
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammed Ahmed Akkaif | + 2432 word(s) | 2432 | 2021-04-21 05:57:23 | | | |
| 2 | Karina Chen | -40 word(s) | 2392 | 2021-05-06 08:16:42 | | | | |
| 3 | Mohammed Ahmed Akkaif | Meta information modification | 2392 | 2021-05-07 23:42:59 | | |
Video Upload Options
Clopidogrel is a widely-used antiplatelet drug. It is important for the treatment and prevention of coronary heart disease. Clopidogrel can effectively reduce platelet activity and therefore reduce stent thrombosis. However, some patients still have ischemic events despite taking the clopidogrel due to the alteration in clopidogrel metabolism attributable to various genetic and non-genetic factors. This review aims to summarise the mechanisms and causes of clopidogrel resistance (CR) and potential strategies to overcome it.
1. Definition of Clopidogrel Resistance
There is currently no uniform definition of CR, but the most accepted is that the drug has lost its target of the action[1]. It is generally believed that CR means that a patient still has a thrombotic event after receiving clopidogrel treatment, and laboratory tests show that platelet function is not inhibited [2]. Some researchers refer to it as clinical resistance among patients who have experienced thromboembolism and other adverse events following long-term oral clopidogrel therapy [3]. The incidence of CR varies among different regions and races. According to literature reports, the incidence of CR in Western countries is 5 to 44%, while in Asian populations, it may be as high as 20 to 65% [3][4][1].
There are several methods commonly used to evaluate platelet function. The oldest and more accurate way is optical turbidimetry, which is often considered the gold standard. This method assesses the responsiveness of platelets to ADP through the function of P2Y1 and P2Y12 receptors. However, because of the repetition rate and the lack of a specific P2Y12 pathway, its use is limited. At present, vasodilator-stimulated phosphoprotein (VASP) phosphorylation assay (VerifyNow) and bedside monitoring are widely used due to the relatively easy operation [5][6]. Tantry and colleagues (2014), in their follow-up studies on CR, confirmed that the available evidence does not support routine screening for hypo/non-responsiveness in patients who started treatment with clopidogrel [7]. So far, there is still a lack of standard experimental methods for diagnosing CR. Clinically, platelet function can be tested to determine the patient’s platelet reaction after medication intake to identify the potential risk of increased cardiovascular or bleeding events. The incidence of CR in elderly patients may be higher than that in younger patients, and the risk of bleeding with clopidogrel is also increased [8][9][10].
The use of platelet function tests (PFTs) to allocate a better selection of antiplatelet drugs to patients with cardiovascular disease has been discussed over the past ten years [11]. These studies mitigated the escalation of antiplatelet therapy according to the results of PFTs for potential clinical benefit. Furthermore, the 2011 American College of Cardiology/American Heart Association guidelines issued a Class IIb recommendation for the use of PFTs among patients taking P2Y12 inhibitors [12]. Still, this classification was downgraded to a Category III recommendation in 2016 [13]. In ACS cases, the latest European guidelines indicate that de-escalation, but not escalation, of P2Y12 inhibitors directed by PFT, with a Class IIb rating, can be considered [14].
2. Factors Associated with CR
The mechanism of CR is still unclear. Relevant studies have shown that CR may be influenced by various factors such as race, age, weight, genetic polymorphism, drug interaction, diabetes, inflammation, immature platelets, atherosclerosis, medication compliance and other factors. Despite these various contributing factors of clopidogrel resistance, the exact mechanism is currently unknown [3][10][15][16][17][18][19][20][21][22].
2.1. Gene Polymorphism
Many studies have been done to determine the relationship between P2Y12 receptor gene polymorphism and CR. Zoheir et al. (2013) found that P2Y12 receptor gene polymorphism is closely related to platelet activity [23]. The P-gp encoded by ABCB1 regulates the absorption of clopidogrel in the intestines. Earlier studies by Mega et al. (2009) found that ABCB1 gene polymorphism affects the degree of platelet inhibition, which is closely related to the risk of major adverse cardiac events (MACE) [24]. However, in recent years, studies on the Chinese population have shown no association between ABCB1 gene polymorphism and CR [25][26][27].
CYP3A4/5 are among the essential enzymes in clopidogrel activation. Previously, Lau et al. (2004) have shown that lower CYP3A4 activity, determined using an erythromycin breath test, is associated with a lower antiplatelet effect of the drug [28].
One study aimed to determine the effect of the CYP3A homologs of sub-enzymes (allelic variants of CYP3A4 * 22 and CYP3A5 * 3) on the efficacy of clopidogrel in patients with ACS undergoing percutaneous coronary intervention. The study results found that CYP3A4 / 5 activity was not associated with platelet aggregation rates, as well as the genotyping and phenotyping of CYP3A4 / CYP3A5 did not predict the antiplatelet effect of clopidogrel. The researcher recommended more extensive research to prove its clinical relevance [29].
The genetic variations in CYP450 isoenzymes genes (CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4), which are involved in drug metabolism, can influence the variation of pharmacodynamic response to clopidogrel, especially the genetic variation in the CYP2C19 isoenzyme. This enzyme contributes significantly to the two sequential oxidative steps in the biotransformation of clopidogrel into active metabolites [30][31]. Hence, genetic polymorphism of CYP2C19 could play a crucial role in wide inter-individual and inter-ethnic variabilities in clinical response towards clopidogrel [32][33][34][35].
