
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Paolo Sapienza | + 2247 word(s) | 2247 | 2021-04-20 08:29:21 | | | |
| 2 | Lily Guo | Meta information modification | 2247 | 2021-04-28 10:25:46 | | |
Video Upload Options
Self-expandable metal stent (SEMS) is commonly accepted in a palliative setting for symptomatic obstructive colorectal cancer.
1. Introduction
While the self-expandable metal stent (SEMS) is commonly accepted in a palliative setting for obstructive colorectal cancer, deciding whether to proceed with endoscopic stent as a bridge to curative surgery or upfront emergency surgery (ES) in case of symptomatic left-sided malignant colonic obstruction is still under debate. Several authors [1],[2] do not recommend the use of SEMS before surgery in resectable patients because it may harm long-term outcomes. Similarly, the 2017 Guidelines of the World Society of Emergency Surgery [3] recognize “interesting advantages” offered by the use of the SEMS, but they highlighted that its use for surgically treatable cases may expose some long-term oncologic issues. Conversely, the recent European Society of Gastrointestinal Endoscopy (ESGE) Guideline [4] recommended the use of SEMS because it is associated with lower mortality rate, shorter hospital stay and a lower rate of related colostomy.
To date, only a few randomized controlled trials (RCTs) have been published on this topic. The last systematic review including only RCTs was published three years ago [5], and the authors did not report a pooled analysis on the survival variables. Furthermore, an additional RCT was published by Elwan et al. [6] in 2020 adding data for future analysis. Recently, the long-term oncologic results of the ESCO Trial were presented [7].
2. Studies on Self-Expandable Metal Stent as a Bridge to Surgery Versus Emergency Surgery in Colorectal Cancer
The PRISMA flow chart for systematic review schematically reported (Figure 1). Briefly, after this screening for relevance, 20 articles remained for further assessment of eligibility. Eight of them were successively excluded [8][9][10][11][12][13][14][15] and a total of 12 articles were eligible for further analyses (Table 1) [6][7][16][17][18][19][20][21][22][23][24][25]. Noticeably, 3 RTCs reported the short-term results in the first publication [16][19][23] and the long-term outcomes in a subsequent publication [7][24][25]. Therefore, for long-term outcomes we considered the second publication reporting an up-to-date follow-up.
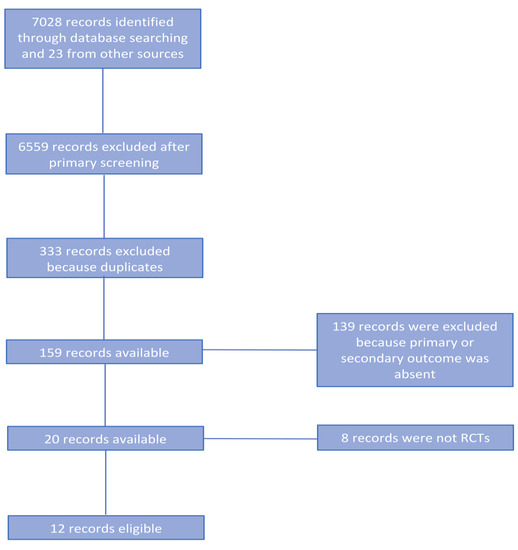
Figure 1. PRISMA flow chart.
Table 1. Characteristics of included studies.
| Author | Country | Number of Centres | Time of Enrollment | Premature Closure of the Trial | Number of Patients Enrolled | |
|---|---|---|---|---|---|---|
| SEMS | Surgery | |||||
| Arezzo et al., 2020 | Italy/Spain | Multicenter | 2008–2015 | No | 56 * | 59 |
| Elwan et al., 2020 | Egypt | Single-centre | 2015–2019 | No | 30 | 30 |
| Arezzo et al., 2017 | Italy/Spain | Multicenter | 2008–2015 | No | 56 * | 59 |
| Sloothaak et al., 2014 | Netherlands | Single-centre | 2007–2009 | Yes | 26 | 32 |
| Thung et al., 2013 | Hong Kong, China | Single-centre | 2002–2005 | No | 24 | 24 |
| Ghazal et al., 2013 | Egypt | Single center | 2009–2012 | No | 30 | 30 |
| Ho et al., 2012 | Singapore | Single-centre | 2004–2008 | No | 20 | 19 |
| Pirlet et al., 2011 | France | Multicenter | 2002–2006 | Yes | 30 | 30 |
| Van Hooft et al., 2011 | Netherlands | Multicenter | 2007–2009 | Yes | 47 | 51 |
| Cui et al., 2011 | China | Single center | 2005–2009 | No | 29 | 15 |
| Alcántara et al., 2011 | Spain | Single-centre | 2004–2006 | Yes | 15 | 13 |
| Cheung et al., 2009 | Hong Kong, China | Single-centre | 2002–2005 | No | 24 | 24 |
2.1. Characteristics of the Studies Included
The majority of studies were performed in Europe (4 studies: 299 patients, 54.36%), followed by Asia (3 studies: 131 patients, 22.82%) and Africa (2 study: 120 patients, 21.82%).
