
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephanie Annett | + 3277 word(s) | 3277 | 2021-04-26 05:07:32 | | | |
| 2 | Camila Xu | Meta information modification | 3277 | 2021-05-05 04:21:03 | | |
Video Upload Options
Ovarian cancer is an aggressive gynaecological cancer with extremely poor prognosis, due to late diagnosis as well as the development of chemoresistance after first-line therapy.
1. Introduction
In 1992, ovarian cancer was termed ‘the most lethal gynaecologic malignancy’ [1], with the overall five-year survival rate reported at 30%. Although the past three decades have seen a significant improvement in diagnostic advances, therapeutic strategies and overall care in ovarian cancer, prognosis continues to remain poor. The current five-year survival rate of 48.6% is the lowest among all gynaecological cancers [2], meriting the dismal title of ovarian cancer being the deadliest gynaecological cancer. Over 90% of all ovarian cancers are of epithelial origin and can be broadly divided further into Type I (including low- grade serous, endometrioid, clear-cell or mucinous carcinomas) and Type II (including high-grade serous or undifferentiated carcinomas).
Population-based cancer incidence and mortality data is compiled by various organisations across the world. For Europe, the European Cancer Information System estimates an age standardised incidence rate of ovarian cancer at 16.1 per 100,000 and an associated mortality rate of 10.4 per 100,000 (Figure 1) [3]. This high mortality-to-incidence ratio is attributable to a combination of late detection and resistance to therapy. The improbability of early diagnosis is a direct consequence of the lack of specific symptoms during the early stages of the disease, as well as the absence of reliable screening strategies. Owing to the success of cervical and breast cancer screening, as well as the rather modest increase in survival from improved treatment, there have been fervent efforts to boost ovarian cancer survival via screening using CA125, an epitope of MUC16, a large glycoprotein marker. However, the accuracy of this biomarker is still questionable, although more effective screening strategies with CA125 are being developed [4]. As outlined before, therapeutic advances have led to only a small increase in ovarian cancer survival rate over the years. Standard treatment for ovarian cancer is cytoreductive surgery along with combination taxane–platinum-based chemotherapy. More recently, the two most promising novel therapeutic approaches are using monoclonal antibodies such as bevacizumab, targeting tumour microenvironmental pathways such as angiogenesis, and inhibitors of the poly (ADP-ribose) polymerase (PARP) enzyme which is involved in critical cellular functions such as DNA repair. Both have been approved by the FDA and show promising outcomes as combinatorial and maintenance drugs in ovarian cancer [5].
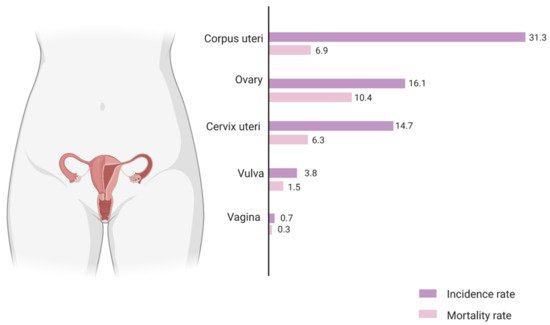
Figure 1. The estimated incidence and mortality rate for gynaecological cancers in European females of all ages, 2020. The values are expressed as age-standardised rate per 100,000 population. The mortality-to-incidence ratio (MIR) for ovarian cancer (0.64) is the highest among all gynaecological cancers and more than twice as high as that for breast cancer (0.25). Source: European Cancer Information System, European Commission.
Although first-line therapy has an initial remission rate of 70–80%, the majority of patients relapse, develop chemoresistance and proceed to respond only very modestly to second-line chemotherapy. The high recurrence rate and chemoresistance associated with ovarian cancer is thought to be due to intra-tumoral heterogeneity, microenvironmental interactions as well as the presence of dynamic cancer stem cell sub-populations. There are three main models proposed to explain the heterogeneity of intra-tumoral cell populations. The two conventional models are the clonal evolution or stochastic model and the stem cell or hierarchical model. It is now understood that the two ideas are not mutually exclusive, and a third model termed the plasticity model conceptualises a more dynamic, flexible understanding of the tumoral niche (Figure 2). Stem cell-like subpopulations existing in the tumoral hemisphere in solid tumours such as ovarian cancer have been found to dynamically interact with the immediate cellular microenvironment so as to induce tumorigenesis, survival and metastases as well as self-renewal leading to an intrinsically generated and maintained tumour niche capable of immunosuppression and therapeutic evasion. Hence, it is vital to study these interactions and devise methods that effectively target these stem cell niches to make substantial strides in the therapeutic targeting and management of aggressive ovarian tumours. This review aims to summarize the current understanding of the ovarian cancer stem cell niche and its interactions with the host immune system and to highlight implications for the development of novel ovarian cancer therapies.
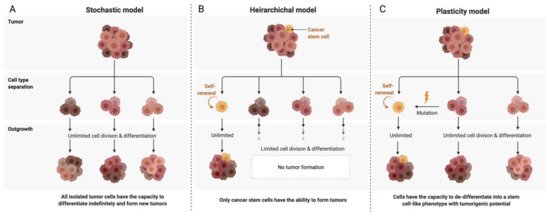
Figure 2. Models of ovarian cancer tumor development and heterogenity. (A) The stochastic model—Each cell is considered biologically equivalent (clonal). Heterogeneity is attributed to genetic mutations propagated through time. All cells have tumorigenic capacity. (B) The hierarchical model—A single cell undergoes a de-differentiating mutation and forms a distinct subpopulation within the niche having stem cell-like tumorigenic potential and leading to the formation of both intermediate progenitor cells as well as terminally differentiated cells, thus contributing to heterogeneity. (C) The plasticity model—Proposes a plastic state of tumorigenic potential in the niche. Differentiated cells can be mutated to re-acquire stem cell-like properties, and the niche contains a dynamic heterogeneous population of differentiated tumour cells as well as stem cells.