The choice of antiplatelet therapy (clopidogrel, ticagrelor, or prasugrel) based on individual patient characteristics, such as treatment choice based on genetic data related to clopidogrel metabolism as well as considerations regarding the clinical features of patients may result in a significantly lower rate of ischemic and hemorrhagic events compared to usual practice [36]. The choice of antiplatelet therapy based on both CYP2C19 gain of function (GOF) and loss of function (LOF) alleles appears to be a preferred approach over universal clopidogrel and universal variant P2Y12 inhibitor therapy for ACS patients with PCI [37][38]. CYP2C19-guided escalation and de-escalation are common as clopidogrel persistence in nonfunctional allele carriers is associated with adverse outcomes [39].
Genetic polymorphisms in CYP2C19 were classified into groups and referred to as alleles. The preliminarily identified alleles include 36 alleles such as CYP2C19 *1,*2, *3, *4, *5, *6, *7 or *8 etc. of which the most significant impact on clopidogrel is *2/*3 mutation sites (weak metabolites) and *17 mutation sites (strong metabolites). The frequency of other variations in most population groups is low [40]. According to clinical guidelines issued by the Clinical Pharmacogenetics Implementation Consortium (CPIC), genotype-related individual variability in metabolic enzyme function is divided into four predicted CYP2C19 metabolic phenotypes: Poor metabolisers (PMs), intermediate metabolisers (IMs), Extensive metabolisers (EMs), and Ultrarapid metabolisers (UMs) [41] .
Many studies have reported wide inter-ethnic variability in CYP2C19 polymorphism. Asian populations (~ 55.0 to 70.0%) have a higher prevalence rate of CYP2C19 LOF variant alleles (CYP2C19 *2 and *3) as compared with white populations (~ 25.0 to 35.0%) and black populations (~35.0 to 45.0%) [42][43]. On the other hand, Asian populations (~4.0%) have a low prevalence of the CYP2C19 GOF variant allele (CYP2C19 *17) as compared to white populations (~18.0%) [44][45].
Recent studies have reported a variation in the prevalence of individuals carrying CYP2C19 alleles among the Asian population (Table 4). The CYP2C19 * 2 allele was found in individuals of the selected countries, with prevalence rates ranging between 4.0–59.6%, with an average prevalence rate of 23.00%. The percentage prevalence of CYP2C19 * 2 allele in Saudi Arabia, Qatar and Jordan was less than 10% (residents of the Arabian Peninsula), which is low compared to others. Meanwhile, the CYP2C19 * 3 allele prevalence was found at rates up to 0–13.03% with an average prevalence rate of 4.61%. It is noticed that the spread of this allele is higher in the countries of Southeast and East Asia. Still, its prevalence rates are lower in India, located in the south of Asia, Russia, which is in its north and most countries in West Asia, excluding Turkey. From the CYP2C19 * 17 allele prevalence data, it is noticed that the prevalence rates ranged between (1- 28.72) %, with an average rate of 15.18%, as it is seen here that there are high prevalence rates in the North, South and West Asia. Medium to low rates are observed in some Central and Southeast Asia (Figure 1).
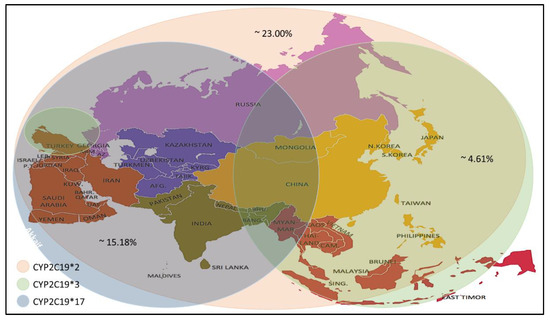
Figure 1. Prevalence of the CYP2C19 * 2/*3/*17 alleles in the Asian population[1].
In general, the high allele frequency of CYP2C19 * 2 and * 17 in the Asian population led to the recommendation of a pre-treatment test to monitor for clopidogrel response, dose and to avoid adverse drug reactions after treatment.
2.2. Drug Interactions
It is known that clopidogrel is converted into an effective product through the metabolic pathway mediated by CYP enzymes. This process involves a variety of isoenzymes. Such as CYP2C19, CYP3A4, CYP1A2, CYP2C9, etc., but the most important ones are CYP3A4 (~40%) and CYP2C19 (~45%) that contribute to the formation of the active metabolite of clopidogrel; so, the combined use of CYP3A4 and CYP2C19 inhibitors may affect the metabolism of clopidogrel [31][46]. Besides clopidogrel, the CYP3A4 pathway also metabolises statins and calcium channel blockers, and the CYP2C19 pathway metabolises proton pump inhibitors (PPIs) [47][48]. Figure 2 illustrates the mechanism by which these three compounds affect clopidogrel.
