rats |
Embryonic neural progenitor cells |
Synthesizing rGO microfibers from GO => assembling rGO microfibers into the 3D gelatin hydrogel for stable implantation |
Microfiber coated with adhesive molecules => interconnected culture ↑ Differentiation in the defect site |
2017[34] Spain |
|
Bone |
GO and chitosan (CHT) |
Mice |
Murine preosteoblasts belonging to the 3T3-E1 cell line |
CHT/GO blend => freeze-drying |
↑ Alkaline phosphatase activity (ALP), ↑ osteogenesis, and ↑ bone morphogenetic protein expression ↑ Differentiation of osteoprogenitor cells ↑ New bone formation |
2017 [35] Romania |
|
Bone |
rGO and nanohydroxyapatite (nHA) |
Rabbits |
Bone mesenchymal stem cells |
Self-assembling of GO and nHA => nHA@RGO |
↑ CPAM, ↑ ALP, and ↑ osteogenic gene expression ↑ Healing circular calvarial defects (optimum: 20% nHA@RGO) ↑ Collagen deposition and ↑ mineralization |
2017 [11] China |
Keys: ↑, increased or improved; ↓, decreased; =>, followed by; CPAM, cellular proliferation, adhesion, and migration; SD, Sprague–Dawley; MPs, mechanical properties; HM, Hummer’s method; ES, electrical stimulation.
Having drastic proliferation and differentiation of stem cells into the specific tissue lineage, considered as the most critical features of an ideal scaffold. As mentioned before, GBMs have considerable potential for cells stimulating in stem cell-based therapy. In Table 2, we summarized the most common stem cells utilized for each tissue.
4.1. Nerve Muscle and Cardiac Tissue Engineering
Electrical signals and external stimuli enhance tissue regeneration for excitable tissue, specifically nerve, muscle, and cardiac tissue. Depolarization and repolarization take place between the two sides of both nerve and muscle cell membranes under an action potential, which leads to their contracting activity and response to electrical signals. Conductive materials, such as GOBMs, with similar electrical conductivity to native tissue, are considered to be promising scaffolds that stimulate cell proliferation and differentiation in stem-cell-based therapy, with decreased cytotoxicity and improved mechanical properties. They are better electron transmitters in comparison to other electronic materials, such as carbon nanotubes [30]. Much effort has been made to repair neuronal injuries, assisted by GOBMs to provide the signaling pathways between cells [36]. Despite the acceptable cellular outcome of GO film in neural tissue regeneration, the precoating of GO by polymers (e.g., collagen, laminin, poly-L-lysine (PLL), or PDL) could improve cell adhesion. There is also no preference between utilizing graphene or GO film because neural differentiation is affected by different fabrication approaches and different cell types [37]. Furthermore, Zhang et al. demonstrated the enhanced neural differentiation of a GO mat compared to an rGO mat by immersing the modified glass in 1.5 mg/mL of GO aqueous solution and then reduced the oxygen-containing groups to obtain an rGO mat [38]. The outstanding properties of the 3D structures of GO, such as electrospun mats, foam, hydrogels, and layer-by-layer casting (LBLC), in addition to their supportive role in cell viability and neural cell differentiation, are its sufficient porosity, which facilitates nutrient exchange and a high surface/volume ratio, which provides highly conductive pathways for charge transport in neural networks [37,38]. Biodegradable materials, including polycaprolactone (PCL) and poly(lactic-co-glycolic acid) (PLGA), are the most popular polymers along with GOBMs proposed in electrospinning nerve tissue engineering in recent years [25]. In the electrospinning design, achieving an optimal GO concentration is substantial, as GO concentration affects the physicochemical structure, mechanical properties, and eventually the biological properties of the material. Thus, manufacturing a scaffold with a varied GO concentration is necessary. GO concentration can affect the fiber diameter in electrospun mats. Subsequently, GO concentration can affect cellular colonization in the scaffold, which is another important parameter in nerve tissue engineering [39]. Apart from the important effect of GO concentration, the dryness or wetness state of the scaffold also affects its final properties. A new study in 2021 revealed that the conductivity, metabolic activity, and cell proliferation of the scaffold, which is a combination of silk fibroin and GO (or rGO), increases after hydration [40]. Due to the limited dispersibility of graphene-based materials (particularly pure graphene) in solvents, the conventional electrospinning method is not considered to be an efficient strategy. Thus, in novel approaches, GOBMs are coated on the surface of the nanofibrous scaffold instead of encapsulated in fibers, with no damaging effect on the nanofibrous structure [25,37,41]. Juan and coworkers demonstrated that Schwann cells can upregulate various myelin gene expressions and also release many neutrophils to support nerve regeneration in rGO-coated electrospun mats. The nanofibrous mat was saturated at the amount of 1.4% rGO, and the electrical conductivity was revealed to be 4.05 × 10−2 S/m [25]. The LBLC method was utilized to fabricate the 3D porous graphene conduit, considered an altered electrospinning method. In this method, adhesive macromolecules coated single and multilayered GO/PCL nerve conduits (Figure 3A). Via an integrated 3D-printing and LBLC procedure, the nanoscaffolds revealed a perfect cellular behavior. Due to the potency of GOBMs in nerve regeneration, there are some endeavors to develop a new class of these biomaterials, particularly in inorganic conditions. A unique hybrid scaffold made of a transition metal, synthetic Mo0.25Co1.257W0.25S3 hybridized with GO (MCWS/GO), was prepared, which led to successful nerve regeneration and recovery within 2–5 weeks after MCWS/GO transplantation [42].