2. Ovarian Cancer Stem Cells (OCSCs): Signaling Pathways and Markers
Like many solid tumours, ovarian cancer has been shown to reflect significant tumoral phenotypic diversity [6]. Key evidence suggests that the high relapse rate inevitably seen in ovarian cancer is linked to chemoresistant stem cell-like subpopulations which persist through therapy and have tumorigenic properties [7]. In 2013, Virant-Klun et al., first discovered very small embryonic-like stem cells identifying stage-specific embryonic antigen-4- (SSEA-4; a marker of human embryonic stem cells) positive cells from cultures of human ovarian cancers and validated their discovery in women with borderline ovarian cancer (a less aggressive form of epithelial ovarian cancer) versus healthy women. The cells from the test group were proliferative and formed tumour-like structures in vitro as well as in vivo [8][9].
2.1. Signaling Pathways
A number of oncogenic signaling pathways have been found to generate and maintain OCSCs, as summarised in Figure 3. Specific inhibition of these pathways has shown promising results in decreasing stemness in ovarian cancer cell lines as well as in animal models and will be discussed later in the review.
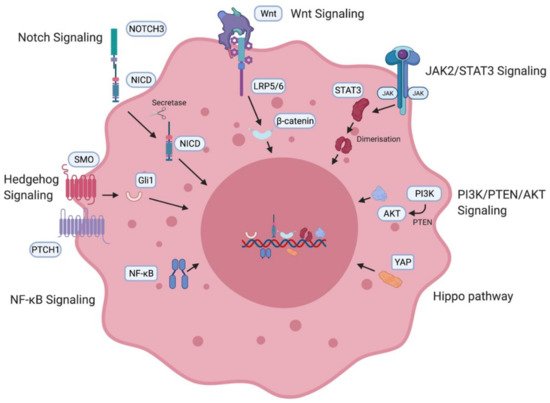
Figure 3. Ovarian cancer stem cell (OCSC)-associated signaling pathways. OCSC signaling pathways involved in the generation and maintenance of OCSCs including the Notch pathway [10][11], Wnt signaling pathway [10][11], JAK2/STAT3 pathway [12][13][14], PI3K/PTEN/AKT pathway [15], Hippo pathway [16], NF-κB [17][18] and the Hedgehog pathway [19]. NICD—intracellular domain of Notch protein; LRP—low-density lipoprotein-related protein; JAK—Janus kinase, STAT—signal transducer and activator of transcription proteins; PI3K—phosphatidylinositol 3-kinase, PTEN—phosphatidylinositol 3,4,5—triphosphate 3-phosphatase, AKT/PKB—protein kinase B; YAP—Yes-associated protein; NF-κB—nuclear factor kappa B.
Notch3 has been found to be overexpressed in high-grade serous ovarian cancer (HGSOC) [20]. In ovarian cancer cell lines, Notch3 overexpression causes upregulation of pathways associated with stem cell generation. Treatment of ovarian cancer cells with notch pathway inhibitors was found to deplete stem cells and when administered in combination with cisplatin, it eliminated the stem cell population as well as the tumour cells [21]. The Wnt pathway has been implicated in the ovarian cancer stem cell niche. Specific G-protein-coupled receptors have been associated with Wnt pathway regulation of stem cells in the ovary [22]. Downstream β-catenin activation leads to upregulation of ABC transporters, which have been linked to the development of taxane–platinum therapy resistance [23]. PTCH1 and Gli1 transcription factors associated with the Hedgehog pathway have also been found to be overexpressed in ovarian cancer patients and correlate with poor prognosis and survival [24]. The effector protein of the Hippo pathway, YAP, is a known oncogene in ovarian cancer [25]. Inhibition of YAP causes in vitro and in vivo suppression of platinum therapy resistance [26]. The PI3K/PTEN/AKT pathway is also activated in HGSOC. PI3K inhibition was found to chemosensitise resistant ovarian cancer patients to platinum-based therapy [27]. Patient-derived CD24+ OCSCs showed increased expression of STAT3, and inhibition of the JAK2/STAT3 pathway correlated with better survival [28]. Taxane and JAK2 inhibitor combination therapy was found to cause a decrease in ovarian cancer stemness [29]. The NF-κB pathway has been implicated in the formation of stem cells [30]. Tumorigenic and stemness-initiating properties were verified in a mouse xenograft model-based study which found that stemness was maintained via both canonical and non-canonical cascades of the NF-κB pathway. Inhibition of the pathway restored sensitivity and response to platinum therapy in ovarian cancer cells [31].
2.2. Cancer Stem Cell Markers
Cancer stem cells can be identified and confirmed by the presence of specific cell surface and non-surface biomarkers. Several cell surface markers have been associated with OCSCs and are summarised in Table 1.