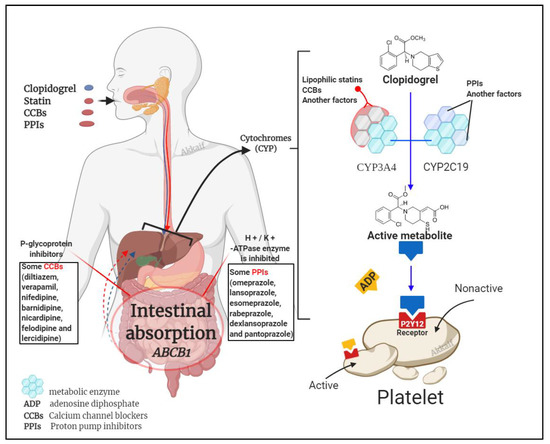
Figure 2. Drug interaction mechanism of clopidogrel with statins, calcium channel blockers (CCBs) and proton pump inhibitors (PPIs)[1].
2.3. Dose Factors
The anti-platelet effect of clopidogrel is dose-dependent [49]. The 300 mg loading dose of clopidogrel reaches a steady state after 4 to 24 h. If there is no load, it takes 4 to 7 days to reach a steady-state [50]. Allier et al. found that the antiplatelet effect of clopidogrel 600 mg administered for the first time was equivalent to that of long-term 75 mg patients. Clopidogrel 600 mg administered during long-term treatment can further inhibit platelet aggregation [51]. Due to the increase in thrombus load before treatment, the standard loading dose is not enough to achieve effective platelet inhibition for patients with severe symptoms. Therefore, CR will still occur with conventional-dose treatment [52].
2.4. Other Factors
Among other factors, patients’ compliance also directly affects the effectiveness of clopidogrel. Other than that, the antiplatelet effect of clopidogrel is limited in type 2 diabetes patients because this disease is often associated with atherosclerotic disease manifestations; clopidogrel is commonly used in these patients [53]. Diabetes is also a risk factor for reduced antiplatelet effects by clopidogrel [53][54]. There is also a vital relationship found between the level of inflammatory factors and CR caused by abnormal platelet function [55][56][57].
3. Strategies to Overcome CR
3.1. Increase the Dose of Clopidogrel
Increasing the dose can increase the biological effect of clopidogrel and reduce the incidence of CR. Simultaneously, large doses of clopidogrel can reduce patients’ platelet aggregation rate with CR [58]. For PCI patients, the 600 mg loading dose has a faster response than the 300 mg loading dose and has a more substantial platelet inhibitory effect. In this way, the incidence of CR is significantly reduced [59][60]. At the same time, studies have shown that CR or platelet hyperresponsiveness is still common after the administration of clopidogrel 600 mg load, but increasing the dose can reduce the risk of death from cardiovascular disease, myocardial infarction, and stent thrombosis [24]. In patients with stable coronary heart disease, CYP2C19*2 heterozygous carriers taking 225 mg of clopidogrel per day were shown to achieve the same antiplatelet effect with CYP2C19 wild-type patients taking 75 mg of clopidogrel per day. In contrast, CYP2C19*2 homozygous patients cannot achieve the desired antiplatelet effect even if they take the 300 mg clopidogrel maintenance dose [61]. CR in patients treated with PCI between high maintenance dose (150 mg · d −1) than conventional maintenance dose (75 mg · d −1) can more effectively prevent major adverse cardiac events (MACE). In the 1-month follow-up after PCI, the incidence of in-stent thrombosis was lower among the group receiving 150 mg · d −1 as compared to the group receiving 75 mg · d −1 (1.1% and 4.9%, p = 0.03). Simultaneously, cardiovascular events incidence was also significantly lower in the group with higher doses (2.7% and 7.6%, p = 0.03) [62]. However, some studies have shown that high-dose clopidogrel after PCI did not reduce the mortality of cardiovascular events or stent thrombosis incidence than standard doses [63]. Moreover, high-dose clopidogrel may lead to an increased probability of bleeding complications; therefore, the use of high-dose clopidogrel maintenance treatment to avoid treatment resistance requires further research.
3.2. Combined Use of Other Antiplatelet Drugs
Ainetdinova et al. [64] found that the probability of resistance to aspirin, clopidogrel, and the combination of these two drugs were 25.7%, 17.1%, and 5.7%, respectively. Therefore, DAPT with aspirin and clopidogrel was shown to reduce the occurrence of drug resistance. Another potential combination therapy uses the GPIIb/IIIa receptor antagonists (such as abciximab, tirofiban and eptifibatide), which can directly block the final pathway of platelet activation, adhesion, and aggregation. Based on clopidogrel therapy, the combined use of GPIIb/IIIa receptor antagonists can further inhibit platelet aggregation [65][66].