Three studies were multicentric while the remaining were single center. All articles describe the duration of the participants’ enrollment comprised between 3 and 12 years. The studies were published between 2009 and 2020.
Three RCTs were prematurely terminated for the unacceptable high complication rate; the first was terminated because emergency surgery group had significantly increased rate of anastomotic leak [17]; the second reported a significantly higher incidence of 30-day morbidity in the SEMS group of patients [19]; the third for a high rate of colonic perforations during stent placement and a high rate of technical failure of stent placement [20]. Patients’ characteristics were similar between the groups (Table S1). Four studies included stage IV patients and in two, the inclusion rate was different (Table S1) [16][21]. The tumor location was reported in all but one [16] study; in seven the cancer was located in the left colon or rectum, and in one [6] the authors also included patients affected with right colon cancer (Table S2).
The laparoscopic colectomy was commonly performed in the SEMS group; conversely, in ES group a traditional open approach was preferred (S3). The intraoperative colonic lavage was performed in a few studies during ES (Table S3).
In ES group, surgical treatment varied deeply; total or subtotal abdominal colectomy with ileorectal anastomosis, Hartmann’s procedure, colorectal resection with primary anastomosis, or derivative colostomy (Table S3). Conversely, all patients undergoing SEMS positioning as a bridge to resective surgery had colorectal resection with primary anastomosis.
In eight studies the type of stent was reported, and in most of the studies the authors used the Wallflex stents; the time intercourse between stent placement and elective surgery was 5 to 10 days (Table S4). The perforation rate varied between 8.9–14% (Table S4).
2.2. Quality Assessment of the Included Studies
The potential risk for bias in each of the trials and a summary of these using the criteria and the “Risk of bias” table are reported in Cochrane Handbook for Systematic Reviews of Interventions Version 5 [26][27]. The risk of bias of RCTs was reported in Figure S5a. (review authors’ judgments about each risk of bias item presented as percentages across all included studies) and Figure S5b (review authors’ judgments about each risk of bias item for each included study).
2.3. Primary Outcomes
2.3.1. Overall Postoperative Mortality Rate
Eight studies including 508 patients (252 SEMS and 256 ES) reported the mortality rates. We registered 16 (6.35%) deaths in the SEMS group and 17 (6.64%) in the ES group. Four studies did not specify when mortality occurred [6][16][18][21]. Three studies reported an overall in-hospital mortality rate [17][20][22], one study a 30-day mortality [19] and one a 60-day mortality [23]. No differences between the mortality rate in the two groups (RR 1.06, 95% CI 0.55 to 2.04; I2 = 0%) (Figure 2a) were recorded.
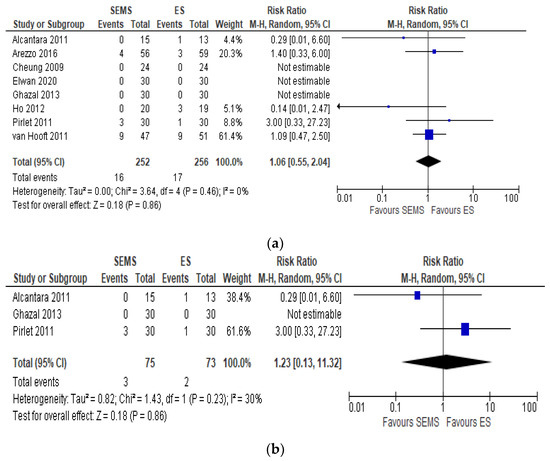
Figure 2. (a) Forest plot of overall postoperative mortality rate. (b) Forest plot of overall postoperative mortality during the hospital stay.