Table 1. Markers associated with OCSCs.
| Marker | Characteristic | Function in Ovarian Cancer | Evidence |
|---|---|---|---|
| CD133 | Transmembrane glycoprotein | Identified by several groups to be expressed in tumour-initiating cells; promotes adhesion to metastatic cancer niche. | Ferrandina et al. [32], Roy et al. [33] |
| ALDH | Aldehyde dehydrogenase enzyme | Correlates with tumourigenicity and spheroid formation; increased expression significantly associated with poor outcomes in patients with serous ovarian cancer. | Ma et al. [34], Ishiguro et al. [35], Deng et al. [36] |
| CD44 | Transmembrane glycoprotein | Positively associated with ovarian cancer migration and metastatic spread; high expression correlates to recurrence and drug resistance. | Bourguignon et al. [37], Carpenter et al. [38], Sacks et al. [39] |
| CD24 | Glycophosphatidylinositol-anchored membrane glycoprotein | Positive marker; cell lines and tumour samples displayed stemness genes, tumourigenicity, spheroid formation. | Burgos-Ojeda, D. et al. [40], Gao, M.Q. et al. [41] |
| CD117 | Receptor tyrosine kinase | Surface marker binding to stem cell factor; consistently formed tumours in mice models | Mazzoldi et al. [42], Luo et al. [43] |
3. The Ovarian Cancer Stem Cell Niche
The intra-tumoral space where stem cells exist and interact with their immediate environment via humoral, neuronal, paracrine, positional and metabolic signals for self-maintenance and overall tumour growth is called the stem cell niche. The cancer stem cell niche interacts with several intra-tumoral processes such as epithelial–mesenchymal transition (EMT), neovascularisation, hypoxic microenvironment and inflammatory networks. The bi-directional communication is biologically dynamic, wherein the cellular processes support the survival, growth and invasive properties of the cells, and the stem cells in turn regulate the cellular processes in the tumour microenvironment for self-benefit.
3.1. Epithelial–Mesenchymal Transition
The process by which an epithelial phenotype undergoes transition first by increasing in dimension and subsequently by acquiring a mesenchymal phenotype is called EMT [44]. One of the very first studies identifying stem-cell like subpopulations in the ovarian epithelium by Virant-Klun et al. found strong evidence that the stem cell niche induced EMT [8]. This transition is a dynamic process occurring in conjunction with persisting surrounding epithelial cells, as well as a wide spectrum of stromal cells (fibroblasts, immune cells) and endothelial cells, and enabling invasive and migratory properties within cancer cell populations [45]. Specific transcription factors are associated with the transitional process and can be mainly categorized into three families—TWIST, Snail and ZEB [46]. They suppress epithelial state-inducing genes like E-cadherin and stimulate mesenchymal state-inducing genes like N-cadherin [46]. These transcription factors have also been associated with expression of stemness-enhancing genes [47][48]. In the ovarian cancer stem cell niche, TGF-β signaling plays a significant role in promoting EMT via regulation of tissue transglutaminase 2 (TTGM2) [49]. A dynamic EMT state leads to increased stemness and enables chemoresistance. OCSCs exist in an intermediate epithelial–mesenchymal state, expressing both kinds of markers and equipping them with unique potential for adhesion and migration, respectively [50]. This dials into the plasticity model for cancer stem cells by proving that stemness is a dynamic interconvertible state [51].
3.2. Hypoxia
While hypoxia has been implicated as a driver in the maintenance of most cancer stem cell niches, it is of particular interest in ovarian cancer due to the presence of ascites which serve as metastasis hotspots for invasive spheroid formation. Ascites contain half the soluble oxygen as blood [52]. This hypoxic condition stimulates the hypoxia-inducible transcription factor-1 alpha (HIF-1α) to initiate hypoxia-responsive downstream signaling of various target genes which allows cells to adapt to environmental insults. Hypoxia drives stemness [53] and induces chemoresistance potential by maintaining OCSCs in a quiescent state, shielding them from drugs intended to target proliferative cells [54]. HIF-1,2 are involved in stimulating fibroblasts to secrete CXCL12, which is believed to initiate the cancerous phenotype in ovarian cancer. [55] These cells are also able to respond to stress [56], whilst also being invasive and migratory, and can promote increased angiogenic potential [52]. Reactive oxygen species (ROS) are produced by cancer cells and can stimulate oncogenes and facilitate new mutations. A recent study verified that ROS levels were eight times higher in tumours from 34 Stage III/IV HGSOC patients than in non-cancerous ovaries [57]. Elevated level of ROS in cancer stem cells has been found to promote cancer metastasis by inducing EMT via the TGF-β pathway [58][59].
3.3. Neovascularisation and Angiogenesis
Hypoxic conditions also induce the expression of vascular endothelial growth factor (VEGF), the most potent pro-angiogenic factor, by various cells (both cancer stem cells and normal cancer cells) in the tumour microenvironment [56]. Specifically, in the ovarian cancer niche, VEGF stimulates the CXCL2 receptor pathway in the endothelial cells, further inducing angiogenesis [60]. Moreover, Alvero et al. showed that OCSCs themselves have the capacity to form new vessels independent of the VEGF pathway [61]. This was further supported in another study demonstrating that OCSCs can self-differentiate into endothelial cells and undergo angiogenesis via activation of NF-κB and JAK2/STAT3 signaling [62]. VEGFA also stimulates upregulation of Bmi1 and loss of miR128-2, which increases stemness [63]. In addition, the vascular niche stimulates the expression of inflammatory cytokines, which further lead to metastatic initiation, and self-renewal and maintenance of the stem cell niche. Hence, the stem cell niche and angiogenic processes trigger and maintain each other in a cyclical manner.
3.4. Inflammation
The tumour microenvironment has been linked to chronic inflammation producing cytokines and pro-angiogenic signals which in turn initiate a cascade of immune responses. Immune signaling in the microenvironment, as outlined previously, feeds back into enrichment and maintenance of the stem cell niche, and this aspect will be discussed in further detail.