3.3. Replacement of New P2Y12 Receptor Antagonists
The new P2Y12 inhibitors, ticagrelor and prasugrel, will substantially reduce platelet hyperresponsiveness and improve clinical outcomes relative to the regular clopidogrel dose. Most patients who do not respond to clopidogrel can significantly inhibit the platelet aggregation rate after switching to prasugrel [67] because prasugrel can better inhibit ADP-induced platelet aggregation, which is faster and stronger than clopidogrel. The longer-lasting antiplatelet effect of prasugrel can significantly reduce the occurrence of ischemic events [68]. On the other hand, ticagrelor does not require liver metabolism and not affected by CYP2C19 gene polymorphism. It was also shown to significantly reduce mortality related to cardiovascular events, myocardial infarction [69]. A study showed that in STEMI patients undergoing PCI for the first time, a loading dose of 180 mg of ticagrelor was more effective than a loading dose of 600 mg of clopidogrel in reducing microvascular damage [70]. There is also literature mentioning that cangrelor has a powerful platelet inhibitory effect. Its effect may be more significant than clopidogrel. Moreover, its half-life is shorter, does not require liver activation, and is a direct antagonist of P2Y12 [71].
3.4. Other Management of CR
Active control of blood sugar in patients with coronary heart disease can reduce the incidence of CR. Avoiding the simultaneous application of other drugs that require CYP metabolisms, such as statins, calcium channel blockers, and PPI, would ensure a better response to clopidogrel therapy.
In a randomised trial of TROPICAL-ACS [72][73], a targeted de-escalation regimen with early switching from prasugrel to clopidogrel was established as an effective alternative treatment strategy in ACS patients. However, the study found that patient age was the primary determinant of outcome after PCI, [74][75], especially when using P2Y12 receptor inhibitors during and after PCI [76][77]. Therefore, TROPICAL-ACS performed a randomised assessment of the effect of age on reducing the escalation of antiplatelet therapy. Significant variation was found among the younger patients who showed an increased net clinical benefit resulting from reduced bleeding complications. These results suggest that targeted de-escalation may be a safe and attractive alternative therapy concept for all ACS patients after PCI, while a significant bleeding benefit could be achieved in younger patients [78].
References
- Mohammed Akkaif; Nur Daud; Abubakar Sha’Aban; Mei Ng; Muhamad Abdul Kader; Dzul Noor; Baharudin Ibrahim; The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 2021, 26, 1987, 10.3390/molecules26071987.
- Patrono, C.; Coller, B.; Dalen, J.E.; Gerald, G.A.F.; Fuster, V.; Gent, M.; Hirsh, J.; Roth, G. Platelet-active drugs: The relationships among dose, effectiveness, and side effects. Chest 2001, 119, 39S–63S.
- Hasan, M.S.; Basri, H.B.; Hin, L.P.; Stanslas, J. Genetic polymorphisms and drug interactions leading to clopidogrel resistance: Why the Asian population requires special attention. Int. J. Neurosci. 2013, 123, 143–154.
- Jiang, X.-L.; Samant, S.; Lesko, L.J.; Schmidt, S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin. Pharmacokinet. 2015, 54, 147–166.
- Bonello, L.; Tantry, U.S.; Marcucci, R.; Blindt, R.; Angiolillo, D.J.; Becker, R.; Bhatt, D.L.; Cattaneo, M.; Collet, J.P.; Cuisset, T. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J. Am. Coll. Cardiol. 2010, 56, 919–933.
- Cuisset, T.; Morange, P.-E.; Alessi, M.-C. Recent advances in the pharmacogenetics of clopidogrel. Human genetics 2012, 131, 653–664.
- Tantry, U.S.; Hennekens, C.H.; Zehnder, J.L.; Gurbel, P.A. Clopidogrel Resistance and Clopidogrel Treatment Failure; Leung, L.L.K., Cutlip, D., Eds.; UpToDate Inc.: Waltham, MA, USA, 2018.
- Cay, S.; Cagirci, G.; Aydogdu, S.; Balbay, Y.; Sen, N.; Maden, O.; Demir, A.D.; Erbay, A.R. Safety of clopidogrel in older patients. Drugs Aging 2011, 28, 119–129.
- Carlquist, J.F.; Knight, S.; Horne, B.D.; Huntinghouse, J.A.; Rollo, J.S.; Muhlestein, J.B.; May, H.; Anderson, J.L. Cardiovascular risk among patients on clopidogrel anti-platelet therapy after placement of drug-eluting stents is modified by genetic variants in both the CYP2C19 and ABCB1 genes. J. Thromb. Haemost. 2013, 109, 744–754.
- Park, J.J.; Park, K.W.; Kang, J.; Jeon, K.-H.; Kang, S.-H.; Ahn, H.S.; Han, J.-K.; Koh, J.-S.; Lee, S.E.; Yang, H.-M. Genetic determinants of clopidogrel responsiveness in Koreans treated with drug-eluting stents. Int. J. Cardiol. 2013, 163, 79–86.
- Fontana, P.; Roffi, M.; Reny, J.-L. Platelet function test use for patients with coronary artery disease in the early 2020s. Med. Clin. Med. 2020, 9, 194.
- Wright, R.S.; Anderson, J.L.; Adams, C.D.; Bridges, C.R.; Casey, D.E.; Ettinger, S.M.; Fesmire, F.M.; Ganiats, T.G.; Jneid, H.; Lincoff, A.M. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non–ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2011, 57, e215–e367.
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2016, 134, e123–e155.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J. 2018 ESC/EACTS Guidelines on myocardial revascularisation. Eur. Heart J. 2019, 40, 87–165.