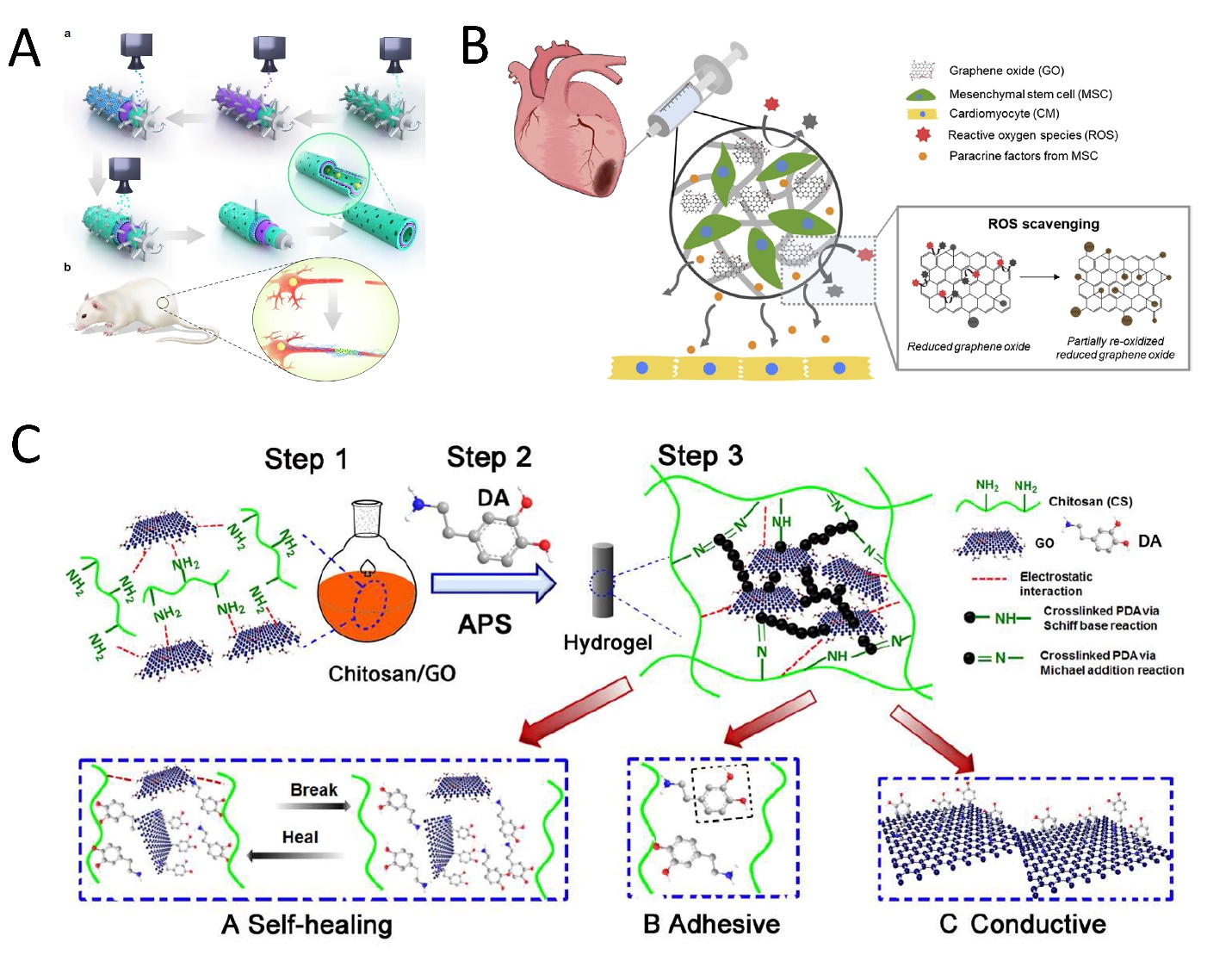
Figure 3. Schematic illustration of (A) the LBLC graphene nerve conduit. (a) The green layers are PDA/RGD adhesive macromolecules. The purple layer is single or multilayered graphene and the PCL-blended layer. The blue layer is the graphene and PCL-blended layer once more. (b) The GO/PCL nerve conduit in a sciatic nerve defect model in SD rats. Reused with permission from [30]. Copyright © 2021, Springer Nature. (B) rGO/alginate microgel embedding MSCs for cardiac tissue repair post-MI. Due to the loaded rGO, it was expected that encapsulated MSCs were protected from the severe oxidative stress in the infarcted tissue and facilitated cardiac regeneration. Reused with permission from [27]. Copyright © 2021, Elsevier Ltd. (C) CS-DA-GO composite hydrogel fabrication in three steps, which causes the enhanced properties of the hydrogel. (A) Self-healing mechanism of the hydrogel. (B) Self-adhesiveness of the hydrogel. (C) Enhanced conductivity. Reused with permission from [29]. Copyright © 2021, Elsevier Ltd.
Similar to the nerves, muscles (skeletal, cardiac, and smooth) have an electrical stimuli-responsive characteristic; thus, the utilization of conductive biomaterials was proposed. There was no difference in cell adhesion and cellular growth in a random and aligned GO electrospun mat, which was modified with oxygen plasma [43]. GO-polyurethane (PU) foam is considered to be a beneficial scaffold for myogenesis in skeletal tissue engineering. This scaffold is fabricated by the dip-coating method with a biocompatible concentration of 10 μg/mL for GO. The results showed enhanced spontaneous myogenic differentiation without any myogenic factors [44]. Xin et al. designed a muscle-inspired, self-healing, conductive, and also self-adhesive hydrogel through the combination of chitosan (CS), GO (lower than 0.75 mg/mL), and polydopamine (PDA). PDA has a great binding ability to a variety of materials. Chemically crosslinked networks enhanced electrical conductivity and also increased adhesive features caused by the presence of DA in the hydrogel (Figure 3C). The electrical conductivity of the hydrogel was revealed as 1.22 ms/cm, matching the native myocardium. The cell results also showed increased stem-cell-derived fibroblast and cardiomyocyte (CM) adhesion [29].
As mentioned, cardiac muscles are electrically conductive (0.03 to 0.6 S/m3); hence, conductive biomaterials (especially with polymeric substrates) are potential candidates that can mimic the native extracellular matrix (ECM) for cell growth, promote electrical signal conduction, and preserve electrical coupling between the myocardium cells [45–47]. The incorporation of GOBMs could treat various cardiac disorders, including cardiac regeneration after myocardial infarction (MI) [31,48], conduction disorders, and restoration [31,46,48] Particularly in the case of MI, GOBMs can overcome the high oxidative stress in the infarcted tissue due to antioxidant activity (Figure 3B) [27]. A combination of other biomaterials, mostly GO and rGO, could be used for developing blood vessels [49] and heart valves [50] to overcome the malfunctions and seems to be an attractive alternative to mechanical and biological prostheses due to high durability, high biocompatibility, and being hemodynamic. Through electrospinning, random and aligned nanofibrous matrices can be fabricated to enable the investigation of isotropic conductivity [48,51]. To this aim, blends of silk fibroin with GO (SF/GO) and rGO (SF/rGO) were prepared for random and aligned electrospinning in a previous study, which resulted in superior electrical conduction properties in SF/rGO (from a mean resistance of 4866.7 MΩ to 4.3 MΩ) in a concentration-dependent manner. The aligned nanofibers not only caused anisotropic conductivity but also aligned and strengthened sarcomeric structures, with an optimum rGO thickness of ~100 nm. The concentration of 0.02–0.08 mg/mL rGO fundamentally enhanced the expression of cardiac-specific proteins, the formation of gap junctions, beating rate, cardiac tissue contraction, and regeneration [48]. Micro- and nanopatterning is another method to develop anisotropic conductive scaffolds to enhance the biomimicry of cardiac tissue constructs. Smith et al. transferred GO films onto polymeric, polyethylene glycol (PEG), and topographic substrates [52]. The electroconductive scaffold had a 7.071 ± 0.124 KΩ resistance in the transverse orientation, which indicated an increment in the myofibril and sarcomere property, cell–cell coupling and calcium-handling protein expression, and action potential [52]. In several studies, other conductive scaffolds (i.e., hydrogels, microgels, sponges, and foams), with a combination of GOBMs, fabricated and fundamentally improved cardiac regeneration and function [27,31,46,53].