The subgroup analysis of hospital mortality [17][20][22] reported the same mortality in the two groups (RR 1.23, 95% CI 0.13 to 11.32; I2 = 30%) (Figure 2b)
2.3.2. Postoperative Complications Rate
Nine studies (567 patients: 281 SEMS and 286 ES) reported the postoperative complications. The overall postoperative complications rate was significantly lower in the SEMS group (32.74%) and in the ES group (48.25%) (RR 0.61, 95% CI 0.41 to 0.91; I2 = 65%) (Figure 3a). Two studies did not specify when the complications occurred [6][21]. Two studies [17][22] reported an overall in-hospital postoperative complication rates without further specification, one study [19] a 30-day and one [23] a 60-day postoperative complication rate.
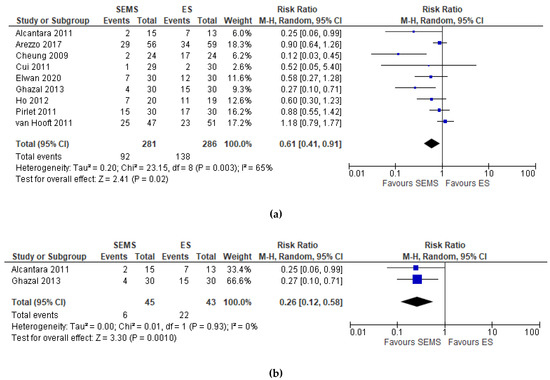
Figure 3. (a) Forest plot of overall postoperative complications. (b) Forest plot of overall postoperative complications during hospital stay.
The subgroup analysis of hospital postoperative complications [17][22] showed a statistically significant lower complication rate in the SEMS group (RR 0.26, 95% CI 0.12 to 0.58; I2 = 0%) as compared to the ES group (Figure 3b).
2.3.3. Clinical Success Rate. Successful Primary Anastomosis
Nine studies (557 patients: 281 SEMS and 276 ES) reported this outcome. The rate of primary anastomosis was significantly higher in of SEMS (69.75%) than in the ES (55.07%) (RR 1.26, 95% CI 1.01 to 1.57; I2 = 86%) (Figure 4).
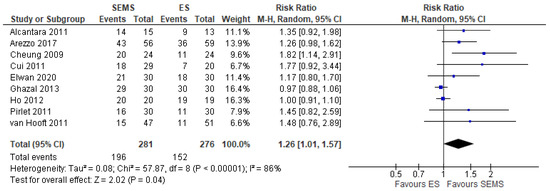
Figure 4. Forest plot of success of primary anastomosis.
2.4. Secondary Outcomes
2.4.1. Short-Term Outcomes
Technical Success Rate
Eight studies (508 patients: 252 SEMS and 256 ES) reported the clinical success rate. The technical success in SEMS group was intended as absence of colonic perforation, bleeding or stent migration, whereas in the ES group was intended as the absence of intraoperative surgical complications. The failure rate in SEMS group was 10.7%, whereas in the ES group there were no intraoperative surgical complications (RR 12.05, 95% CI 2.83 to 51.23; I2 = 0%).
Clinical Success Rate
Eight studies including 508 patients (252 SEMS and 256 ES) reported the clinical success rate intended as colonic decompression. The colonic decompression was significantly higher in patients who underwent ES (100%) than in of the patients undergoing SEMS (86.5%) (RR 9.18, 95% CI 2.06 to 27.59; I2 = 0%).
Anastomotic Leakage Rate
Eight studies (345 patients: 190 SEMS and 155 ES) reported the anastomotic leakage rate. It was lower in SEMS group (5.8%) as compared to the ES group (7.7%) (RR 0.78, 95% CI 0.32 to 1.91; I2 = 4%) (Figure 5).
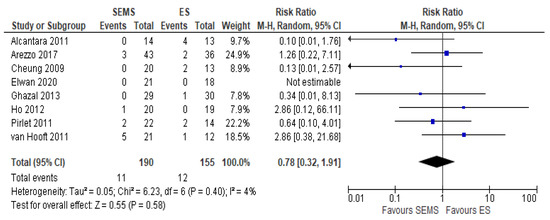
Figure 5. Forest plot of anastomotic leak.