- Wang, Y.; Zhao, X.; Lin, J.; Li, H.; Johnston, S.C.; Lin, Y.; Pan, Y.; Liu, L.; Wang, D.; Wang, C. Association between CYP2C19 loss-of-function allele status and efficacy of clopidogrel for risk reduction among patients with minor stroke or transient ischemic attack. Jama 2016, 316, 70–78.
- Sarno, G.; Garg, S.; Onuma, Y.; Buszman, P.; Linke, A.; Ischinger, T.; Klauss, V.; Eberli, F.; Corti, R.; Wijns, W. The impact of body mass index on the one year outcomes of patients treated by percutaneous coronary intervention with Biolimus-and Sirolimus-eluting stents (from the LEADERS Trial). Am. J. Cardiol. 2010, 105, 475–479.
- Weisz, G.; Smilowitz, N.R.; Kirtane, A.J.; Rinaldi, M.J.; Parvataneni, R.; Xu, K.; Stuckey, T.D.; Maehara, A.; Witzenbichler, B.; Neumann, F.-J. Proton pump inhibitors, platelet reactivity, and cardiovascular outcomes after drug-eluting stents in clopidogrel-treated patients: The ADAPT-DES study. Circ. Cardiovasc. Interv. 2015, 8, e001952.
- Mizobe, M.; Hokimoto, S.; Akasaka, T.; Arima, Y.; Kaikita, K.; Morita, K.; Miyazaki, H.; Oniki, K.; Nakagawa, K.; Ogawa, H. Impact of CYP2C19 polymorphism on clinical outcome following coronary stenting is more important in non-diabetic than diabetic patients. Thromb. Res. 2014, 134, 72–77.
- Elkind, M.S.; Luna, J.M.; McClure, L.A.; Zhang, Y.; Coffey, C.S.; Roldan, A.; Del Brutto, O.H.; Pretell, E.J.; Pettigrew, L.C.; Meyer, B.C. C-reactive protein as a prognostic marker after lacunar stroke: Levels of inflammatory markers in the treatment of stroke study. Stroke 2014, 45, 707–716.
- Ibrahim, H.; Schutt, R.C.; Hannawi, B.; DeLao, T.; Barker, C.M.; Kleiman, N.S. Association of immature platelets with adverse cardiovascular outcomes. J. Am. Coll. Cardiol. 2014, 64, 2122–2129.
- Chirumamilla, A.P.; Maehara, A.; Mintz, G.S.; Mehran, R.; Kanwal, S.; Weisz, G.; Hassanin, A.; Hakim, D.; Guo, N.; Baber, U. High platelet reactivity on clopidogrel therapy correlates with increased coronary atherosclerosis and calcification: A volumetric intravascular ultrasound study. JACC: Cardiovasc. Imaging 2012, 5, 540–549.
- Rinfret, S.; Rodés-Cabau, J.; Bagur, R.; Déry, J.-P.; Dorais, M.; Larose, É.; Barbeau, G.; Gleeton, O.; Nguyen, C.-M.; Noël, B. Telephone contact to improve adherence to dual antiplatelet therapy after drug-eluting stent implantation. Heart 2013, 99, 562–569.
- Zoheir, N.; Abd Elhamid, S.; Abulata, N.; Sobky, M.E.; Khafagy, D.; Mostafa, A. P2Y12 receptor gene polymorphism and antiplatelet effect of clopidogrel in patients with coronary artery disease after coronary stenting. Blood Coagul. Fibrinolysis 2013, 24, 525–531.
- Mega, J.L.; Close, S.L.; Wiviott, S.D.; Shen, L.; Hockett, R.D.; Brandt, J.T.; Walker, J.R.; Antman, E.M.; Macias, W.L.; Braunwald, E. CLINICAL PERSPECTIVE. Circulation 2009, 119, 2553–2560.
- Xie, C.; Ding, X.; Gao, J.; Wang, H.; Hang, Y.; Zhang, H.; Zhang, J.; Jiang, B.; Miao, L. The effects of CES1A2 A (− 816) C and CYP2C19 loss-of-function polymorphisms on clopidogrel response variability among Chinese patients with coronary heart disease. Pharmacogenetics Genom. 2014, 24, 204–210.
- Tang, X.-F.; Wang, J.; Zhang, J.-H.; Meng, X.-M.; Xu, B.; Qiao, S.-B.; Wu, Y.-J.; Chen, J.; Wu, Y.; Chen, J.-L. Effect of the CYP2C19* 2 and* 3 genotypes, ABCB1 C3435T and PON1 Q192R alleles on the pharmacodynamics and adverse clinical events of clopidogrel in Chinese people after percutaneous coronary intervention. Eur. J. Clin. Pharmacol. 2013, 69, 1103–1112.
- Zhuo, Z.-L.; Xian, H.-P.; Long, Y.; Liu, C.; Sun, Y.-Y.; Ma, Y.-T.; Gao, H.; Zhao, J.-Z.; Zhao, X.-T. Association between CYP2C19 and ABCB1 polymorphisms and clopidogrel resistance in clopidogrel-treated Chinese patients. Anatol. J. Cardiol. 2018, 19, 123–129.