4.2. Bone Tissue Engineering
The bone has a prominent ability to regenerate; however, the human skeleton has a limited capacity of self-regeneration when bone defects are large enough or critical sized. Several different approaches have been utilized for bone tissue engineering (BTE); the most common were those that tried to mimic the natural process of bone repair using 3D osteoconductive scaffolds. Recently, the potential of GOBMs has gained tremendous attention to facilitate and improve BTE [54–59] in its various forms, namely scaffolds, coatings, guided bone membranes, and drug delivery systems [60]. Moreover, GOBMs, particularly with low oxygen contents, have a pivotal influence on adult mesenchymal stem cells (MSCs) to increase osteoinductivity and osteoconductivity [11,61]. Bone is a piezoelectric tissue; hence, using GOBMs, they supply either essential mechanical and biological requirements or electrical conductivity [28]. GOBMs, rGO in particular, could enhance levels of biocompatibility, alkaline phosphatase activity, and calcium deposits, which are essential for bone regeneration [62]. Biphasic calcium phosphate (BCP) coated with rGO (BCP-rGO) at various concentrations was fabricated in a previous study. Briefly, the prepared bone graft materials were implanted in rats with calvarial defects, the common model for investigating new bone regeneration (Figure 4B). The grafting resulted in a new volume of bone formation after eight weeks, particularly in a specimen with a concentration ratio of 4:1000 rGO:BCP (7.65 ± 1.39 mm3). The results indicated rGO enhances bone regeneration, although when the rGO percentage exceeded a certain threshold level (≥100 μg/mL), it became severely cytotoxic, [63] which complies with other studies [11,28,60,64]. Recently, researchers have developed 3D-printed scaffolds entailing GOBMs to mimic not only bone geometry but also bone remodeling. Wang et al. fabricated a novel 3D-printed scaffold made of poly(ɛ-caprolactone)/graphene. As expected, the addition of small concentrations of GO enhanced mechanical properties and osteogenesis (by applying electrical stimulation, 10 μA), which led to a better bone defect treatment and bone deposition [28]. Additionally, GOBMs (GO and rGO in particular) presented antimicrobial activity without compromising osteoblasts’ viability, attachment, and proliferation (Figure 4A) [58,65]. A key characteristic of GOBMs is the ability to induce bone differentiation, which leads to rapid bone repair. It has been reported that bioinspired composite scaffolds based on gelatin and GO (GG) could induce the bidirectional differentiation of bone marrow stromal cells (BMSCs) by activating the Erk1/2 and AKT pathway. The presence of GG created the initial hypoxia, which gradually transformed into a well-vasculature robust condition with the bidifferentiation of BMSCs in calvarial defect models [66].
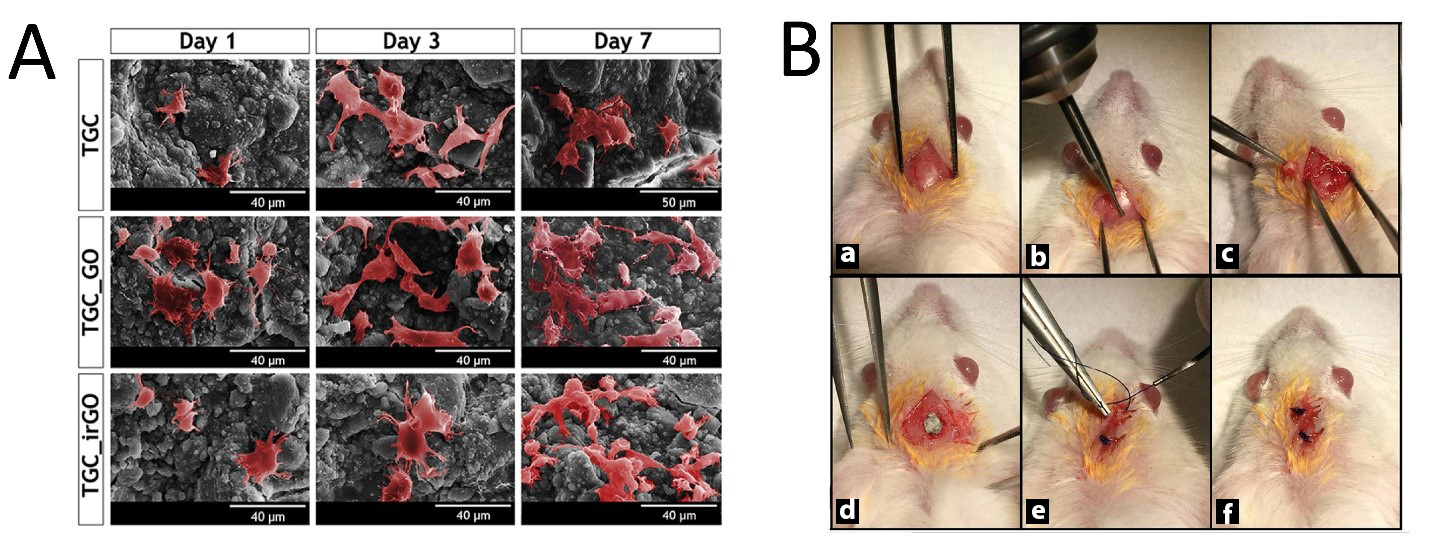
Figure 4. (A) Pseudocolored SEM images of human osteoblasts that successfully adhered to the surface of scaffolds at an upward trend (Days 1, 3, and 7). Reused with permission from [58]. Copyright © 2021, Elsevier Ltd. (B) Surgical procedure of the preparation of the surgical size defect (5 mm) critical in the rat calvarium (a–c), placement of the chitosan–graphene oxide scaffold (d), and closure of the periosteum and skin (e,f). Reused with permission from [64]. Copyright © 2021, Springer Nature.
4.3. Skin Tissue Engineering
The skin acts as a vital barrier, blocking the entrance of dangerous foreign matter into the body [67]. Because of this significance, GOBMs have been applied for skin regeneration by wound dressings [68] or skin monitoring via electronic skin [69]. Wound dressings, in the form of electrospun mats [70,71], hydrogels [68,72], and sponges, [73] are the best option to accelerate wound healing; hence, GOBMs have been incorporated within these forms of dressings, although other shapes (e.g., modified rGO nanosheets [74]) could also provide drastic novel treatment options. Tang and coworkers fabricated an inspired scaffold by mussel chemistry, based on polydopamine-rGO (pGO) that was incorporated in chitosan (CS) and silk fibroin (SF) hydrogel (pGO-CS/SF). Due to the pGO, electroactivity responded to electrical signals and increased cytological behavior (Figure 5A) [68]. Antioxidant activity reduced cellular oxidation by removing excessive radical oxygen species (ROS) [68,75]. It is a plus that rGO could substantially enhance angiogenesis, which is massively beneficial for slow-healing/chronic wounds [76]. Alongside these applications, it is necessary to recognize the GOBMs and their interactions with the skin, as it is one of the main exposing routes of materials [67,77]. The effects of GO and few-layer graphene (FLG) with HaCaT keratinocytes were investigated. FLG (>5 µg/mL) and mainly GO (~2–5 µg/mL) in long exposure times increased the level of ROS and led to mitochondrial and plasma membrane damages [67,77]. Our own experience with skin regeneration is the development of a membrane from an FGO-based nanocomposite with a hydrophobic outer layer with low porosities to keep bacteria away from the skin, while the inner layer was hydrophilic and more porous to seed with stem cells and growth factors.