Upfront Hartmann Procedure or Another Derivative Colostomy Rate
Eight studies (508 patients: 252 SEMS and 256 ES) reported the Hartmann or derivative colostomy procedure rate. The Hartmann or derivative colostomy procedure rate was statistically higher in ES group (39.1%) when compared to the SEMS group (22.41%) (RR 0.62, 95% CI 0.45 to 0.85; I2 = 23%) (Figure 6).
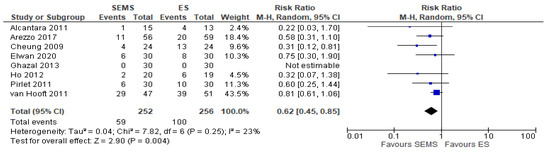
Figure 6. Forest plot of upfront Hartmann procedure or another derivative colostomy rate.
Permanent Hartmann Procedure or Another Derivative Colostomy Rate
Five studies (324 patients: 164 SEMS and 160 ES) reported the covering stoma. The covering stoma rate was higher in the ES group (35.62%) as compared to the SEMS group (22.56%) (RR 0.64, 95% CI 0.33 to 1.25; I2 = 47%) (Figure 7).
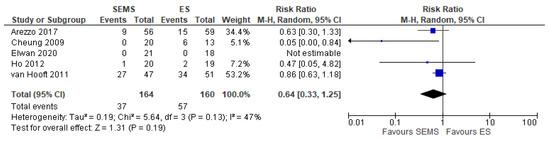
Figure 7. Forest plot of permanent Hartmann procedure or another derivative colostomy rate.
2.4.2. Long-Term Outcomes
Overall Recurrence
The overall recurrence rate was reported in five studies (302 patients: 148 SEMS and 154 ES). The rate was higher in the ES group (24.67%) as compared to the SEMS group (35.14%) (RR 1.63, 95% CI 0.88 to 2.04; I2 = 57%) (Figure 8).
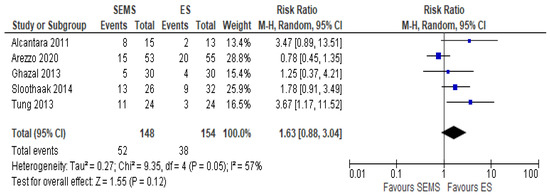
Figure 8. Forest plot of overall recurrence rate.
Local Recurrence
The local recurrence rate was reported in three studies (225 patients: 108 SEMS and 117 ES). The recurrence rate was higher in the SEMS group (11.11%) as compared to the ES group (8.54%) (RR 1.34, 95% CI 0.52 to 2.43; I2 = 0%) (Figure 9).
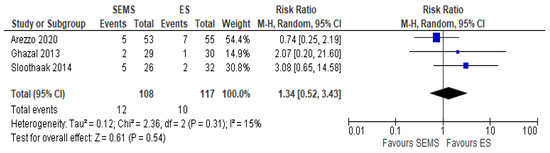
Figure 9. Forest plot of local recurrence rate.
Systemic Recurrence
The systemic recurrence rate was reported in three studies (225 patients: 108 SEMS and 117 ES). The rate was not statistically significant different between the two groups (21.77% in SEMS group vs 20% in ES group) (RR 1.01, 95% CI 0.60 to 1.71; I2 = 0%) (Figure 10).
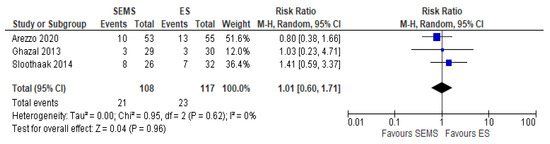
Figure 10. Forest plot of systemic recurrence rate.
Three Years OS
The 3-year OS was reported in four studies (235 patients: 118 SEMS and 117 ES). Three years’ survival rate was higher in ES group (72.5%) when compared to SEMS group (67.8%), but the result was not statistically significant (RR 1.20, 95% CI 0.80 to 1.79; I2 = 33%) (Figure 11).
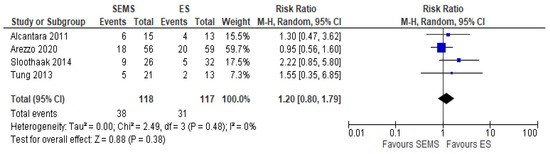
Figure 11. Forest plot of overall survival.
