
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liming Yan | + 2218 word(s) | 2218 | 2021-04-14 10:45:16 | | | |
| 2 | Vivi Li | + 10 word(s) | 2228 | 2021-05-06 03:55:27 | | |
Video Upload Options
Tubules of the endoplasmic reticulum (ER) spread into the buds of yeast by an actin-based mechanism and, upon entry, become attached to the polarisome, a proteinaceous micro-compartment below the tip of the bud. The minimal tether between polarisome and cortical ER is formed by a protein complex consisting of Epo1, a member of the polarisome, Scs2, a membrane protein of the ER and Cdc42 guanosine triphosphatase-activating protein Bem3. Here, we report the crystal structure of a complex between Epo1 and Bem3.
1. Introduction
Polarized growth is crucial for various biological processes across yeast and filamentous fungi, which is achieved through the cytoskeleton-based directional transport of cargo to polarized domains [1]. As a result of its asymmetric growth and the polar delivery of organelles, the budding yeast, Saccharomyces cerevisiae, is considered to be the preferred model system for studying the mechanisms and molecules of polarized growth and faithful organelle inheritance in eukaryotic cells [1][2]. Rho GTPase Cdc42 is essential for the control of polarized growth during bud emergence, by recruitment of a yeast-specific complex called the polarisome, which is comprised of formin Bni1, nucleation-promoting factor (NPF) Bud6, Pea2, scaffolding protein Spa2 and receptor protein Epo1 [2][3][4]. During budding, Cdc42 also initiates the formation of a physical diffusion barrier at the neck, comprising septins, which compartmentalizes the bud plasma membrane (PM) from the mother [5][6]. However, distinct and not fully characterized protein complexes organize the contact sites between the PM and the endoplasmic reticulum.
Bem3 localizes to the sites of polarisome growth through its C-terminal Rho GTPase-activating protein domain, which negatively regulates a Rho-type GTPase Cdc42 [7][8][9]. This domain is preceded by a lipid-binding pleckstrin homology (PH) domain, a PX (phox) domain and an N-terminal region that harbors a predicted coiled-coil domain [8][10][11]. A previous study showed that the N-terminal coiled-coil domain of Bem3 interacts directly with the C-terminal coiled-coil domains of Epo1, which is a new member of the polarisome [2][12], suggesting a novel role for the polarisome in linking Bem3 to its functional target, Cdc42, during the budding process.
Scs2 is a homolog of mammalian synaptobrevin-associated protein, which is a conserved integral ER protein and a component of a lipid-sensing complex [12][13]. Scs2 serves as anchors to the ER for cytoplasmic proteins (including Opi1p), through a conserved motif known as FFAT, two phenylalanines (FF) in an acidic tract [14][15][16]. Scs2 also contributes to the tethering of the ER to the septins and to the robust inheritance of the cER, in that its single deletion already leads to a severe reduction in the number of cER-PM contact sites and an up-regulated unfolded protein response [12][14][16][17]. Epo1, which was founded to be the PM-located receptor for Scs2, can promote cER tethering at sites of polarized growth [2][12]. In budding yeast, there exists an Scs2–Epo1–Bem3 polarisome complex that is required to keep ER tubules or the PM-attached cER close to the tip of the bud during tip growth. The Epo1-Scs2 connection might pull the cER actively into the bud, and then the connection between Epo1 and Scs2 is dissolved during the M phase (Mitosis phase) of the cell cycle [2]. Whether and how Epo1 assists Scs2 in its newly discovered roles as an ER-septin tether and in spindle positioning remain open questions for future experiments.
To investigate the mechanism by which Epo1 anchors cER to the bud tip in yeast, we determine the X-ray structure of the C-terminal coiled-coil domains 2, 3 and 4 of Epo1 (named Epo1CC2-CC4) in complex with the N-terminal coiled-coil domain of Bem3 (named Bem3CC1). The structure reveals that the Bem3CC1 domain forms a homodimer to bind four Epo1CC2-CC4 molecules, with two CC3 domains of Epo1 providing an interface for binding to each Bem3CC1. Moreover, through H/D exchange assay, we determine that the N-terminus 12 residue of Scs2 is responsible for binding to Epo1. Thus, Epo1 serves as a key receptor link between Scs2 and Bem3.