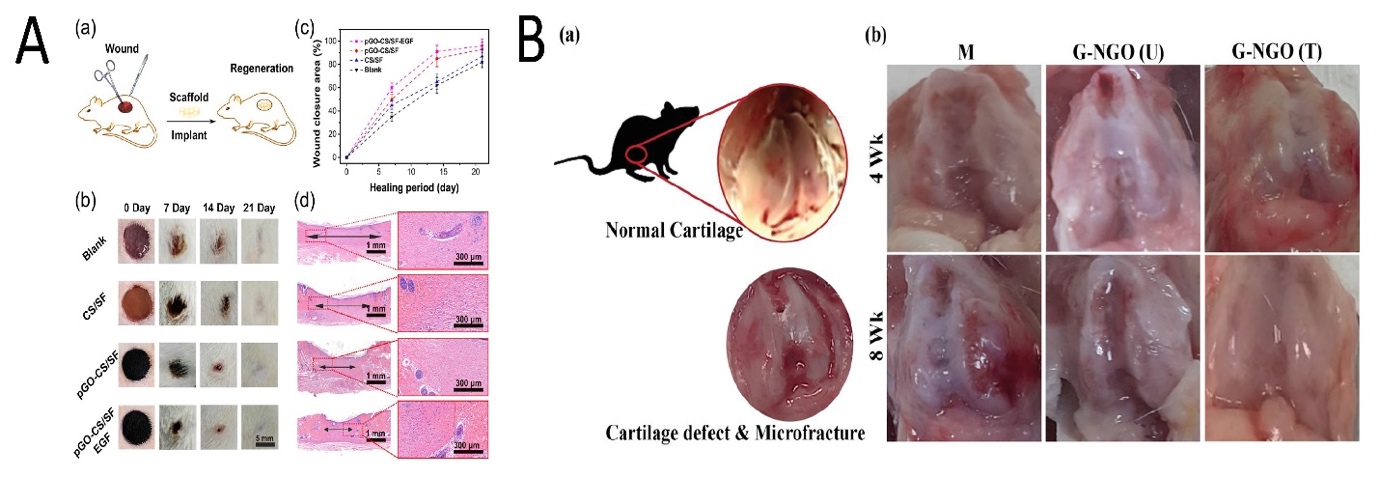
Figure 5. (A) Wound healing of the skin through the treatment of various scaffolds. (a) Model of wound regeneration. Digital images (b) and closure rate (c) of the wound defects; multiple treatments at Days 0, 7, 14, and 21. (d) Depictive images of H&E-stained histological sections after 21 days (arrows indicate the granulation tissue). Reused with permission from [68]. Copyright © 2021 American Chemical Society. (B) (a) A normal rat knee joint; (b) knee joint restoration in different experimental groups (M: without implant; GO-NGO (U): non-crosslinked hydrogel; GO-NGO (T): microplasma crosslinked hydrogel) i 4 and 8 weeks. Reused with permission from [24]. Copyright © 2021 American Chemical Society.
4.4. Cartilage Tissue Engineering
Cartilage is an avascular tissue that has limited self-healing potential compared to other tissues [78]. Thus, utilizing a proper microenvironment for chondrogenic differentiation will eventually lead to a hyaline-cartilage-like formation. GOBMs in cartilage tissue engineering (CTE) are recognized as promising substitutes due to their self-lubricating and antiwear properties, as well as enhanced mechanical properties, in addition to improved biological behavior [79]. Chitosan/poly(vinyl alcohol) (PVA)/GO (6 wt %) electrospun mats with a 1.81 MPa tensile strength are considered promising when used to reinforce scaffolds in CTE [80]. Yeqiao et al. fabricated a PVA/GO-PEG nanocomposite hydrogel using the freezing/thawing method. The presence of PEG caused the efficient grafting of PVA molecules on the GO surface. The tensile strength, elongation at break, and compressive modulus increased. Furthermore, for samples with a 1.5% GO content, the maximum force retention and dynamic stiffness were improved. In composite hydrogel, the friction coefficient decreased by more than 50% [79]. The small amount of nanographene oxide (NGO) (1 wt %) enhances both the mechanical and biomedical features of microplasma crosslinked gelatin-based hydrogel and provides hyaline cartilage formation without expensive growth factors (Figure 5B). Gelatin methacrylate (GelMa)-poly(ethylene glycol) diacrylate (PEGDA)-GO is a novel cartilage printing ink that increased glycosaminoglycan and collagen levels after the GO-induced chondrogenic differentiation of hMSCs [81]. Seifalian and coworkers have been working on the development of the ear for auricular cartilage reconstruction for children with microtia [82]. Recently, they used an FGO-based nanocomposite as a 3D microporous scaffold with adipose-derived stem cells [83].
4.5. Dental Application
Most studies in dental tissue engineering have emphasized the role of GOBMs in the osteogenic differentiation of stem cells after dental implantation, which is an essential function in regenerative dentistry. The potential of GO was demonstrated to induce the odontoblastic or osteogenic differentiation of dental pulp stem cells (DPSCs) without the use of any chemical inductors [84]. However, GOBMs are also utilized in the inhibition of bacterial biofilm formation, membranes, resin, cement, and teeth whitening [85]. Ti, as a metal-based implant, is the best replacement for teeth due to its excellent biocompatibility, high corrosion resistance, and long-term performance; however, Ti has a relatively weak shear strength. The surface treatment of Ti with GOBMs enhances the mechanical implant properties and also exhibits a uniform and widely spread roughness, which affects stem cell [81,85–87] bacterial contamination, causes impaired osteogenesis, and is an essential challenge after dental implantation [88]. The presence of GO, coated on Ti(GO-Ti), has shown enhanced antibacterial properties. Immobilized GO on Ti limits the inhibition growth of bacteria, although the addition of antibiotics (e.g., minocycline hydrochloride) or silver nanoparticles is recommended [79,86] (Figure 6). Compared with Ti-based implants, the combination of zirconia (ZrO2) and GO in dental implants resulted in healthier surrounding tissue and fewer bacteria growth, in addition to possessing enhanced mechanical features (bending strength increased by 200%, toughness increased by 41%) [89]. The combination of GO with resins, cement, and adhesives inhibits bacterial growth in the oral cavity [85]. Barrier membranes are used in oral surgical procedures for bone augmentation. Membranes can be made of different materials, such as collagen. The presence of GO on the collagen membrane affects the mechanical properties that promote osteoblastic differentiation and decrease inflammation [87]. The last application of GOBMs in the dental field is in teeth whitening, which is based on hydrogen peroxide (H2O2) and GOBM nanocomposites. This combination enhanced the whitening effect of H2O2 and decreased treatment time [85].
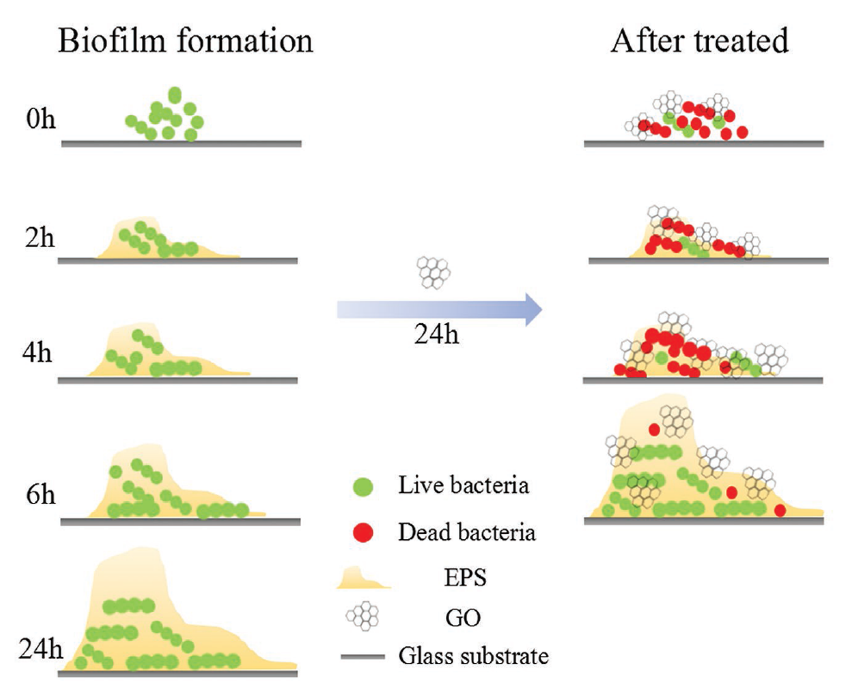
Figure 6. The prohibition effect of GO on biofilm formation. Reused with permission from [90]. Copyright © 2021 WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim.