Three Years DFS
The 3-year DFS was reported in three studies (204 patients: 102 SEMS and 102 ES). The DFS rate was better in the ES group (62.46%) when compared to the SEMS group (58.65%) (RR 1.22, 95% CI 0.87 to 1.69; I2 = 0%) (Figure 12).
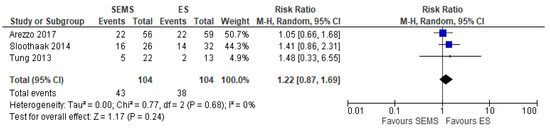
Figure 12. Forest plot of 3-year Disease Free Survival.
3. Discussion
The use of SEMS as a bridge to curative surgery is still controversial because it has some advantages but also some disadvantages [28]. Our up-to-date systematic review and meta-analysis demonstrated that the use of SEMS is associated with low in-hospital mortality, high rate of primary anastomosis and decreased need for Hartmann procedure or derivative colostomies.
Data regarding mortality greatly vary among the different published systematic reviews [29][30][31][32][33][34][35]. However, the most recent meta-analyses on this topic seem to be in line with our results, demonstrating a benefit in terms of mortality rates with the use of SEMS when compared to ES [36][37]. Particularly, our results on mortality rates were obtained from RCTs, which conferred a robust evidence in favor of SEMS in the presence of malignant left-sided colonic obstruction as a bridge to surgery.
The RCTs included in the papers are somewhat different. In effect, the recent systematic review and meta-analysis performed from Spannenburg et al. ES [36] included studies (RCTs and CCTs) in which the surgical treatment was performed with curative or palliative aim. Differently, our review included only RCTs in which the patients underwent curative surgery.
The higher rate of primary anastomosis in the SEMS group as compared to the ES group is also a clear advantage of this treatment, and our observations are consistent with the majority of previous studies (RR 1.26, 95% CI 1.01 to 1.57; I2 = 86%). Analyzing the available literature, we also should take into account when choosing one of the two different strategies: SEMS or ES both the clinical success rate (defined as the ability of the procedure to decompress the bowel) and the technical success rate (defined as intraprocedural versus intraoperative complications). This point represents an important limitation of this meta-analysis for the few different definitions of outcomes such as technical and clinical success in the included studies. For this reason, we have aggregated similar conditions reported in the literature in two outcome groups of this review (Table S6).
Our analysis suggests a preferential use of ES when considering these variables. Furthermore, SEMS might be a more complex and challenging procedure that is operator-dependent, affected by the expertise in operative endoscopy and it should be reserved to a tertiary care center.
Overall, our results support the use of SEMS whenever feasible, leaving the choice of ES for patients at a high risk of clinical/technical failure. At present, few studies tried to investigate the predictors of technical failure, but it seems that a stenosis greater than 8 cm in length and the need for endoscopic guidance may be associated with higher rates of technical and/or clinical stenting failure [38]. Finally, we believe that further analyses are required in order to identify and select the patients who might benefit from SEMS prior to resective surgery.
The main disadvantage in deploying SEMS as a bridge to surgery is the possibility to jeopardize long-term outcome [2][37][39][40][41][42]. Some authors sustain the hypothesis that SEMS deployment may cause microperforation leading to a higher risk of peritoneal carcinomatosis [37][39][40][41][42]; others support the possibility that a tumor’s compression by SEMS causes tumoral spreading into the nearby vessels, favoring hematogenous diffusion. However, if these hypotheses have had a reliable basis, our study should have produced consistent evidence in decreased survival and disease-free survival in the SEMS group.
Moreover, some authors [7][43] reported a higher rate of harvested lymph nodes, although not reaching statistical significance, in the resected patients after SEMS deployment, assuming that there was a strict relationship between delayed surgery and the availability of a more experienced colorectal surgeon in an elective setting.
However, our analysis found that the overall recurrence along with local and systemic recurrence and the three-year overall survival rate were similar among the two groups. According to these observations, we are confident to reinforce the use of SEMS in the presence of malignant left-sided colonic obstruction.