2. Bem3-Epo1 Complex Is a Hexamer
The N-terminal coiled-coil domain of Bem3 was previously reported to interact directly with the C-terminal domain of Epo1 [2] (Figure 1A). We started with the co-expression of the N-terminal domain of Bem3 (residue 1–99, CC1 domain) and the C-terminal domain of Epo1 (residue 746–943, CC2-CC4 domain) in E. coli BL21(DE3) cells, and purified the Bem3-Epo1 complex using size-exclusion chromatography. The fractions were analyzed using SDS-PAGE, which showed that Bem3CC1 and Epo1CC2-CC4 can form a stable complex (Figure 1B). To determine the accurate mass of the Bem3CC1 and Epo1CC2-CC4 complex, we performed analytical gel filtration combined with MALS. Interestingly, while both Bem3CC1 and Epo1CC2-CC4 in isolation act as dimers in solution, the mixture of Bem3CC1 and Epo1CC2-CC4 eluted from MALS corresponded to a hexamer (about 100 kDa molecular mass) (Figure 1C).
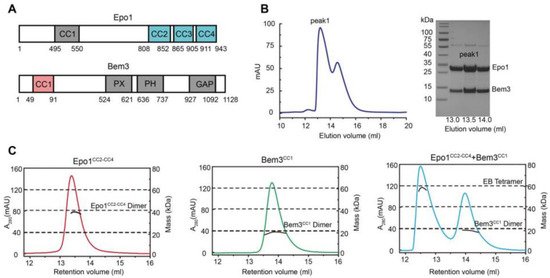
To determine the atomic structure of the protein complex between Bem3CC1 and Epo1CC2-CC4, we crystallized the Bem3CC1–Epo1CC2-CC4 complex and determined its structure to 3.5 Å resolution by X-ray crystallography (Figure 2A and Table S1, supplementary could be found in https://www.mdpi.com/1422-0067/22/8/3812). The model of the Epo1CC2-CC4–Bem3CC1 complex was built manually via several rounds of restrained individual atomic displacement parameter refinement, which allowed us to visualize the structure of the Epo1–Bem3 complex in detail. The final complex structure contained the heterologous hexamer of two Bem3CC1 molecules and four Epo1CC2-CC4 molecules in one asymmetric unit (Figure 2A), which was consistent with the aforementioned data obtained from MALS (Figure 1C), but the superposition of two Epo1CC2-CC4 had obvious conformation changes (Figure S1).
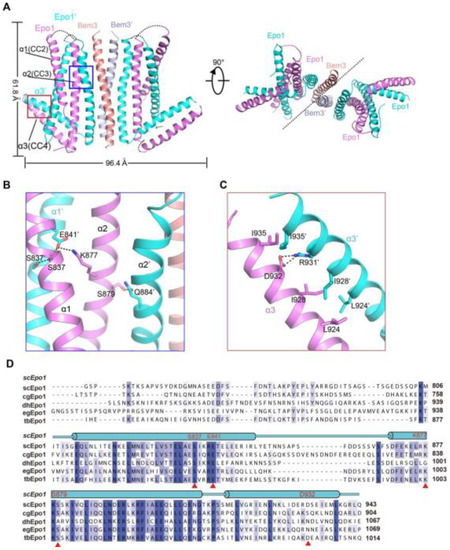
Figure 2. Structure of the Epo1 and Bem3 complex. (A) Epo1 and Bem3 form the hexamer structure in the asymmetric unit, one Bem3 in complex with an Epo1 homodimer and two trimers side-by-side to form a heterologous hexamer; two promoters of Epo1 are colored violet and cyan, respectively. The Bem3 dimers show in the colors salmon and light blue (Bem3 and Bem3′). (B) The CC2 dimer interface of Epo1 is shown as sticks with the key residues highlighted. (C) The interface of the Epo1 CC4 dimer. (D) The sequence alignment of scEpo1 and different species are conserved and similar residues are highlighted with blue and light blue; residues involved in the CC3 dimer interface are indicated by a red triangle. “sc”, “cg”, “dh”, “eg” and “tb” represent Saccharomyces cerevisiae, Candida glabrata, Debaryomyces hansenii, Eremothecium gossypii and Tetrapisispora blattae, respectively.