Table 2. The most common stem cells utilized in each tissue [24,26,32,90].
|
Tissue |
Stem cells |
|
Bone |
hMSCs, hADMSCs, MC3T3-E1, DPSCs, PDLSCs |
|
Nerve |
NSCs, hMSCs, hADMSCs, ESCs, iPSCs, SCAP |
|
Muscle and cardiac |
C2C12, MSCs, hMSCs, cardiomyocytes, and EC |
|
Cartilage |
Human mesenchymal stem cell |
|
Skin |
MSCs, human dermal fibroblasts (HDFs) |
|
Dental |
DPSCs, PDLSCs, hMSCs, BMSCs |
Keys: hADMSCs, human adipose-derived mesenchymal stem cells; DPSCs, dental pulp stem cells; PDLSCs, periodontal ligament stem cells; NSCs, neural stem cells; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; SCAP, stem cell from apical papilla; BMSCs
bone-marrow-derived stem cells; C2C12, mouse myoblasts; EC, endothelial cells.
5. Conclusions
Graphene oxide has raised considerable interest in tissue engineering and regenerative medicine due to its specific characteristics, e.g., exceptional mechanical properties and electrical conductivity, as well as physiochemical, antibacterial, and biological capabilities. The biological features of GOBMs depend on many parameters, including concentration, size, shape, and surface chemistry. Within GOBMs, FGO and rGO exhibited the greatest antibacterial capability. Moreover, GOBMs in various forms of 2D and 3D structures could stimulate the proliferation and differentiation of cells to a specific lineage, designated to chemical reactions with biomolecules. Growth factors, small molecules, and ECM proteins force cell differentiation via GOBMs’ π–π and hydrophobic interactions. The main challenges associated with utilizing GOBMs are toxicity, the lack of a detailed mechanism of GOBMs’ biological features, and the explanation of the biological pathway, which has still not been clarified in depth. Moreover, most research has concentrated on toxicity at the cellular level instead of at the genetic level. GOBMs can interact with different biomolecules, especially the DNA; therefore, studies are needed to elucidate the apoptosis pathway. ROS production, which causes cell death, has been studied more, along with lung, liver, intestine, and kidney tissues, than other tissues, e.g., nerve, muscle, cardiac, bone, skin, cartilage, and dental tissue. The application of GOBMs in biomedicine is summarized in Figure 7.
Figure 7. Graphical conclusion.
6. Future Direction
The long-term toxicity of GOBMs, known as a primary hurdle, has limited experimental data, and further research investigating their effects is necessary (Figure 8). This uncertainty has impeded widespread access to clinics; therefore, ensuring human safety is a priority for these biomaterials. In other words, to achieve the best clinical outcomes, it is essential to consider solutions to overcome the possible challenges of GOBMs, such as their toxicity and biodegradability; for example, by developing standardized parameters for GOBMs utilization, as their toxicity and biological features are intertwined with physicochemical parameters. Another solution that could minimize these drawbacks is fabricating biocomposites. It is worth noting that the dose- and time-dependent effects of GOBMs dictate whether or not ROS scavenging or oxidative stress affects cells detrimentally. The interaction of GOBMs at the genetic level should be considered as well, as they can interact with DNA and result in alterations of the genetics in the human population. Although research on GOBMs is still in an early stage, due to its versatile properties, it will provide extensive applications for biomaterial science and regenerative medicines. We have been working on the development of functionalized graphene oxide (FGO)-based nanocomposite materials for biomedical applications [82]. The two families of materials developed are Hastalex™, a nonbiodegrable nanocomposite for applications such as heart valves [50], tendons, and breast implants, and a bioabsorbance version (BioHastalex™), which has been used for nerve regeneration and tissue engineering applications, as well as for other industries such as the textile industry, with sustainably produced, nontoxic, and biodegradable materials in the ocean (Nanoloom.co.uk).
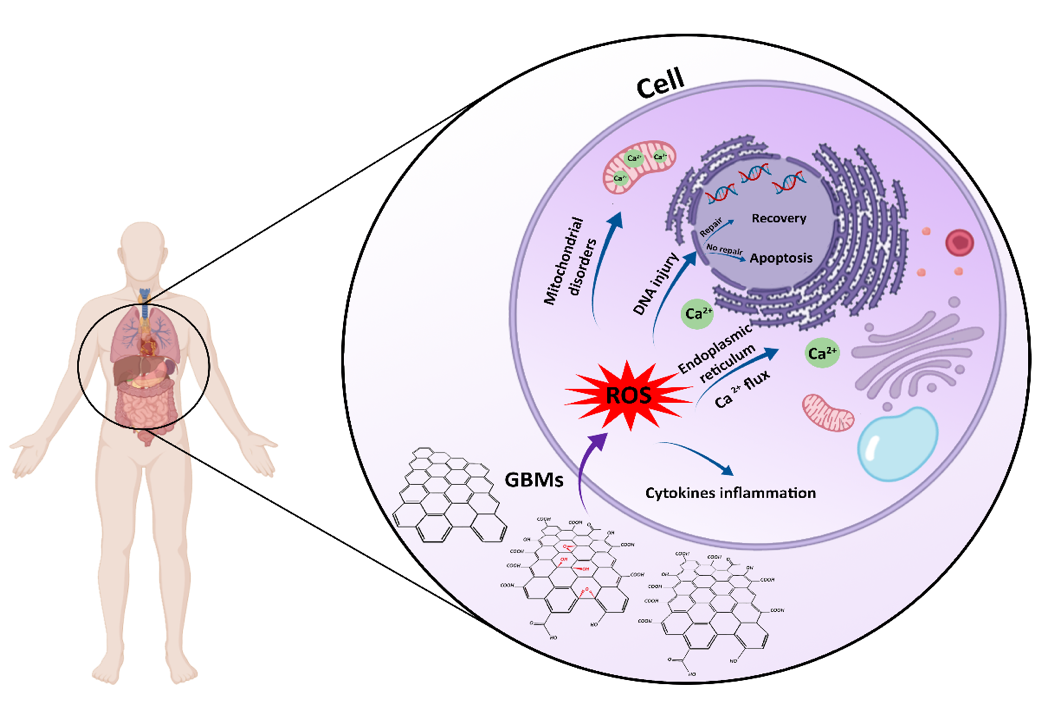
Figure 8. Schematic illustration of the cellular toxicity of GOBMs when exposed to the cell. GOBMs enter the cell through various pathways, which affects their shape, size, and surface chemistry and eventually results in ROS production. An increased ROS level may cause mitochondrial membrane depolarization and Ca2+ release; lipid, protein, and DNA damage; and inflammation response by releasing cytokines and chemokines.
Author Contributions: The research topic was initiated by A.M.S. and highlighted the subtitle. P.Z. and M.A. equally worked on it and drafted the review. A.S. and Z.B. analysed the data and tabulated the tables with P.Z. & M.A. and help with the discussion. A.M.S. critically reviewed the data and expanded the review with his own experience and work on graphene oxide. Overall supervision of research carried out by Z.B. and A.M.S. All authors have read and agreed to the published version of the manuscript.
Funding: This research received no external funding.
Data Availability Statement: Physicochemical properties of Hastalex and BioHastalex is available on www.NanoRegMed.com.
Conflicts of Interest: The authors declare no conflicts of interest.
References
- Dag Line, P. The Fundamental Challenges in Organ Transplantation. OBM Transpl. 2017, 1, 6, doi:10.21926/obm.transplant.1704006.
- Aleemardani, M.; Bagher, Z.; Farhadi, M.; Chahsetareh, H.; Najafi, R.; Eftekhari, B.; Seifalian, A.M. Can tissue engineering bring hope in the development of human tympanic membrane? Tissue Eng. Part B Rev. 2020, 1–62, doi:10.1089/ten.teb.2020.0176.
- Peng, Z.; Zhao, T.; Zhou, Y.; Li, S.; Li, J.; Leblanc, R.M. Bone Tissue Engineering via Carbon‐Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495.