The quality of life, a crucial variable [44][45][46][47] which at least theoretically might favor the SEMS group of patients, was not considered in the RCTs included in the present analysis. Therefore, further studies are warranted to investigate the impact of the stoma creation rate, the risk of reintervention and the incidence of persistent stomas on patient-reported outcome.
References
- Dastur, J.K.; Forshaw, M.J.; Modarai, B.; Solkar, M.M.; Raymond, T.; Parker, M.C. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech. Coloproctol. 2008, 12, 51–55.
- Ng, K.C.; Law, W.L.; Lee, Y.M.; Choi, H.K.; Seto, C.L.; Ho, J.W. Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: A case-matched study. J. Gastrointest. Surg. 2006, 10, 798–803.
- Occhionorelli, S.; Tartarini, D.; Cappellari, L.; Stano, R.; Vasquez, G. Colonic stent placement as a bridge to surgery in patients with left-sided malignant large bowel obstruction. An observational study. G. Chir. 2014, 35, 283–289.
- Rodrigues-Pinto, E.; Morais, R.; Coelho, C.; Pereira, P.; Repici, A.; Macedo, G. Bridge-to-surgery versus emergency surgery in the management of left-sided acute malignant colorectal obstruction—Efficacy, safety and long-term outcomes. Dig. Liver Dis. 2019, 51, 364–372.
- Veld, J.V.; Amelung, F.J.; Borstlap, W.A.A.; Van Halsema, E.E.; Consten, E.C.J.; Siersema, P.D.; Ter Borg, F.; Van der Zaag, E.S.; De Wilt, J.H.W.; Fockens, P.; et al. For the Dutch Snapshot Research. Comparison of decompressing stoma vs stent as a bridge to surgery for left-sided obstructive colon cancer. JAMA Surg. 2020, 155, 206–215.
- Tanis, P.J.; Paulino Pereira, N.R.; Van Hooft, J.E.; Consten, E.C.; Bemelman, W.A. Dutch Surgical Colorectal Audit. Resection of obstructive left-sided colon cancer at a national level: A prospective analysis of short-term outcomes in 1816 patients. Dig. Surg. 2015, 32, 317–324.
- Flor-Lorente, B.; Baguena, G.; Frasson, M.; Garcia-Granero, A.; Cervantes, A.; Sanchiz, V.; Pena, A.; Espi, A.; Esclapez, P.; Garcia-Granero, E. Self-expanding metallic stent as a bridge to surgery in the treatment of left colon cancer obstruction: Cost-benefit analysis and oncologic results. Cir. Esp. 2017, 95, 143–151.
- Gorissen, K.J.; Tuynman, J.B.; Fryer, E.; Wang, L.; Uberoi, R.; Jones, O.M.; Cunningham, C.; Lindsey, I. Local recurrence after stenting for obstructing left-sided colonic cancer. Br. J. Surg. 2013, 100, 1805–1809.
- Elwan, T.H.; Zaher, N.A. Endoscopic stenting as a bridge to elective surgery versus emergency laparotomy for patients with acute malignant large bowel obstruction. Egypt. J. Surg. 2020, 39, 529–535.
- Arezzo, A.; Forcignanò, E.; Bonino, M.A.; Balagué, C.; Targarona, E.; Borghi, F.; Giraudo, G.; Ghezzo, L.; Passera, R.; Morino, M.; et al. Long-term Oncologic Results After Stenting as a Bridge to Surgery Versus Emergency Surgery for Malignant Left-sided Colonic Obstruction: A Multicenter Randomized Controlled Trial (ESCO Trial). Annu. Surg. 2020, 272, 703–708.
- Cheung, H.Y.; Chung, C.C.; Tsang, W.W.C.; Hoo Wong, J.C.; Kay Yau, K.K.; Wah Li, M.K. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: A randomised controlled trial. Arch. Surg. 2009, 144, 1127–1132.
- Alcántara, M.; Serra-Aracil, X.; Falcó, J.; Mora, L.; Bombardò, J.; Navarro, S. Prospective, controlled, randomised study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J. Surg. 2011, 35, 1904–1910.
- Cui, J.; Zhang, J.L.; Wang, S.; Sun, Z.Q.; Jiang, X.L. A preliminary study of stenting followed by laparoscopic surgery for obstructing left-sided colon cancer. Chin. J. Gastrointest. Surg. 2011, 14, 40–43.