4. Ovarian Cancer Stem Cell Niche and Inflammatory Networks
Although there has been a long apparent association between inflammation and cancer, it was only introduced as one of the ‘Hallmarks of Cancer’ in Hanahan and Weinberg’s second, revised magnum opus [64]. Chronic inflammation has been established as a cause of several cancers [65], and the phenotypes, processes and pathways associated with various immune cells and interactions contribute to the dynamic maintenance of the tumours at a microenvironmental level [66]. These correlations have been verified in vitro, in zebrafish [67] and mouse models [68] as well as in patient prognostic data [69]. Specifically, cancer stem cells can use immune surveillance evasion to enhance their survival and invasive properties. Growing evidence suggests that cancer stem cells are able to not only circumvent key immune checkpoints, but also manipulate inflammatory networks to promote self-sustenance, tumorigenesis and cellular invasion [68]. Ovarian cancer, in particular, is a classic example of a stem cell-driven cancer. It metastasises via a trans-coelomic route spreading to the peritoneal organs in the form of persistent spherical multicellular aggregates. The primary tumour is capable of metastasising very early due to the ability to form spheroids from ascites, which proliferate and persist even in the absence of organ adhesion, and displays key stemness attributes [70]. These cells invade the extracellular matrix where they interact with the cellular microenvironment consisting broadly of immune (cytokines, macrophages, lymphocytes) and non-immune (adipocytes, fibroblasts, endothelial) cell components (Figure 4).
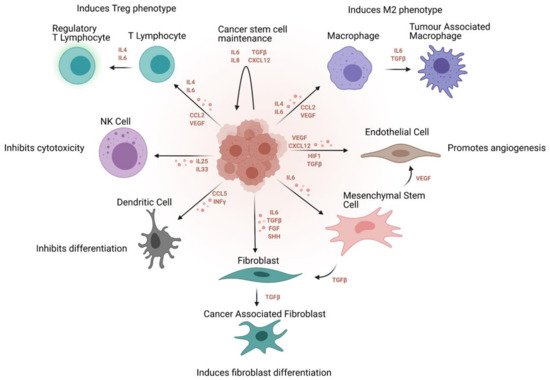
Figure 4. Immunosuppressive effect of the cancer stem cell niche on the tumour immune and non-immune microenvironment. Signaling molecules regulating these processes and the overall effect of the stem cell niche are outlined.
4.1. Cytokine Signaling
Not only have cytokines been identified in ovarian cancer patient ascites and cysts [71], they have also been found in the tumour stroma and epithelium [72]. This indicates that active cytokine-mediated signaling is part of the microenvironmental interactions in the ovarian tumour niche. Non-tumoral cells like adipocytes in the omentum and endothelial cells of the vasculature also trigger the release of cytokine signaling. Adipocyte-mediated cytokine signaling induces a change in lipid metabolism and allows cancer cells to use fatty acids as fuel for proliferation [73]. In ovarian cancer, adipocytes express IL-6, increasing the expression of BCLxl that provides the ability to cancer stem cells to become resistant to drug therapy [74]. Endothelial cells on the other hand, enhance inflammation and angiogenetic potential along with cell migration in the tumoral niche via the release of TNF-α, VEGF and interleukins (IL) [75]. IL-17 was one of the first cytokines identified in the ovarian cancer niche which was found to promote self-renewal of OCSCs [76][77]. Upon further investigation, it was found that OCSCs expressed the IL-17 receptor which promotes self-sustenance and growth via the NF-κB and MAPK pathways [76]. The NF-κB pathway has also been implicated via the release of IL-23 [78] and CCL5 [79] by OCSCs, which further enriches the angiogenic potential of tumour cells within the niche.
4.2. Tumour-Associated Macrophages (TAMs)
TAMs constitute the highest percentage of immune cells in the tumour niche. JAK2/STAT3 activation within TAMs promotes increased tumorigenicity, chemoresistance and stemness within tumours [80]. Subsequently, anti-tumour CD8+ responses from chemotherapeutic targeting are blocked by the cancer stem cell niche and the polarisation of the TAMs towards an anti-inflammatory M2 phenotype [80]. M2 macrophages in general have been seen to have a notable positive impact on the progression of tumours in different cancers [81]. In particular, among patients with high-grade ovarian cancer, M1 macrophages were significantly associated with better outcomes, while the M2 phenotype was associated with worse outcomes [82]. A co-culture study proved that OCSCs are capable of polarising the macrophage phenotype towards an M2 state via COX-2 overexpression and cytokine production, involving the JAK2/STAT3 pathway [83]. Furthermore, the M2 phenotype stimulates cancer stem cell self-renewal and growth via various signaling pathways e.g., EGF, TGF-β, IL-6 and IL-10, that lead to STAT3 activation [84]. NF-κB signaling pathways are activated, causing subsequent recruitment of M2 macrophages and also contributing to supplementary production of cytokines, and hence feedback into the self-sustaining cycle of the cancer stem cell niche [84]. Furthermore, an immunosuppressive microenvironment may originate as an outcome of the responses of CD4+ Treg T cells that are stimulated by M2 macrophages [85]. Additionally, macrophages make the tumour microenvironment amicable for cancer stem cell seeding as well as migration [86][87].
4.3. Tumour-Infiltrating Lymphocytes (TILs)
Tumour-infiltrating lymphocytes (TILs) includes cells such as CD8+ T cells, T regulatory cells and B regulatory cells, and they are recruited to the tumour mass. The presence of these infiltrates in the tumour microenvironment has a varied effect on tumour progression and prognosis, depending on the timeline of tumour growth as well as the subtype of ovarian cancer. In HGSOC, CD8+ T cells were found to correlate with better overall survival [85]. While a significant association was also observed between CD8+ T cells and overall survival in LGSOC, there was no such correlation in endometrioid or clear cell carcinomas [88]. B cells also contribute to tumour regulation both as tumour suppressive immune response cells, and immunosuppressive tumour-promoting cells [89]. In conjunction with T cells, B cells were found to co-localise in the niche, produce markers, and improve overall survival [90]. They also have a counter-regulatory effect on CD8+ T cells [91], contribute to cytokine signaling [92], and hence the overall proportion of these cells correlates with disease progression in a dynamic way.