- Aydin, T.; Gurcan, C.; Taheri, H.; Yilmazer, A. Graphene based materials in neural tissue regeneration. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; Volume 1107, pp. 129–142.
- Bellet, P.; Gasparotto, M.; Pressi, S.; Fortunato, A.; Scapin, G.; Mba, M.; Menna, E.; Filippini, F. Graphene-based scaffolds for regenerative medicine. Nanomaterials 2021, 11, 404.
- Bacakova, L.; Pajorova, J.; Tomkova, M.; Matejka, R.; Broz, A.; Stepanovska, J.; Prazak, S.; Skogberg, A.; Siljander, S.; Kallio, P. Applications of nanocellulose/nanocarbon composites: Focus on biotechnology and medicine. Nanomaterials 2020, 10, 196, doi:10.3390/nano10020196.
- Jiang, X.; Ruan, G.; Huang, Y.; Chen, Z.; Yuan, H.; Du, F. Assembly and Application Advancement of Organic-Functionalized Graphene-Based Materials: A Review. J. Sep. Sci. 2020, 43, 1544–1557.
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, doi:10.3390/ijms19113564.
- Lakshmanan, R.; Maulik, N. Graphene-based drug delivery systems in tissue engineering and nanomedicine. Can. J. Physiol. Pharmacol. 2018, 96, 869–878, doi:10.1139/cjpp-2018-0225.
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921, doi:10.1021/acsami.7b08585.
- Nie, W.; Peng, C.; Zhou, X.; Chen, L.; Wang, W.; Zhang, Y.; Ma, P.X.; He, C. Three-dimensional porous scaffold by self-assembly of reduced graphene oxide and nano-hydroxyapatite composites for bone tissue engineering. Carbon 2017, 116, 325–337, doi:10.1016/j.carbon.2017.02.013.
- Li, J.; Liu, X.; Crook, J.M.; Wallace, G.G. 3D graphene-containing structures for tissue engineering. Mater. Today Chem. 2019, 14, 100199.
- Zhao, H.; Ding, R.; Zhao, X.; Li, Y.; Qu, L.; Pei, H.; Yildirimer, L.; Wu, Z.; Zhang, W. Graphene-based nanomaterials for drug and/or gene delivery, bioimaging, and tissue engineering. Drug Discov. Today 2017, 22, 1302–1317.
- Shang, L.; Qi, Y.; Lu, H.; Pei, H.; Li, Y.; Qu, L.; Wu, Z.; Zhang, W. Graphene and Graphene Oxide for Tissue Engineering and Regeneration. In Theranostic Bionanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–185.
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843.
- Sayyar, S.; Officer, D.L.; Wallace, G.G. Fabrication of 3D structures from graphene-based biocomposites. J. Mater. Chem. B 2017, 5, 3462–3482.
- Martín, C.; Kostarelos, K.; Prato, M.; Bianco, A. Biocompatibility and biodegradability of 2D materials: Graphene and beyond. Chem. Commun. 2019, 55, 5540–5546.
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-Based Antimicrobial Biomedical Surfaces. ChemPhysChem 2020, 22, 250–263, doi:10.1002/cphc.202000769.
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737.
- Han, J.; Kim, Y.S.; Lim, M.-Y.; Kim, H.Y.; Kong, S.; Kang, M.; Choo, Y.W.; Jun, J.H.; Ryu, S.; Jeong, H.-Y.; et al. Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair. ACS Nano 2018, 12, 1959–1977.
- Amrollahi-Sharifabadi, M.; Koohi, M.K.; Zayerzadeh, E.; Hablolvarid, M.H.; Hassan, J.; Seifalian, A.M. In vivo toxicological evaluation of graphene oxide nanoplatelets for clinical application. Int. J. Nanomed. 2018, 13, 4757–4769, doi:10.2147/IJN.S168731.
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416.
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of carbon nanotubes, graphene, and their derivatives. Trends Biotechnol. 2017, 35, 836–846.
- Satapathy, M.K.; Manga, Y.B.; Ostrikov, K.K.; Chiang, W.H.; Pandey, A.; Lekha, R.; Nyambat, B.; Chuang, E.Y.; Chen, C.H. Microplasma Cross-Linked Graphene Oxide-Gelatin Hydrogel for Cartilage Reconstructive Surgery. ACS Appl. Mater. Interfaces 2020, 12, 86–95, doi:10.1021/acsami.9b14073.
- Wang, J.; Cheng, Y.; Chen, L.; Zhu, T.; Ye, K.; Jia, C.; Wang, H.; Zhu, M.; Fan, C.; Mo, X. In vitro and in vivo studies of electroactive reduced graphene oxide-modified nanofiber scaffolds for peripheral nerve regeneration. Acta Biomater. 2019, 84, 98–113, doi:10.1016/j.actbio.2018.11.032.
- Park, J.; Choi, J.H.; Kim, S.; Jang, I.; Jeong, S.; Lee, J.Y. Micropatterned conductive hydrogels as multifunctional muscle-mimicking biomaterials: Graphene-incorporated hydrogels directly patterned with femtosecond laser ablation. Acta Biomater. 2019, 97, 141–153, doi:10.1016/j.actbio.2019.07.044.
- Choe, G.; Kim, S.-W.; Park, J.; Park, J.; Kim, S.; Kim, Y.S.; Ahn, Y.; Jung, D.-W.; Williams, D.R.; Lee, J.Y. Anti-oxidant activity reinforced reduced graphene oxide/alginate microgels: Mesenchymal stem cell encapsulation and regeneration of infarcted hearts. Biomaterials 2019, 225, 119513.
- Wang, W.; Junior, J.R.P.; Nalesso, P.R.L.; Musson, D.; Cornish, J.; Mendonça, F.; Caetano, G.F.; Bártolo, P. Engineered 3D printed poly (ɛ-caprolactone)/graphene scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 100, 759–770.
- Jing, X.; Mi, H.Y.; Napiwocki, B.N.; Peng, X.F.; Turng, L.S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570, doi:10.1016/j.carbon.2017.09.071.
- Qian, Y.; Zhao, X.; Han, Q.; Chen, W.; Li, H.; Yuan, W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018, 9, doi:10.1038/s41467-017-02598-7.
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018, 8, 1–13.
- Suo, L.; Jiang, N.; Wang, Y.; Wang, P.; Chen, J.; Pei, X.; Wang, J.; Wan, Q. The enhancement of osseointegration using a graphene oxide/chitosan/hydroxyapatite composite coating on titanium fabricated by electrophoretic deposition. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 635–645, doi:10.1002/jbm.b.34156.
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class II furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376, doi:10.2147/IJN.S163206.
- Mayorga, A.G.; Dolado, E.L.; Gutierrez, M.C.; Collazos-Castro, J.E.; Luisa Ferrer, M.; Del Monte, F.; Serrano, M.C. Favorable biological responses of neural cells and tissue interacting with graphene oxide microfibers. ACS Omega 2017, 2, 8253–8263, doi:10.1021/acsomega.7b01354.
- Huang, C.T.; Kumar Shrestha, L.; Ariga, K.; Hsu, S.H. A graphene-polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864, doi:10.1039/c7tb01594a.
- Burnstine‐Townley, A.; Eshel, Y.; Amdursky, N. Conductive Scaffolds for Cardiac and Neuronal Tissue Engineering: Governing Factors and Mechanisms. Adv. Funct. Mater. 2019, 30, 1901369, doi:10.1002/adfm.201901369.