3. The Overall Structure of Epo1CC2-CC4–Bem3CC1
The Epo1CC2-CC4–Bem3CC1 complex is a heterologous hexamer with four Epo1CC2-CC4 molecules and a Bem3CC1 homodimer. The structure of Epo1CC2-CC4 comprises three α-helix, a CC2 helix (amino acids 821 to 851), a longer CC3 (amino acids 866 to 905) and a C-terminal CC4 (amino acids 912 to 939). Similar to Bem3CC1, two Epo1 molecules are aligned in parallel and interact directly with each other to form homodimers; the observed buried area between the two promoters is 3522 Å2. Two Epo1 homodimers located at the two sides of the Bem3 homodimer finally assemble into a heterologous hexamer (Figure 2A).
The complex structure reveals that the two Epo1 homodimers interacted with each other via two interfaces. In the first interface, residue S837 from Epo1 α1 stabilized α1′ by forming hydrogen bonds with S837, E841 and α2 S879 formed a hydrogen bond with α2′ Q844, while α2 K877 and α1 E841 formed a salt bridge (Figure 2B). The second region of intermolecular interactions was that of L924, I928 and I935 forming several hydrophobic interactions, which were buttressed by a salt bridge between α3 D932 and α3′ R931 (Figure 2C). Moreover, Epo1CC2-CC4 was a highly conserved cross-species (Figure 2D).
4. Interaction of Epo1CC2-CC4–Bem3CC1
As mentioned above, Bem3 formed a homodimer mainly via its CC1 helices; CC1 and CC1′ form the parallel contents between the coiled-coil dimer (Figure 2A and Figure 3A). The Bem3CC1 folded into a dimeric parallel coiled-coil that was 61.8 Å long, with a buried solvent-assessable area of 914 Å2 (Figure 3A). Mutations of Bem3 N56 and Y66A disrupted the Bem3 dimer (Figure 3C) and these parts constituted the primary interface for Bem3 bound to Epo1 (Figure 4A). The Epo1–Bem3 interface regions could be subdivided into a central compartment and a side compartment. The major interface, a hydrophilic region, comprised Epo1 α2 and α2′ to form a zipper with Bem3, consisting mainly of hydrogen bonds, involving residues from Bem3 (K58, Q62 and E69), Epo1 α2 (E872 and R876) and α2′ (Q888) (Figure 4A,B), and a salt bridge between E65 of Bem3 and K881 of the Epo1 α2′ helix (Figure 4B). The minor interface contained a local intermolecular hydrogen bond network, involving the side chains of three critical residues (Q906 of Epo1 α2′, R84 and E85 of Bem3′) and the carbonyl oxygen of Epo1 A899 (Figure 4C). In order to investigate the role of the above-described key residues involved in interactions between Epo1CC2-CC4 and Bem3CC1, we performed site-directed mutagenesis and a subsequent SPR experiment.
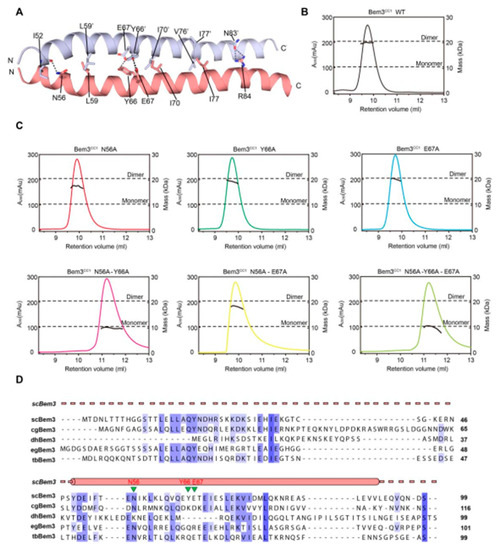
Figure 3. The dimer interaction of Bem3CC1. (A) The interface residues between the Bem3CC1 dimer. (B,C) The sizes of wild-type (wt) Bem3CC1 (theoretical molecular mass 11.39 kDa) and some key residue mutants were determined by MALS coupled with gel filtration. The estimated molecular masses are shown on the right axis. (D) Sequence alignment of Bem3 from different species: Saccharomyces cerevisiae (sc), Candida glabrata (cg), Debaryomyces hansenii (dh), Eremothecium gossypii (eg) and Tetrapisispora blattae (tb). The scBem3 are numbered and aligned, while the secondary structures of Bem3 are labeled on top.