4.4. Natural Killer Cells (NK)
Similar to T cells, NK cells are capable of acquiring memory functional phenotypes once target cells are encountered, thereby bridging the gap between adaptive and innate immune systems [93]. Cancer stem cells can be killed in a major histocompatibility complex (MHC)-unrestricted manner by NK cells [94] via the release of TNF family members [95]. Immunoglobulin Fc, inflammatory cytokines and endogenous ligands activate these NK cells [96]. A range of activating and inhibitory receptors modulate NK cell function. These receptors sense changes, such as loss of MHC in tumour cells, and subsequently allow NK cells to respond accordingly [93]. It was found that OCSCs downregulate NK cell function. Ascites of ovarian cancer patients have been found to have increased levels of NK cells. However, due to the immunosuppressive effects of the ovarian cancer stem cell niche and the dysregulation of natural and cell-mediated cytotoxicity, these cells are functionally impaired [97].
References
- Brucks, J.A. Ovarian cancer. The most lethal gynecologic malignancy. Nurs. Clin. N. Am. 1992, 27, 835–845.
- American Cancer Society. 2020. Available online: (accessed on 2 February 2021).
- European Cancer Information System. 2020. Available online: (accessed on 3 February 2021).
- Nash, Z.; Menon, U. Ovarian cancer screening: Current status and future directions. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 32–45.
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38.
- Schwarz, R.F.; Ng, C.K.; Cooke, S.L.; Newman, S.; Temple, J.; Piskorz, A.M.; Gale, D.; Sayal, K.; Murtaza, M.; Baldwin, P.J.; et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015, 12, e1001789.
- Alvero, A.B.; Chen, R.; Fu, H.H.; Montagna, M.; Schwartz, P.E.; Rutherford, T.; Silasi, D.A.; Steffensen, K.D.; Waldstrom, M.; Visintin, I.; et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle 2009, 8, 158–166.
- Virant-Klun, I.; Stimpfel, M.; Cvjeticanin, B.; Vrtacnik-Bokal, E.; Skutella, T. Small SSEA-4-positive cells from human ovarian cell cultures: Related to embryonic stem cells and germinal lineage? J. Ovarian Res. 2013, 6, 24.
- Virant-Klun, I.; Stimpfel, M. Novel population of small tumour-initiating stem cells in the ovaries of women with borderline ovarian cancer. Sci. Rep. 2016, 6, 34730.
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106.
- Kessler, M.; Hoffmann, K.; Brinkmann, V.; Thieck, O.; Jackisch, S.; Toelle, B.; Berger, H.; Mollenkopf, H.J.; Mangler, M.; Sehouli, J.; et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat. Commun. 2015, 6, 8989.
- Liang, R.; Chen, X.; Chen, L.; Wan, F.; Chen, K.; Sun, Y.; Zhu, X. STAT3 signaling in ovarian cancer: A potential therapeutic target. J. Cancer 2020, 11, 837–848.
- Qureshy, Z.; Johnson, D.E.; Grandis, J.R. Targeting the JAK/STAT pathway in solid tumors. J. Cancer Metastasis Treat. 2020, 6, 27.
- Chan, E.; Luwor, R.; Burns, C.; Kannourakis, G.; Findlay, J.K.; Ahmed, N. Momelotinib decreased cancer stem cell associated tumour burden and prolonged disease-free remission period in a mouse model of human ovarian cancer. Oncotarget 2018, 9, 16599–16618.
- Cai, J.; Xu, L.; Tang, H.; Yang, Q.; Yi, X.; Fang, Y.; Zhu, Y.; Wang, Z. The role of the PTEN/PI3K/Akt pathway on prognosis in epithelial ovarian cancer: A meta-analysis. Oncologist 2014, 19, 528–535.
- Zhang, X.; George, J.; Deb, S.; Degoutin, J.L.; Takano, E.A.; Fox, S.B.; Bowtell, D.D.; Harvey, K.F.; AOCS Study Group. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene 2011, 30, 2810–2822.
- Gonzalez-Torres, C.; Gaytan-Cervantes, J.; Vazquez-Santillan, K.; Mandujano-Tinoco, E.A.; Ceballos-Cancino, G.; Garcia-Venzor, A.; Zampedri, C.; Sanchez-Maldonado, P.; Mojica-Espinosa, R.; Jimenez-Hernandez, L.E.; et al. NF-kappaB Participates in the Stem Cell Phenotype of Ovarian Cancer Cells. Arch. Med. Res. 2017, 48, 343–351.
- Leizer, A.L.; Alvero, A.B.; Fu, H.H.; Holmberg, J.C.; Cheng, Y.C.; Silasi, D.A.; Rutherford, T.; Mor, G. Regulation of inflammation by the NF-kappaB pathway in ovarian cancer stem cells. Am. J. Reprod. Immunol. 2011, 65, 438–447.
- Merchant, A.A.; Matsui, W. Targeting Hedgehog—A cancer stem cell pathway. Clin. Cancer Res. 2010, 16, 3130–3140.
- Park, J.T.; Li, M.; Nakayama, K.; Mao, T.L.; Davidson, B.; Zhang, Z.; Kurman, R.J.; Eberhart, C.G.; Shih Ie, M.; Wang, T.L. Notch3 gene amplification in ovarian cancer. Cancer Res. 2006, 66, 6312–6318.
- McAuliffe, S.M.; Morgan, S.L.; Wyant, G.A.; Tran, L.T.; Muto, K.W.; Chen, Y.S.; Chin, K.T.; Partridge, J.C.; Poole, B.B.; Cheng, K.H.; et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumours to platinum therapy. Proc. Natl. Acad. Sci. USA 2012, 109, E2939–E2948.