- Lau, W.C.; Gurbel, P.A.; Watkins, P.B.; Neer, C.J.; Hopp, A.S.; Carville, D.G.; Guyer, K.E.; Tait, A.R.; Bates, E.R. Contribution of hepatic cytochrome P450 3A4 metabolic activity to the phenomenon of clopidogrel resistance. Circulation 2004, 109, 166–171.
- Mirzaev, K.; Samsonova, K.; Potapov, P.; Andreev, D.; Grishina, E.; Ryzhikova, K.; Sychev, D. Genotyping and phenotyping CYP3A4\CYP3A5: No association with antiplatelet effect of clopidogrel. Mjol. Biol. Resp. 2019, 46, 4195–4199.
- Brandt, J.T.; Close, S.; Iturria, S.; Payne, C.; Farid, N.; Ernest, C.; Lachno, D.; Salazar, D.; Winters, K. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007, 5, 2429–2436.
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 2010, 38, 92–99.
- Kuliczkowski, W.; Witkowski, A.; Polonski, L.; Watala, C.; Filipiak, K.; Budaj, A.; Golanski, J.; Sitkiewicz, D.; Pregowski, J.; Gorski, J. Interindividual variability in the response to oral antiplatelet drugs: A position paper of the Working Group on antiplatelet drugs resistance appointed by the Section of Cardiovascular Interventions of the Polish Cardiac Society, endorsed by the Working Group on Thrombosis of the European Society of Cardiology. Eur. Heart J. 2009, 30, 426–435.
- Simon, T.; Verstuyft, C.; Mary-Krause, M.; Quteineh, L.; Drouet, E.; Méneveau, N.; Steg, P.G.; Ferrières, J.; Danchin, N.; Becquemont, L. Genetic determinants of response to clopidogrel and cardiovascular events. N. Engl. J. Med. 2009, 360, 363–375.
- Chan, M.Y. Clopidogrel pharmacogenetics of east, south and other Asian populations. Eur. Heart J. Suppl. 2012, 14, A41–A42.
- Brown, S.-A.; Pereira, N. Pharmacogenomic impact of CYP2C19 variation on clopidogrel therapy in precision cardiovascular medicine. J. Pers. Med. 2018, 8, 8.
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A. Pharmacogenomic approach to selecting antiplatelet therapy in patients with acute coronary syndromes: The PHARMCLO trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877.
- Jiang, M.; You, J.H. CYP2C19 LOF and GOF-guided antiplatelet therapy in patients with acute coronary syndrome: A cost-effectiveness analysis. Cardiovasc. Drug Ther. 2017, 31, 39–49.
- Máchal, J.; Hlinomaz, O. Efficacy of P2Y12 receptor blockers after myocardial infarction and genetic variability of their metabolic pathways. Curr. Vasc. Pharmacol. 2019, 17, 35–40.
- Martin, J.; Williams, A.K.; Klein, M.D.; Sriramoju, V.B.; Madan, S.; Rossi, J.S.; Clarke, M.; Cicci, J.D.; Cavallari, L.H.; Weck, K.E. Frequency and clinical outcomes of CYP2C19 genotype-guided escalation and de-escalation of antiplatelet therapy in a real-world clinical setting. Genet. Med. 2020, 22, 160–169.
- Chen, D.-Y.; Wang, C.-Y.; Wen, M.-S.; Lee, T.-H.; Chu, Y.; Hsieh, M.-J.; Chang, S.-H.; Lee, C.-H.; Wang, J.-L.; Chen, C.-C. Paraoxonase-1 is not a major determinant of stent thrombosis in a Taiwanese population. PLoS ONE 2012, 7, e39178.
- Scott, S.; Sangkuhl, K.; Stein, C.; Hulot, J.S.; Mega, J.; Roden, D.; Klein, T.; Sabatine, M.; Johnson, J.; Shuldiner, A. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin. Pharmacol. Ther. 2013, 94, 317–323.
- Desta, Z.; Zhao, X.; Shin, J.-G.; Flockhart, D.A. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin. Pharmacokinet. 2002, 41, 913–958.
- Hwang, S.-J.; Jeong, Y.-H.; Kim, I.-S.; Koh, J.-S.; Kang, M.-K.; Park, Y.; Kwak, C.H.; Hwang, J.-Y. The cytochrome 2C19* 2 and* 3 alleles attenuate response to clopidogrel similarly in East Asian patients undergoing elective percutaneous coronary intervention. Thromb. Res. 2011, 127, 23–28.
- Sim, S.C.; Risinger, C.; Dahl, M.L.; Aklillu, E.; Christensen, M.; Bertilsson, L.; Ingelman-Sundberg, M. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin. Pharmacol. Ther. 2006, 79, 103–113.
- Sugimoto, K.; Uno, T.; Yamazaki, H.; Tateishi, T. Limited frequency of the CYP2C19* 17 allele and its minor role in a Japanese population. Br. J. Clin. Pharmacol. 2008, 65, 437–439.
- Wang, Z.-Y.; Chen, M.; Zhu, L.-L.; Yu, L.-S.; Zeng, S.; Xiang, M.-X.; Zhou, Q. Pharmacokinetic drug interactions with clopidogrel: Updated review and risk management in combination therapy. Ther. Clin. Risk Manag. 2015, 11, 449–467.