- Zhang, Z.; Klausen, L.H.; Chen, M.; Dong, M. Electroactive Scaffolds for Neurogenesis and Myogenesis: Graphene-Based Nanomaterials. Small 2018, 14, 1801983, doi:10.1002/smll.201801983.
- Feng, Z.Q.; Yan, K.; Shi, C.; Xu, X.; Wang, T.; Li, R.; Dong, W.; Zheng, J. Neurogenic differentiation of adipose derived stem cells on graphene-based mat. Mater. Sci. Eng. C 2018, 90, 685–692, doi:10.1016/j.msec.2018.05.019.
- Ginestra, P. Journal of the Mechanical Behavior of Biomedical Materials Manufacturing of polycaprolactone—Graphene fi bers for nerve tissue engineering. J. Mech. Behav. Biomed. Mater. 2019, 100, 103387.
- Magaz, A.; Li, X.; Gough, J.E.; Blaker, J.J. Graphene oxide and electroactive reduced graphene oxide-based composite fibrous scaffolds for engineering excitable nerve tissue. Mater. Sci. Eng. C 2021, 119, 111632, doi:10.1016/j.msec.2020.111632.
- Zhang, Z.; Xu, R.; Wang, Z.; Dong, M.; Cui, B.; Chen, M. Visible-Light Neural Stimulation on Graphitic-Carbon Nitride/Graphene Photocatalytic Fibers. ACS Appl. Mater. Interfaces 2017, 9, 34736–34743, doi:10.1021/acsami.7b12733.
- Askari, N.; Askari, M.B.; Shafieipour, A. Investigation the molecular structure of novel graphene hybrid scaffold in nerve regeneration. J. Mol. Struct. 2019, 1186, 393–403, doi:10.1016/j.molstruc.2019.03.058.
- Uehara, T.M.; Paino, I.M.M.; Santos, F.A.; Scagion, V.P.; Correa, D.S.; Zucolotto, V. Fabrication of random and aligned electrospun nanofibers containing graphene oxide for skeletal muscle cells scaffold. Polym. Adv. Technol. 2020, 31, 1437–1443, doi:10.1002/pat.4874.
- Shin, Y.C.; Kang, S.H.; Lee, J.H.; Kim, B.; Hong, S.W.; Han, D.W. Three-dimensional graphene oxide-coated polyurethane foams beneficial to myogenesis. J. Biomater. Sci. Polym. Ed. 2018, 29, 762–774, doi:10.1080/09205063.2017.1348738.
- Bahrami, S.; Solouk, A.; Mirzadeh, H.; Seifalian, A.M. Electroconductive polyurethane/graphene nanocomposite for biomedical applications. Compos. Part B Eng. 2019, 168, 421–431.
- Norahan, M.H.; Pourmokhtari, M.; Saeb, M.R.; Bakhshi, B.; Zomorrod, M.S.; Baheiraei, N. Electroactive cardiac patch containing reduced graphene oxide with potential antibacterial properties. Mater. Sci. Eng. C 2019, 104, 109921.
- Bao, R.; Tan, B.; Liang, S.; Zhang, N.; Wang, W.; Liu, W. A π-π conjugation-containing soft and conductive injectable polymer hydrogel highly efficiently rebuilds cardiac function after myocardial infarction. Biomaterials 2017, 122, 63–71.
- Zhao, G.; Qing, H.; Huang, G.; Genin, G.M.; Lu, T.J.; Luo, Z.; Xu, F.; Zhang, X. Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues. NPG Asia Mater. 2018, 10, 982–994.
- Huo, D.; Liu, G.; Li, Y.; Wang, Y.; Guan, G.; Yang, M.; Wei, K.; Yang, J.; Zeng, L.; Li, G. Construction of antithrombotic tissue-engineered blood vessel via reduced graphene oxide based dual-enzyme biomimetic cascade. ACS Nano 2017, 11, 10964–10973.
- Ovcharenko, E.A.; Seifalian, A.; Rezvova, M.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Akenteva, T.N.; Antonova, L.V.; Velikanova, E.A.; Chernonosova, V.S.; Shevelev, G.Y.; et al. A new nanocomposite copolymer Based on functionalised Graphene oxide for Development of Heart Valves. Sci. Rep. 2020, 10, 5271.
- Nazari, H.; Azadi, S.; Hatamie, S.; Zomorrod, M.S.; Ashtari, K.; Soleimani, M.; Hosseinzadeh, S. Fabrication of graphene‐silver/polyurethane nanofibrous scaffolds for cardiac tissue engineering. Polym. Adv. Technol. 2019, 30, 2086–2099.
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.-H. Micro-and nano-patterned conductive graphene–PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. 2017, 53, 7412–7415.
- Bahrami, S.; Baheiraei, N.; Mohseni, M.; Razavi, M.; Ghaderi, A.; Azizi, B.; Rabiee, N.; Karimi, M. Three-dimensional graphene foam as a conductive scaffold for cardiac tissue engineering. J. Biomater. Appl. 2019, 34, 74–85.
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing cell proliferation and osteogenic differentiation of MC3T3-E1 pre-osteoblasts by BMP-2 delivery in graphene oxide-incorporated PLGA/HA biodegradable microcarriers. Sci. Rep. 2017, 7, 1–13.
- Luo, H.; Ao, H.; Peng, M.; Yao, F.; Yang, Z.; Wan, Y. Effect of highly dispersed graphene and graphene oxide in 3D nanofibrous bacterial cellulose scaffold on cell responses: A comparative study. Mater. Chem. Phys. 2019, 235, doi:10.1016/j.matchemphys.2019.121774.
- Shie, M.-Y.; Chiang, W.-H.; Chen, I.-W.P.; Liu, W.-Y.; Chen, Y.-W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C 2017, 73, 726–735.
- Wang, P.; Yu, T.; Lv, Q.; Li, S.; Ma, X.; Yang, G.; Xu, D.; Liu, X.; Wang, G.; Chen, Z.; et al. Fabrication of hydroxyapatite/hydrophilic graphene composites and their modulation to cell behavior toward bone reconstruction engineering. Colloids Surf. B Biointerfaces 2019, 173, 512–520.
- Cabral, C.S.D.; Miguel, S.P.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. In situ green reduced graphene oxide functionalized 3D printed scaffolds for bone tissue regeneration. Carbon 2019, 146, 513–523.
- Daneshmandi, L.; Barajaa, M.; Tahmasbi Rad, A.; Sydlik, S.A.; Laurencin, C.T. Graphene-Based Biomaterials for Bone Regenerative Engineering: A Comprehensive Review of the Field and Considerations Regarding Biocompatibility and Biodegradation. Adv. Healthc. Mater. 2021, 10, e2001414, doi:10.1002/adhm.202001414.
- Cheng, X.; Wan, Q.; Pei, X. Graphene family materials in bone tissue regeneration: Perspectives and challenges. Nanoscale Res. Lett. 2018, 13, 289.
- Elkhenany, H.; Bourdo, S.; Hecht, S.; Donnell, R.; Gerard, D.; Abdelwahed, R.; Lafont, A.; Alghazali, K.; Watanabe, F.; Biris, A.S.; et al. Graphene nanoparticles as osteoinductive and osteoconductive platform for stem cell and bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2117–2126.
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and in vivo study. Nanomed. Nanotechnol. Biol. Med. 2020, 29, doi:10.1016/j.nano.2020.102251.
- Kim, J.-W.; Shin, Y.C.; Lee, J.-J.; Bae, E.-B.; Jeon, Y.-C.; Jeong, C.-M.; Yun, M.-J.; Lee, S.-H.; Han, D.-W.; Huh, J.-B. The effect of reduced graphene oxide-coated biphasic calcium phosphate bone graft material on osteogenesis. Int. J. Mol. Sci. 2017, 18, 1725.