- Ng, A.; Tan, S.; Singh, G.; Rizk, P.; Swathi, Y.; Tan, T.Z.; Huang, R.Y.; Leushacke, M.; Barker, N. Lgr5 marks stem/progenitor cells in ovary and tubal epithelia. Nat. Cell Biol. 2014, 16, 745–757.
- Chau, W.K.; Lp, C.K.; Mak, A.S.C.; Lai, H.-C.; Wong, A.S.T. c-Kit mediates chemoresistance and tumour-initiating capacity of ovarian cancer cells through activation of Wnt/β-catenin–ATP-binding cassette G2 signaling. Oncogene 2013, 32, 2767–2781.
- Liao, X.; Siu, M.K.; Au, C.W.; Wong, E.S.; Chan, H.Y.; Ip, P.P.; Ngan, H.Y.; Cheung, A.N. Aberrant activation of hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis 2009, 30, 131–140.
- Hall, C.A.; Wang, R.; Miao, J.; Oliva, E.; Shen, X.; Wheeler, T.; Hilsenbeck, S.G.; Orsulic, S.; Goode, S. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010, 70, 8517–8525.
- Munoz-Galvan, S.; Felipe-Abrio, B.; Verdugo-Sivianes, E.M.; Perez, M.; Jimenez-Garcia, M.P.; Suarez-Martinez, E.; Estevez-Garcia, P.; Carnero, A. Downregulation of MYPT1 increases tumour resistance in ovarian cancer by targeting the Hippo pathway and increasing the stemness. Mol. Cancer 2020, 19, 7.
- Altomare, D.A.; Wang, H.Q.; Skele, K.L.; De Rienzo, A.; Klein-Szanto, A.J.; Godwin, A.K.; Testa, J.R. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumour cell growth. Oncogene 2004, 23, 5853–5857.
- Abubaker, K.; Luwor, R.B.; Zhu, H.; McNally, O.; Quinn, M.A.; Burns, C.J.; Thompson, E.W.; Findlay, J.K.; Ahmed, N. Inhibition of the JAK2/STAT3 pathway in ovarian cancer results in the loss of cancer stem cell-like characteristics and a reduced tumour burden. BMC Cancer 2014, 14, 317.
- Muñoz-Galván, S.; Carnero, A. Targeting Cancer Stem Cells to Overcome Therapy Resistance in Ovarian Cancer. Cells 2020, 9, 1402.
- Rinkenbaugh, A.L.; Baldwin, A.S. The NF-kappaB Pathway and Cancer Stem Cells. Cells 2016, 5, 16.
- House, C.D.; Jordan, E.; Hernandez, L.; Ozaki, M.; James, J.M.; Kim, M.; Kruhlak, M.J.; Batchelor, E.; Elloumi, F.; Cam, M.C.; et al. NFkappaB Promotes Ovarian Tumourigenesis via Classical Pathways That Support Proliferative Cancer Cells and Alternative Pathways That Support ALDH(+) Cancer Stem-like Cells. Cancer Res. 2017, 77, 6927–6940.
- Ferrandina, G.; Bonanno, G.; Pierelli, L.; Perillo, A.; Procoli, A.; Mariotti, A.; Corallo, M.; Martinelli, E.; Rutella, S.; Paglia, A.; et al. Expression of CD133–1 and CD133–2 in ovarian cancer. Int. J. Gynecol. Cancer 2008, 18, 506–514.
- Roy, L.; Bobbs, A.; Sattler, R.; Kurkewich, J.L.; Dausinas, P.B.; Nallathamby, P.; Cowden Dahl, K.D. CD133 Promotes Adhesion to the Ovarian Cancer Metastatic Niche. Cancer Growth Metastasis 2018, 11, 1179064418767882.
- Ma, I.; Allan, A.L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011, 7, 292–306.
- Ishiguro, T.; Sato, A.; Ohata, H.; Ikarashi, Y.; Takahashi, R.U.; Ochiya, T.; Kato, T.; Kasamatsu, T.; Enomot, T.; Tanaka, K.; et al. Establishment and characterization of an in vitro model of ovarian cancer stem-like cells with an enhanced proliferative capacity. Cancer Res. 2016, 76, 150–160.
- Deng, S.; Yang, X.; Lassus, H.; Liang, S.; Kaur, S.; Ye, Q.; Li, C.; Wang, L.P.; Roby, K.F.; Orsulic, S.; et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE 2010, 5, e10277.
- Bourguignon, L.Y.; Zhu, H.; Zhou, B.; Diedrich, F.; Singleton, P.A.; Hung, M.C. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumour cell migration and growth. J. Biol. Chem. 2001, 276, 48679–48692.
- Carpenter, P.M.; Dao, A.V. The role of hyaluronan in mesothelium-induced motility of ovarian carcinoma cells. Anticancer Res. 2003, 23, 3985–3990.
- Sacks, J.D.; Barbolina, M.V. Expression and function of CD44 in epithelial ovarian carcinoma. Biomolecules 2015, 5, 3051–3066.
- Burgos-Ojeda, D.; Wu, R.; McLean, K.; Chen, Y.C.; Talpaz, M.; Yoon, E.; Cho, K.R.; Buckanovich, R.J. CD24+ ovarian cancer cells are enriched for cancer-initiating cells and dependent on jak2 signaling for growth and metastasis. Mol. Cancer Ther. 2015, 14, 1717–1727.
- Gao, M.Q.; Choi, Y.P.; Kang, S.; Youn, J.H.; Cho, N.H. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene 2010, 29, 2672–2680.
