Tubules of the endoplasmic reticulum (ER) spread into the buds of yeast by an actin-based mechanism and, upon entry, become attached to the polarisome, a proteinaceous micro-compartment below the tip of the bud. The minimal tether between polarisome and cortical ER is formed by a protein complex consisting of Epo1, a member of the polarisome, Scs2, a membrane protein of the ER and Cdc42 guanosine triphosphatase-activating protein Bem3. Here, we report the crystal structure of a complex between Epo1 and Bem3.
- budding yeast
- polarisome
- Epo1-Bem3
- Scs2p
- complex
1. Introduction
Polarized growth is crucial for various biological processes across yeast and filamentous fungi, which is achieved through the cytoskeleton-based directional transport of cargo to polarized domains [1]. As a result of its asymmetric growth and the polar delivery of organelles, the budding yeast, Saccharomyces cerevisiae, is considered to be the preferred model system for studying the mechanisms and molecules of polarized growth and faithful organelle inheritance in eukaryotic cells [1,2]. Rho GTPase Cdc42 is essential for the control of polarized growth during bud emergence, by recruitment of a yeast-specific complex called the polarisome, which is comprised of formin Bni1, nucleation-promoting factor (NPF) Bud6, Pea2, scaffolding protein Spa2 and receptor protein Epo1 [2,3,4]. During budding, Cdc42 also initiates the formation of a physical diffusion barrier at the neck, comprising septins, which compartmentalizes the bud plasma membrane (PM) from the mother [5,6]. However, distinct and not fully characterized protein complexes organize the contact sites between the PM and the endoplasmic reticulum.
Bem3 localizes to the sites of polarisome growth through its C-terminal Rho GTPase-activating protein domain, which negatively regulates a Rho-type GTPase Cdc42 [7,8,9]. This domain is preceded by a lipid-binding pleckstrin homology (PH) domain, a PX (phox) domain and an N-terminal region that harbors a predicted coiled-coil domain [8,10,11]. A previous study showed that the N-terminal coiled-coil domain of Bem3 interacts directly with the C-terminal coiled-coil domains of Epo1, which is a new member of the polarisome [2,12], suggesting a novel role for the polarisome in linking Bem3 to its functional target, Cdc42, during the budding process.
Scs2 is a homolog of mammalian synaptobrevin-associated protein, which is a conserved integral ER protein and a component of a lipid-sensing complex [12,13]. Scs2 serves as anchors to the ER for cytoplasmic proteins (including Opi1p), through a conserved motif known as FFAT, two phenylalanines (FF) in an acidic tract [14,15,16]. Scs2 also contributes to the tethering of the ER to the septins and to the robust inheritance of the cER, in that its single deletion already leads to a severe reduction in the number of cER-PM contact sites and an up-regulated unfolded protein response [12,14,16,17]. Epo1, which was founded to be the PM-located receptor for Scs2, can promote cER tethering at sites of polarized growth [2,12]. In budding yeast, there exists an Scs2–Epo1–Bem3 polarisome complex that is required to keep ER tubules or the PM-attached cER close to the tip of the bud during tip growth. The Epo1-Scs2 connection might pull the cER actively into the bud, and then the connection between Epo1 and Scs2 is dissolved during the M phase (Mitosis phase) of the cell cycle [2]. Whether and how Epo1 assists Scs2 in its newly discovered roles as an ER-septin tether and in spindle positioning remain open questions for future experiments.
To investigate the mechanism by which Epo1 anchors cER to the bud tip in yeast, we determine the X-ray structure of the C-terminal coiled-coil domains 2, 3 and 4 of Epo1 (named Epo1CC2-CC4) in complex with the N-terminal coiled-coil domain of Bem3 (named Bem3CC1). The structure reveals that the Bem3CC1 domain forms a homodimer to bind four Epo1CC2-CC4 molecules, with two CC3 domains of Epo1 providing an interface for binding to each Bem3CC1. Moreover, through H/D exchange assay, we determine that the N-terminus 12 residue of Scs2 is responsible for binding to Epo1. Thus, Epo1 serves as a key receptor link between Scs2 and Bem3.