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-Graphene Oxide 3D scaffolds as Promising Tools for Bone Regeneration in Critical-Size Mouse Calvarial Defects. Sci. Rep. 2017, 7, doi:10.1038/s41598-017-16599-5.
- Jaidev, L.R.; Kumar, S.; Chatterjee, K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf. B Biointerfaces 2017, 159, 293–302.
- Jiao, D.; Zheng, A.; Liu, Y.; Zhang, X.; Wang, X.; Wu, J.; She, W.; Lv, K.; Cao, L.; Jiang, X. Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact. Mater. 2021, 6, 2011–2028, doi:10.1016/j.bioactmat.2020.12.003.
- Pelin, M.; Fusco, L.; León, V.; Martín, C.; Criado, A.; Sosa, S.; Vázquez, E.; Tubaro, A.; Prato, M. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes. Sci. Rep. 2017, 7, 40572.
- Tang, P.; Han, L.; Li, P.; Jia, Z.; Wang, K.; Zhang, H.; Tan, H.; Guo, T.; Lu, X. Mussel-inspired electroactive and antioxidative scaffolds with incorporation of polydopamine-reduced graphene oxide for enhancing skin wound healing. ACS Appl. Mater. Interfaces 2019, 11, 7703–7714.
- An, J.; Le, T.-S.D.; Huang, Y.; Zhan, Z.; Li, Y.; Zheng, L.; Huang, W.; Sun, G.; Kim, Y.-J. All-graphene-based highly flexible noncontact electronic skin. ACS Appl. Mater. Interfaces 2017, 9, 44593–44601.
- Zhang, Q.; Du, Q.; Zhao, Y.; Chen, F.; Wang, Z.; Zhang, Y.; Ni, H.; Deng, H.; Li, Y.; Chen, Y. Graphene oxide-modified electrospun polyvinyl alcohol nanofibrous scaffolds with potential as skin wound dressings. RSC Adv. 2017, 7, 28826–28836.
- Narayanan, K.B.; Park, G.T.; Han, S.S. Electrospun poly (vinyl alcohol)/reduced graphene oxide nanofibrous scaffolds for skin tissue engineering. Colloids Surf. B Biointerfaces 2020, 191, 110994.
- Narayanan, K.B.; Choi, S.M.; Han, S.S. Biofabrication of Lysinibacillus sphaericus-reduced graphene oxide in three-dimensional polyacrylamide/carbon nanocomposite hydrogels for skin tissue engineering. Colloids Surfaces B Biointerfaces 2019, 181, 539–548.
- Nyambat, B.; Chen, C.-H.; Wong, P.-C.; Chiang, C.-W.; Satapathy, M.K.; Chuang, E.-Y. Genipin-crosslinked adipose stem cell derived extracellular matrix-nano graphene oxide composite sponge for skin tissue engineering. J. Mater. Chem. B 2018, 6, 979–990.
- Gnanasekar, S.; Palanisamy, P.; Jha, P.K.; Murugaraj, J.; Kandasamy, M.; Mohamed Hussain, A.M.K.; Sivaperumal, S. Natural Honeycomb Flavone Chrysin (5, 7-dihydroxyflavone)-Reduced Graphene Oxide Nanosheets Fabrication for Improved Bactericidal and Skin Regeneration. ACS Sustain. Chem. Eng. 2018, 6, 349–363.
- Di Luca, M.; Vittorio, O.; Cirillo, G.; Curcio, M.; Czuban, M.; Farfalla, A.; Hampel, S.; Nicoletta, F.P.; Iemma, F.; Voli, F. Electro-responsive graphene oxide hydrogels for skin bandages: The outcome of gelatin and trypsin immobilization. Int. J. Pharm. 2018, 546, 50–60.
- Ur Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617.
- Frontiñán-Rubio, J.; Gómez, M.V.; Martín, C.; González-Domínguez, J.M.; Durán-Prado, M.; Vázquez, E. Differential effects of graphene materials on the metabolism and function of human skin cells. Nanoscale 2018, 10, 11604–11615.
- Bagher, Z.; Asgari, N.; Bozorgmehr, P.; Kamrava, S.K.; Alizadeh, R.; Seifalian, A. Will Tissue-Engineering Strategies Bring New Hope for the Reconstruction of Nasal Septal Cartilage? Curr. Stem Cell Res. Ther. 2019, 15, 144–154, doi:10.2174/1574888x14666191212160757.
- Meng, Y.; Ye, L.; Coates, P.; Twigg, P. In Situ Cross-Linking of Poly(vinyl alcohol)/Graphene Oxide-Polyethylene Glycol Nanocomposite Hydrogels as Artificial Cartilage Replacement: Intercalation Structure, Unconfined Compressive Behavior, and Biotribological Behaviors. J. Phys. Chem. C 2018, 122, 3157–3167.
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701, doi:10.1016/j.msec.2017.05.056.
- Zhou, X.; Nowicki, M.; Cui, H.; Zhu, W.; Fang, X.; Miao, S.; Lee, S.J.; Keidar, M.; Zhang, L.G. 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells. Carbon N. Y. 2017, 116, 615–624, doi:10.1016/j.carbon.2017.02.049.
- Nayyer, L.; Jell, G.; Esmaeili, A.; Birchall, M.; Seifalian, A.M. A Biodesigned Nanocomposite Biomaterial for Auricular Cartilage Reconstruction. Adv. Healthc. Mater. 2016, 5, 1203–1212, doi:10.1002/adhm.201500968.
- Seifalian, A.M.; Hancock, S. Composite Material and Its Method of Production. GB Patent Application No. 1811090, 2017; WO Patent Application No. 2019008381A1; EP Patent Application No. 3648808A1; US Patent Application No. 20200123381A1, 2020.
- Xie, H.; Chua, M.; Islam, I.; Bentini, R.; Cao, T.; Viana-Gomes, J.C.; Neto, A.H.C.; Rosa, V. CVD-grown monolayer graphene induces osteogenic but not odontoblastic differentiation of dental pulp stem cells. Dent. Mater. 2017, 33, e13–e21.
- Tahriri, M.; Del Monico, M.; Moghanian, A.; Tavakkoli Yaraki, M.; Torres, R.; Yadegari, A.; Tayebi, L. Graphene and its derivatives: Opportunities and challenges in dentistry. Mater. Sci. Eng. C 2019, 102, 171–185, doi:10.1016/j.msec.2019.04.051.
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and microRNAs expression. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 953–965, doi:10.1002/jbm.b.33628.
- De Marco, P.; Zara, S.; De Colli, M.; Radunovic, M.; Lazović, V.; Ettorre, V.; Di Crescenzo, A.; Piattelli, A.; Cataldi, A.; Fontana, A. Graphene oxide improves the biocompatibility of collagen membranes in an in vitro model of human primary gingival fibroblasts. Biomed. Mater. 2017, 12, doi:10.1088/1748-605X/aa7907.
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57, doi:10.1016/j.dental.2017.09.007.
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946, doi:10.1016/j.ceramint.2020.11.041.
- He, J.; Zhu, X.; Qi, Z.; Wang, L.; Aldalbahi, A.; Shi, J.; Song, S.; Fan, C.; Lv, M.; Tang, Z. The Inhibition Effect of Graphene Oxide Nanosheets on the Development of Streptococcus mutans Biofilms. Part. Part. Syst. Charact. 2017, 34, 1–8, doi:10.1002/ppsc.201700001.
This entry is adapted from the peer-reviewed paper 10.3390/nano11051083