- Mazzoldi, E.L.; Pavan, S.; Pilotto, G.; Leone, K.; Pagotto, A.; Frezzini, S.; Nicoletto, M.O.; Amadori, A.; Pastò, A. A juxtacrine/paracrine loop between C-Kit and stem cell factor promotes cancer stem cell survival in epithelial ovarian cancer. Cell Death Dis. 2019, 10, 412.
- Luo, L.; Zeng, J.; Liang, B.; Zhao, Z.; Sun, L.; Cao, D.; Yang, J.; Shen, K. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Exp. Mol. Pathol. 2011, 91, 596–602.
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428.
- Loret, N.; Denys, H.; Tummers, P.; Berx, G. The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance. Cancers 2019, 11, 838.
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110.
- Liao, T.T.; Yang, M.H. Revisiting epithelial-mesenchymal transition in cancer metastasis: The connection be-tween epithelial plasticity and stemness. Mol. Oncol. 2017, 11, 792–804.
- Ahmed, N.; Kadife, E.; Raza, A.; Short, M.; Jubinsky, P.T.; Kannourakis, G. Ovarian Cancer, Cancer Stem Cells and Current Treatment Strategies: A Potential Role of Magmas in the Current Treatment Methods. Cells 2020, 9, 719.
- Rafehi, S.; Ramos Valdes, Y.; Bertrand, M.; McGee, J.; Prefontaine, M.; Sugimoto, A.; DiMattia, G.E.; Shepherd, T.G. TGFbeta signaling regulates epithelial-mesenchymal plasticity in ovarian cancer ascites-derived spheroids. Endocr. Relat. Cancer 2016, 23, 147–159.
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629.
- Strauss, R.; Li, Z.Y.; Liu, Y.; Beyer, I.; Persson, J.; Sova, P.; Möller, T.; Pesonen, S.; Hemminki, A.; Hamerlik, P.; et al. Analysis of epithelial and mesenchymal markers in ovarian cancer reveals phenotypic heterogeneity and plasticity. PLoS ONE 2011, 6, e16186.
- Jolly, M.K.; Boareto, M.; Huang, B.; Jia, D.; Lu, M.; Ben-Jacob, E.; Onuchic, J.N.; Levine, H. Implications of the hybrid epithelial/mesenchymal phenotype in metastasis. Front. Oncol. 2015, 5, 155.
- Qin, J.; Liu, Y.; Lu, Y.; Liu, M.; Li, M.; Li, J.; Wu, L. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci. Rep. 2017, 7, 10592.
- He, M.; Wu, H.; Jiang, Q.; Liu, Y.; Han, L.; Yan, Y.; Wei, B.; Liu, F.; Deng, X.; Chen, H.; et al. Hypoxia-inducible factor-2α directly promotes BCRP expression and mediates the resistance of ovarian cancer stem cells to adriamycin. Mol. Oncol. 2019, 13, 403–421.
- Yeo, C.D.; Kang, N.; Choi, S.Y.; Kim, B.N.; Park, C.K.; Kim, J.W.; Kim, Y.K.; Kim, S.J. The role of hypoxia on the acquisition of epithelial-mesenchymal transition and cancer stemness: A possible link to epigenetic regulation. Korean J. Intern. Med. 2017, 32, 589–599.
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2010, 29, 625–634.
- Kitajima, S.; Lee, K.L.; Hikasa, H.; Sun, W.; Huang, R.Y.; Yang, H.; Matsunaga, S.; Yamaguchi, T.; Araki, M.; Kato, H.; et al. Hypoxia-inducible factor-1alpha promotes cell survival during ammonia stress response in ovarian cancer stem-like cells. Oncotarget 2017, 8, 114481–114494.
- Cohen, S.; Mehrabi, S.; Yao, X.; Millingen, S.; Aikhionbare, F.O. Reactive Oxygen Species and Serous Epithelial Ovarian Adenocarcinoma. Cancer Res. J. 2016, 4, 106–114.
- Bensimon, J.; Biard, D.; Paget, V.; Goislard, M.; Morel-Altmeyer, S.; Konge, J.; Chevillard, S.; Lebeau, J. Forced extinction of CD24 stem-like breast cancer marker alone promotes radiation resistance through the control of oxidative stress. Mol. Carcinog. 2016, 55, 245–254.
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumour growth and angiogenesis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 1011–1027.
- Kryczek, I.; Lange, A.; Mottram, P.; Alvarez, X.; Cheng, P.; Hogan, M.; Moons, L.; Wei., S.; Zou, L.; Machelon, V.; et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005, 65, 465–472.
- Alvero, A.B.; Fu, H.H.; Holmberg, J.; Visintin, I.; Mor, L.; Marquina, C.C.; Oidtman, J.; Silasi, D.A.; Mor, G. Stem-like ovarian cancer cells can serve as tumour vascular progenitors. Stem Cells 2009, 27, 2405–2413.
- Tang, S.; Xiang, T.; Huang, S.; Zhou, J.; Wang, Z.; Xie, R.; Long, H.; Zhu, B. Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumour angiogenesis through autocrine CCL5 signaling. Cancer Lett. 2016, 376, 137–147.
- Jang, K.; Kim, M.; Gilbert, C.A.; Simpkins, F.; Ince, T.A.; Slingerland, J.M. VEGFA activates an epigenetic pathway upregulating ovarian cancer-initiating cells. EMBO Mol. Med. 2017, 9, 304–318.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- O’Byrne, K.J.; Dalgleish, A.G. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer 2001, 85, 473–483.
- Mantovani, A. The inflammation-cancer connection. FEBS J. 2018, 285, 638–640.
- Freisinger, C.M.; Huttenlocher, A. Live imaging and gene expression analysis in zebrafish identifies a link between neutrophils and epithelial to mesenchymal transition. PLoS ONE 2014, 9, e112183.