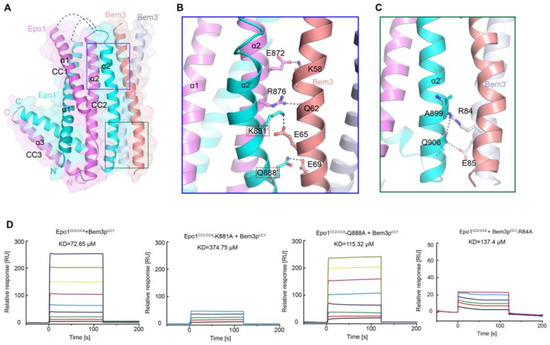
Figure 4. Heterologous interaction of Epo1 and Bem3. (A) Specific interaction between Epo1 and Bem3, with key regions boxed. (B,C) CC3 of two Epo1 present at the interaction surface with Bem3-CC; a stick representation of the key residue is shown. (D) The specific interaction between Bem3-CC and different Epo1-CC (WT and mutant) is characterized by SPR. Epo1-CC is seen binding to Bem3, Epo1-CC-K881A to Bem3, Epo1-CC Q88A to Bem3 and Epo1-CC R84A to Bem3.
Consistent with our structural observations, a mutation of K881 disrupted the interactions between Epo1 and Bem3, while Q884A still maintained the stable interaction (Figure 4D). In addition, we tested the binding of Bem3 mutants to Epo1 using SPR, and found that the single-substitution of R84A, as well as inter-domain hydrogen bond network disruption, severely attenuated the interactions between Bem3 and Epo1 (Figure 4D).
5. Discussion
Epo1 proteins contain four coiled-coil (CC) domains: CC1 located at its N-terminus and CC2 to CC4 at its C-terminus. In this study, we determined the CC2-CC4 to be in a complex with the Bem3 CC1 domain. Protein folding propensity analyses indicated that CC2-CC4 region formed a dimer with an “L” shaped structure, for novelty, in the polarisome complex. In order to identify the key Epo1 CC domain for interaction with Bem3, we isolated CC2, CC3, and CC2-CC3, respectively. While the CC2 and CC3 clones showed no expression, CC2-CC3 could be expressed and bind with Epo1. Though Bem3 CC1 binding was not expected to interfere with the CC4 domain of Epo1, a deletion of CC4 would cause the Epo1–Bem3 interaction ability to decrease. This result suggested that the interaction between Bem3 and Epo1 might have been dependent on their coiled-coil domains. Indeed, as the CC1 domain of Bem3 docked on the CC3 surface of the Epo1 dimer, the overall Epo1-Bem3 structure may be relatively rigid for a hexamer. We did not find a detectable electron density between residues 746 and 808, indicating structural flexibility in this region.
Epo1 is critical in yeast bud development due to its function as a receptor that recruits Scs2 [2][12]. This article reports a key complex structure of Epo1–Bem3, which forms a specific contact to meet the specific demands of rapid membrane and cell wall extension at the cell tip. Specifically, this complex provides a platform for Scs2 interaction and contributes to the establishment of cell polarity, which are a fundamental processes in the life of a yeast bud.
In conclusion, our structure-function studies on the Epo1–Bem3–Scs2 complex reveal several important features. First, the N-terminus of Scs2 bound with Epo1 acts as a receptor of Scs2, and ORP1 and Opi1 also bind to Scs2 via the FFAT motif, which plays an important role in maintaining ER morphology. While we can only speculate on the dual function of Scs2, this is crucial for various biological processes. Second, the Bem3CC1 dimer is the major determinant that is crucial for its polarized localization, as this segment can recruit the Epo1 protein in the absence of other structural elements of Bem3.
References
- Pruyne, D.; Legesse-Miller, A.; Gao, L.; Dong, Y.; Bretscher, A. Mechanisms of polarized growth and organelle segregation in yeast. Annu. Rev. Cell Dev. Biol. 2004, 20, 559–591.