- Bates, E.R.; Lau, W.C.; Angiolillo, D.J. Clopidogrel–drug interactions. J. Am. Coll. Cardiol. 2011, 57, 1251–1263.
- El Rouby, N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 2018, 14, 447–460.
- Müller, I.; Besta, F.; Schulz, C.; Massberg, S.; Schönig, A.; Gawaz, M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. J. Thromb. Haemost. 2003, 89, 783–787.
- Patrono, C.; Bachmann, F.; Baigent, C.; Bode, C.; De Caterina, R.; Charbonnier, B.; Fitzgerald, D.; Hirsh, J.; Husted, S.; Kvasnicka, J. Expert consensus document on the use of antiplatelet agents: The Task Force on the Use of Antiplatelet Agents in Patients With Atherosclerotic Cardiovascular Disease of the European Society of Cardiology. Eur. Heart J. 2004, 25, 166–181.
- L’Allier, P.L.; Ducrocq, G.; Pranno, N.; Noble, S.; Ibrahim, R.; Grégoire, J.C.; Azzari, F.; Nozza, A.; Berry, C.; Doucet, S. Clopidogrel 600-mg double loading dose achieves stronger platelet inhibition than conventional regimens: Results from the PREPAIR randomized study. J. Am. Coll. Cardiol. 2008, 51, 1066–1072.
- De Miguel, A.; Ibanez, B.; Badimón, J.J. Clinical implications of clopidogrel resistance. J. Thromb. Haemost. 2008, 100, 196–203.
- Angiolillo, D.J.; Jakubowski, J.A.; Ferreiro, J.L.; Tello-Montoliu, A.; Rollini, F.; Franchi, F.; Ueno, M.; Darlington, A.; Desai, B.; Moser, B.A. Impaired responsiveness to the platelet P2Y12 receptor antagonist clopidogrel in patients with type 2 diabetes and coronary artery disease. J. Am. Coll. Cardiol. 2014, 64, 1005–1014.
- Capodanno, D.; Angiolillo, D.J. Antithrombotic Therapy for Atherosclerotic Cardiovascular Disease Risk Mitigation in Patients With Coronary Artery Disease and Diabetes Mellitus. Circulation 2020, 142, 2172–2188.
- Gori, A.; Cesari, F.; Marcucci, R.; Giusti, B.; Paniccia, R.; Antonucci, E.; Gensini, G.; Abbate, R. The balance between pro-and anti-inflammatory cytokines is associated with platelet aggregability in acute coronary syndrome patients. Atherosclerosis 2009, 202, 255–262.
- Ge, H.; Zhou, Y.; Liu, X.; Nie, X.; Wang, Z.; Guo, Y.; Chen, W.; Yang, Q. Relationship between plasma inflammatory markers and platelet aggregation in patients with clopidogrel resistance after angioplasty. Angiology 2012, 63, 62–66.
- Cirillo, P.; Taglialatela, V.; Pellegrino, G.; Morello, A.; Conte, S.; Di Serafino, L.; Cimmino, G. Effects of colchicine on platelet aggregation in patients on dual antiplatelet therapy with aspirin and clopidogrel. J. Thromb. Thrombolysis 2020, 50, 468–472.
- Choi, H.; Ryu, J.; Seo, H.; Kang, M.; Kim, E. Is a high maintenance dose of clopidogrel suitable for overcoming clopidogrel resistance in patients? Int. J. Clin. Pharm. 2015, 37, 758–761.
- Montalescot, G.; Sideris, G.; Meuleman, C.; Bal-dit-Sollier, C.; Lellouche, N.; Steg, P.G.; Slama, M.; Milleron, O.; Collet, J.-P.; Henry, P. A randomised comparison of high clopidogrel loading doses in patients with non–ST-segment elevation acute coronary syndromes: The ALBION (Assessment of the Best Loading Dose of Clopidogrel to Blunt Platelet Activation, Inflammation and Ongoing Necrosis) trial. J. Am. Coll. Cardiol. 2006, 48, 931–938.
- Snoep, J.D.; Hovens, M.M.; Eikenboom, J.C.; van der Bom, J.G.; Jukema, J.W.; Huisman, M.V. Clopidogrel non-responsiveness in patients undergoing percutaneous coronary intervention with stenting: A systematic review and meta-analysis. Am. Heart J. 2007, 154, 221–231.
- Powers, W.J.; Clarke, W.R.; Grubb, R.L.; Videen, T.O.; Adams, H.P.; Derdeyn, C.P.; COSS Investigators, F.T. Extracranial-intracranial bypass surgery for stroke prevention in hemodynamic cerebral ischemia: The Carotid Occlusion Surgery Study randomised trial. Jama 2011, 306, 1983–1992.
- Liang, J.; Wang, Z.; Shi, D.; Liu, Y.; Zhao, Y.; Han, H.; Li, Y.; Liu, W.; Zhang, L.; Yang, L. High clopidogrel dose in patients with chronic kidney disease having clopidogrel resistance after percutaneous coronary intervention. Angiology 2015, 66, 319–325.