- Noy, R.; Pollard, J.W. Tumour-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61.
- Gentles, A.; Newman, A.; Liu, C.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945.
- Ghoneum, A.; Afify, H.; Salih, Z.; Kelly, M.; Said, N. Role of tumour microenvironment in the pathobiology of ovarian cancer: Insights and therapeutic opportunities. Cancer Med. 2018, 7, 5047–5056.
- Clendenen, T.V.; Lundin, E.; Zeleniuch-Jacquotte, A.; Koenig, K.L.; Berrino, F.; Lukanova, A.; Lokshin, A.E.; Idahl, A.; Ohlson, N.; Hallmans, G.; et al. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 799–810.
- Jammal, M.P.; Martins-Filho, A.; Silveira, T.P.; Murta, E.F.; Nomelini, R.S. Cytokines and prognostic factors in epithelial ovarian cancer. Clin. Med. Insights Oncol. 2016, 10, 71–76.
- Rodriguez, G.M.; Galpin, K.J.C.; McCloskey, C.W.; Vanderhyden, B.C. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers 2018, 24, 242.
- Cardenas, C.; Montagna, M.K.; Pitruzzello, M.; Lima, E.; Mor, G.; Alvero, A.B. Adipocyte microenvironment promotes Bclxl expression and confers chemoresistance in ovarian cancer cells. Apoptosis 2017, 22, 558–569.
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front Oncol. 2019, 9, 1399.
- Kato, T.; Furumoto, H.; Ogura, T.; Onishi, Y.; Irahara, M.; Yamano, S.; Kamada, M.; Aono, T. Expression of IL-17 mRNA in ovarian cancer. Biochem. Biophys. Res. Commun. 2001, 282, 735–738.
- Xiang, T.; Long, H.; He, L.; Han, X.; Lin, K.; Liang, Z.; Zhuo, W.; Xie, R.; Zhu, B. Interleukin-17 produced by tumor microenvironment promotes self-renewal of CD133+ cancer stem-like cells in ovarian cancer. Oncogene 2015, 34, 165–176.
- Wang, D.; Xiang, T.; Zhao, Z.; Lin, K.; Yin, P.; Jiang, L.; Liang, Z.; Zhu, B. Autocrine interleukin-23 promotes self-renewal of CD133+ ovarian cancer stem-like cells. Oncotarget 2016, 7, 76006–76020.
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells 2012, 30, 2309–2319.
- Mitchem, J.B.; Brennan, D.J.; Knolhoff, B.L.; Belt, B.A.; Zhu, Y.; Sanford, D.E.; Belaygorod, L.; Carpenter, D.; Collins, L.; Piwnica-Worms, D.; et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res. 2013, 73, 1128–1141.
- Coffelt, S.B.; Hughes, R.; Lewis, C.E. Tumor-associated macrophages: Effectors of angiogenesis and tumor progression. Biochim. Biophys. Acta 2009, 1796, 11–18.
- Gao, Y.; Chen, L.; Cai, G.; Xiong, X.; Wu, Y.; Ma, D.; Li, S.C.; Gao, Q. Heterogeneity of immune microenvironment in ovarian cancer and its clinical significance: A retrospective study. Oncoimmunology 2020, 9, 1760067.
- Zhang, Q.; Cai, D.J.; Li, B. Ovarian cancer stem-like cells elicit the polarization of M2 macrophages. Mol. Med. Rep. 2015, 11, 4685–4693.
- Korkaya, H.; Liu, S.; Wicha, M.S. Regulation of cancer stem cells by cytokine networks: Attacking cancer’s inflammatory roots. Clin. Cancer Res. 2011, 17, 6125–6129.
- Dionne, L.K.; Driver, E.R.; Wang, X.J. Head and neck cancer stem cells: From identification to tumor immune network. J. Dent. Res. 2015, 94, 1524–1531.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570.
- Pinto, M.P.; Balmaceda, C.; Bravo, M.L.; Kato, S.; Villarroel, A.; Owen, G.I.; Roa, J.C.; Cuello, M.A.; Ibañez, C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018, 151, 10–17.
- Clarke, B.; Tinker, A.V.; Lee, C.H.; Subramanian, S.; van de Rijn, M.; Turbin, D.; Kalloger, S.; Han, G.; Ceballos, K.; Cadungog, M.G.; et al. Intraepithelial T cells and prognosis in ovarian carcinoma: Novel associations with stage, tumor type, and BRCA1 loss. Mod. Pathol. 2009, 22, 393–402.
- Tokunaga, R.; Naseem, M.; Lo, J.H.; Battaglin, F.; Soni, S.; Puccini, A.; Berger, M.D.; Zhang, W.; Baba, H.; Lenz, H.J. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat. Rev. 2019, 73, 10–19.
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20C tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8C T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292.
- Mauri, C.; Menon, M.J. Human regulatory B cells in health and disease: Therapeutic potential. Clin. Investig. 2017, 127, 772–779.
- Nielsen, J.S.; Nelson, B.H. Tumor-infiltrating B cells and T cells: Working together to promote patient survival. Oncoimmunology 2012, 1, 1623–1625.
- Rosenberg, J.; Huang, J. CD8(+) T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20.
- Luna, J.I.; Grossenbacher, S.K.; Murphy, W.J.; Canter, R.J. Targeting cancer stem cells with natural killer cell immunotherapy. Expert Opin. Biol. Ther. 2017, 17, 313–324.
- Rezvani, K.; Rouce, R.; Liu, E.; Shpall, E. Engineering Natural Killer Cells for Cancer Immunotherapy. Mol. Ther. 2017, 25, 1769–1781.
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158.