- Neller, J.; Dünkler, A.; Rösler, R.; Johnsson, N. A protein complex containing Epo1p anchors the cortical endoplasmic reticulum to the yeast bud tip. J. Cell Biol. 2015, 208, 71–87.
- Nie, W.-C.; He, F.; Yuan, S.-M.; Jia, Z.-W.; Wang, R.-R.; Gao, X.-D. Roles of an N-terminal coiled-coil-containing domain in the localization and function of Bem3, a Rho GTPase-activating protein in budding yeast. Fungal Genet. Biol. 2017, 99, 40–51.
- Etienne-Manneville, S. Cdc42—The centre of polarity. J. Cell Sci. 2004, 117, 1291–1300.
- Dobbelaere, J.; Barral, Y. Spatial Coordination of Cytokinetic Events by Compartmentalization of the Cell Cortex. Science 2004, 305, 393–396.
- Luedeke, C.; Frei, S.B.; Sbalzarini, I.; Schwarz, H.; Spang, A.; Barral, Y. Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J. Cell Biol. 2005, 169, 897–908.
- Knaus, M.; Pelli-Gulli, M.-P.; Van Drogen, F.; Springer, S.; Jaquenoud, M.; Peter, M. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 2007, 26, 4501–4513.
- Mukherjee, D.; Sen, A.; Boettner, D.R.; Fairn, G.D.; Schlam, D.; Valentin, F.J.B.; McCaffery, J.M.; Hazbun, T.; Staiger, C.J.; Grinstein, S.; et al. Bem3, a Cdc42 GTPase-activating protein, traffics to an intracellular compartment and recruits the secretory Rab GTPase Sec4 to endomembranes. J. Cell Sci. 2013, 126, 4560–4571.
- Aguilar, R.C.; Longhi, S.A.; Shaw, J.D.; Yeh, L.-Y.; Kim, S.H.; Schon, A.; Freire, E.; Hsu, A.; McCormick, W.K.; Watson, H.A.; et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 2006, 103, 4116–4121.
- Burkhard, P.; Stetefeld, J.; Strelkov, S.V. Coiled coils: A highly versatile protein folding motif. Trends Cell Biol. 2001, 11, 82–88.
- Smith, G.R.; Givan, S.A.; Cullen, P.; Sprague, G.F., Jr. GTPase-Activating Proteins for Cdc42. Eukaryot. Cell 2002, 1, 469–480.
- Chao, J.T.; Wong, A.K.; Tavassoli, S.; Young, B.P.; Chruscicki, A.; Fang, N.N.; Howe, L.J.; Mayor, T.; Foster, L.J.; Loewen, C.J. Polarization of the Endoplasmic Reticulum by ER-Septin Tethering. Cell 2014, 158, 620–632.
- Loewen, C.J.R.; Gaspar, M.L.; Jesch, S.A.; Delon, C.; Ktistakis, N.T.; Henry, S.A.; Levine, T.P. Phospholipid Metabolism Regulated by a Transcription Factor Sensing Phosphatidic Acid. Science 2004, 304, 1644–1647.
- Loewen, C.J.; Roy, A.; Levine, T.P. A conserved ER targeting motif in three families of lipid binding proteins and in Opi1p binds VAP. EMBO J. 2003, 22, 2025–2035.
- Nikawa, J.-I.; Murakami, A.; Esumi, E.; Hosaka, K. Cloning and Sequence of the SCS2 Gene, Which Can Suppress the Defect of IN01 Expression in an Inositol Auxotrophic Mutant of Saccharomyces cerevisiae. J. Biochem. 1995, 118, 39–45.
- Lowe, M.; Barr, F.A. Inheritance and biogenesis of organelles in the secretory pathway. Nat. Rev. Mol. Cell Biol. 2007, 8, 429–439.
- Manford, A.G.; Stefan, C.J.; Yuan, H.L.; MacGurn, J.A.; Emr, S.D. ER-to-Plasma Membrane Tethering Proteins Regulate Cell Signaling and ER Morphology. Dev. Cell 2012, 23, 1129–1140.