- Stellbaum, C.; Ayral, Y.; Morguet, A.; Schultheiss, H.-P.; Rauch, U. Doubling the clopidogrel dose in patients with reduced responsiveness to the standard dose is associated with a limited effectiveness as evaluated by impedance aggregometry. Cardiovasc. Revascularization Med. 2012, 13, 159–166.
- Aĭnetdinova, D.; Udovichenko, A.; Sulimov, V. Resistance to antiplatelet drugs in patients with non ST elevation acute coronary syndrome. Kardiologiia 2008, 48, 35–39.
- Fong, A.Y.Y.; Ling, H.S. Dual Antiplatelet and Glycoprotein Inhibitors in Emergency PCI. In Primary Angioplasty: A Practical Guide; Watson, T., Ong, P., Tcheng, J., Eds.; Springer: Gateway East, Singapore, 2018; Chapter 8; pp. 99–108.
- Schneider, D.J. Anti-platelet therapy: Glycoprotein IIb-IIIa antagonists. Br. J. Clin. Pharmacol. 2011, 72, 672–682.
- Jernberg, T.; Payne, C.D.; Winters, K.J.; Darstein, C.; Brandt, J.T.; Jakubowski, J.A.; Naganuma, H.; Siegbahn, A.; Wallentin, L. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin-treated patients with stable coronary artery disease. Eur. Heart J. 2006, 27, 1166–1173.
- Shimamatsu, J.; Sasaki, K.-I.; Katsuki, Y.; Kawasaki, T.; Murasato, Y.; Ajisaka, H.; Yokoi, H.; Tashiro, H.; Harada, A.; Hirakawa, Y. Prasugrel effectively reduces the platelet reactivity units in patients with genetically metabolic dysfunction of cytochrome P450 2C19 who are treated with long-term dual antiplatelet therapy after undergoing drug-eluting stent implantation. Heart Vessels 2020, 35, 312–322.
- Huber, K.; Hamad, B.; Kirkpatrick, P. Ticagrelor. Nar. Rev. Drug Discov. 2011, 10, 255–256.
- Park, S.-D.; Lee, M.-J.; Baek, Y.-S.; Kwon, S.-W.; Shin, S.-H.; Woo, S.-I.; Kim, D.-H.; Kwan, J.; Park, K.-S. Randomised trial to compare a protective effect of Clopidogrel Versus TIcagrelor on coronary Microvascular injury in ST-segment Elevation myocardial infarction (CV-TIME trial). EuroIntervention J. Eur. Colab. Work. Group Interv. Cadriol. Eur. Soc. Cadriol. 2016, 12, e964–e971.
- Wang, T.H.; Bhatt, D.L.; Topol, E.J. Aspirin and clopidogrel resistance: An emerging clinical entity. Eur. Heart J. 2006, 27, 647–654.
- Sibbing, D.; Aradi, D.; Jacobshagen, C. TROPICAL-ACS Investigators. A randomised trial on platelet function-guided de-escalation of antiplatelet treatment in ACS patients undergoing PCI. Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) Trial. J. Thromb. Haemost. 2017, 117, 188–195.
- Sibbing, D.; Aradi, D.; Jacobshagen, C.; Gross, L.; Trenk, D.; Geisler, T.; Orban, M.; Hadamitzky, M.; Merkely, B.; Kiss, R.G. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): A randomised, open-label, multicentre trial. Lancet 2017, 390, 1747–1757.
- Boersma, E.; Pieper, K.S.; Steyerberg, E.W.; Wilcox, R.G.; Chang, W.-C.; Lee, K.L.; Akkerhuis, K.M.; Harrington, R.A.; Deckers, J.W.; Armstrong, P.W. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation: Results from an international trial of 9461 patients. Circulation 2000, 101, 2557–2567.
- Eagle, K.A.; Lim, M.J.; Dabbous, O.H.; Pieper, K.S.; Goldberg, R.J.; Van de Werf, F.; Goodman, S.G.; Granger, C.B.; Steg, P.G.; Gore, J.M. A validated prediction model for all forms of acute coronary syndrome: Estimating the risk of 6-month postdischarge death in an international registry. Jama 2004, 291, 2727–2733.
- Larmore, C.; Effron, M.B.; Molife, C.; DeKoven, M.; Zhu, Y.; Lu, J.; Karkare, S.; Lieu, H.D.; Lee, W.C.; Vetrovec, G.W. “Real-world” comparison of prasugrel with ticagrelor in patients with acute coronary syndrome treated with percutaneous coronary intervention in the United States. Catherter. Cardiovasc. Interv. 2016, 88, 535–544.
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Clin. Med. 2007, 357, 2001–2015.
- Sibbing, D.; Gross, L.; Trenk, D.; Jacobshagen, C.; Geisler, T.; Hadamitzky, M.; Merkely, B.; Kiss, R.G.; Komócsi, A.; Parma, R. Age and outcomes following guided de-escalation of antiplatelet treatment in acute coronary syndrome patients undergoing percutaneous coronary intervention: Results from the randomised TROPICAL-ACS trial. Eur. Heart J. 2018, 39, 2749–2758.