2. Bem3-Epo1 Complex Is a Hexamer
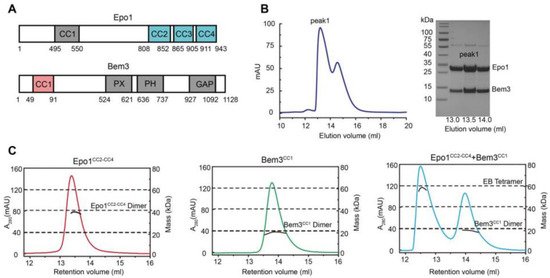
3. The Overall Structure of Epo1CC2-CC4–Bem3CC1
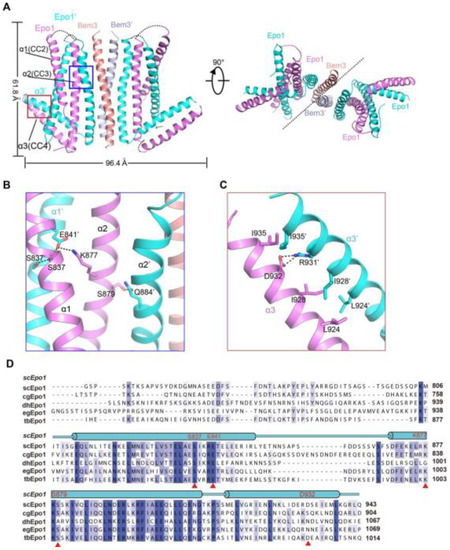
The Epo1CC2-CC4–Bem3CC1 complex is a heterologous hexamer with four Epo1CC2-CC4 molecules and a Bem3CC1 homodimer. The structure of Epo1CC2-CC4 comprises three α-helix, a CC2 helix (amino acids 821 to 851), a longer CC3 (amino acids 866 to 905) and a C-terminal CC4 (amino acids 912 to 939). Similar to Bem3CC1, two Epo1 molecules are aligned in parallel and interact directly with each other to form homodimers; the observed buried area between the two promoters is 3522 Å2. Two Epo1 homodimers located at the two sides of the Bem3 homodimer finally assemble into a heterologous hexamer (Figure 2A).
The complex structure reveals that the two Epo1 homodimers interacted with each other via two interfaces. In the first interface, residue S837 from Epo1 α1 stabilized α1′ by forming hydrogen bonds with S837, E841 and α2 S879 formed a hydrogen bond with α2′ Q844, while α2 K877 and α1 E841 formed a salt bridge (Figure 2B). The second region of intermolecular interactions was that of L924, I928 and I935 forming several hydrophobic interactions, which were buttressed by a salt bridge between α3 D932 and α3′ R931 (Figure 2C). Moreover, Epo1CC2-CC4 was a highly conserved cross-species (Figure 2D).
4. Interaction of Epo1CC2-CC4–Bem3CC1
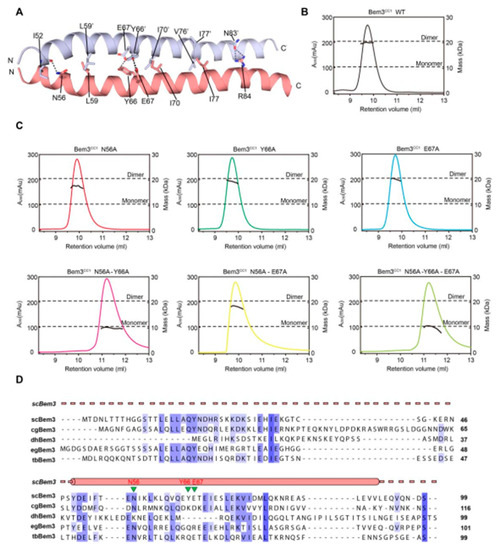
As mentioned above, Bem3 formed a homodimer mainly via its CC1 helices; CC1 and CC1′ form the parallel contents between the coiled-coil dimer (Figure 2A and Figure 3A). The Bem3CC1 folded into a dimeric parallel coiled-coil that was 61.8 Å long, with a buried solvent-assessable area of 914 Å2 (Figure 3A). Mutations of Bem3 N56 and Y66A disrupted the Bem3 dimer (Figure 3C) and these parts constituted the primary interface for Bem3 bound to Epo1 (Figure 4A). The Epo1–Bem3 interface regions could be subdivided into a central compartment and a side compartment. The major interface, a hydrophilic region, comprised Epo1 α2 and α2′ to form a zipper with Bem3, consisting mainly of hydrogen bonds, involving residues from Bem3 (K58, Q62 and E69), Epo1 α2 (E872 and R876) and α2′ (Q888) (Figure 4A,B), and a salt bridge between E65 of Bem3 and K881 of the Epo1 α2′ helix (Figure 4B). The minor interface contained a local intermolecular hydrogen bond network, involving the side chains of three critical residues (Q906 of Epo1 α2′, R84 and E85 of Bem3′) and the carbonyl oxygen of Epo1 A899 (Figure 4C). In order to investigate the role of the above-described key residues involved in interactions between Epo1CC2-CC4 and Bem3CC1, we performed site-directed mutagenesis and a subsequent SPR experiment.
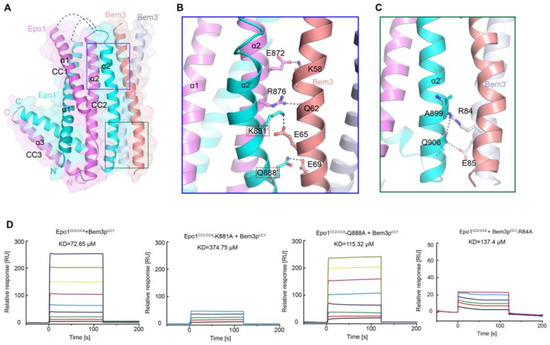
Consistent with our structural observations, a mutation of K881 disrupted the interactions between Epo1 and Bem3, while Q884A still maintained the stable interaction (Figure 4D). In addition, we tested the binding of Bem3 mutants to Epo1 using SPR, and found that the single-substitution of R84A, as well as inter-domain hydrogen bond network disruption, severely attenuated the interactions between Bem3 and Epo1 (Figure 4D).
5. Discussion
Epo1 proteins contain four coiled-coil (CC) domains: CC1 located at its N-terminus and CC2 to CC4 at its C-terminus. In this study, we determined the CC2-CC4 to be in a complex with the Bem3 CC1 domain. Protein folding propensity analyses indicated that CC2-CC4 region formed a dimer with an “L” shaped structure, for novelty, in the polarisome complex. In order to identify the key Epo1 CC domain for interaction with Bem3, we isolated CC2, CC3, and CC2-CC3, respectively. While the CC2 and CC3 clones showed no expression, CC2-CC3 could be expressed and bind with Epo1. Though Bem3 CC1 binding was not expected to interfere with the CC4 domain of Epo1, a deletion of CC4 would cause the Epo1–Bem3 interaction ability to decrease. This result suggested that the interaction between Bem3 and Epo1 might have been dependent on their coiled-coil domains. Indeed, as the CC1 domain of Bem3 docked on the CC3 surface of the Epo1 dimer, the overall Epo1-Bem3 structure may be relatively rigid for a hexamer. We did not find a detectable electron density between residues 746 and 808, indicating structural flexibility in this region.
Epo1 is critical in yeast bud development due to its function as a receptor that recruits Scs2 [2,12]. This article reports a key complex structure of Epo1–Bem3, which forms a specific contact to meet the specific demands of rapid membrane and cell wall extension at the cell tip. Specifically, this complex provides a platform for Scs2 interaction and contributes to the establishment of cell polarity, which are a fundamental processes in the life of a yeast bud.
In conclusion, our structure-function studies on the Epo1–Bem3–Scs2 complex reveal several important features. First, the N-terminus of Scs2 bound with Epo1 acts as a receptor of Scs2, and ORP1 and Opi1 also bind to Scs2 via the FFAT motif, which plays an important role in maintaining ER morphology. While we can only speculate on the dual function of Scs2, this is crucial for various biological processes. Second, the Bem3CC1 dimer is the major determinant that is crucial for its polarized localization, as this segment can recruit the Epo1 protein in the absence of other structural elements of Bem3.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22083812