- Van Hooft, J.E.; Bemelman, W.A.; Oldenburg, B.; Marinelli, A.W.; Lutke Holzik, M.F.; Grubben, M.J.; Sprangers, M.A.; Dijkgraaf, M.G.; Fockens, P. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: A multicentre randomised trial. Lancet Oncol. 2011, 12, 344–352.
- Pirlet, I.A.; Slim, K.; Kwiatkowski, F.; Michot, F.; Millat, B.L. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: A multicenter randomised controlled trial. Surg. Endosc. 2011, 25, 1814–1821.
- Ho, K.S.; Quah, H.M.; Lim, J.F.; Tang, C.L.; Eu, K.W. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: A prospective randomised trial. Int. J. Colorectal Dis. 2012, 27, 355–362.
- Ghazal, A.H.; El-Shazly, W.G.; Bessa, S.S.; El-Riwini, M.T.; Hussein, A.M. Colonic endoluminal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J. Gastrointest. Surg. 2013, 17, 1123–1129.
- Arezzo, A.; Balague, C.; Targarona, E.; Borghi, F.; Giraudo, G.; Ghezzo, L.; Arroyo, A.; Sola-Vera, J.; De Paolis, P.; Bossotti, M. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: Results of a multicentre randomised controlled trial (ESCO trial). Surg. Endosc. 2017, 31, 3297–3305.
- Tung, K.L.; Cheung, H.Y.; Ng, L.W.; Chung, C.C.; Li, M.K. Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: Long-term follow-up of a randomised trial. Asian J. Endosc. Surg. 2013, 6, 78–81.
- Sloothaak, D.A.; van den Berg, M.W.; Dijkgraaf, M.G.; Fockens, P.; Tanis, P.J.; van Hooft, J.E.; Bemelman, W.A. Collaborative Dutch Stent-In study group. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br. J. Surg. 2014, 101, 1751–1757.
- Higgins, J.P.T.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0; Cochrane: London, UK, 2017; updated June 2017.
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343.
- Di Saverio, S.; Birindelli, A.; Segalini, E.; Novello, M.; Larocca, A.; Ferrara, F.; Binda, G.A.; Bassi, M. “To stent or not to stent?”: Immediate emergency surgery with laparoscopic radical colectomy with CME and primary anastomosis is feasible for obstructing left colon carcinoma. Surg. Endosc. 2018, 32, 2151–2155.
- Boland, P.A.; Kell, M.E.; Donlon, N.E.; Rausa, E.; Beddy, D.P.; McCormick, P.H.; Mehigan, B.J.; Larkin, J.O. Outcomes following colonic stenting for malignant left-sided bowel obstruction: A systematic review of randomised controlled trials. Int. J. Colorectal Dis. 2019, 34, 1625–1632.
- Tan, C.J.; Dasari, B.V.; Gardiner, K. Systematic review and meta-analysis of randomised clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br. J. Surg. 2012, 99, 469–476.
- Cirocchi, R.; Farinella, E.; Trastulli, S.; Desiderio, J.; Listorti, C.; Boselli, C.; Parisi, A.; Noya, G.; Sagar, J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2013, 22, 14–21.
- Allievi, N.; Ceresoli, M.; Fugazzola, P.; Montori, G.; Coccolini, F.; Ansaloni, L. Endoscopic stenting as bridge to surgery versus emergency resection for left-sided malignant colorectal obstruction: An updated meta-analysis. Int. J. Surg. Oncol. 2017, 2, 863272.
- Huang, X.; Lv, B.; Zhang, S.; Meng, L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: A meta-analysis. J. Gastrointest. Surg. 2014, 18, 584–591.
- Liu, Z.; Kang, L.; Li, C.; Huang, M.; Zhang, X.; Wang, J. Meta-analysis of complications of colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, 73–79.
- Sagar, J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst. Rev. 2011, 9, CD007378.
- Spannenburg, L.; Gonzalez, M.S.; Brooks, A.; Wei, S.; Li, X.; Liang, X.; Gao, W.; Wang, H. Surgical outcomes of colonic stents as a bridge to surgery versus emergency surgery for malignant colorectal obstruction: A systematic review and meta-analysis of high quality prospective and randomised controlled trials. Eur. J. Surg. Omcol. 2020, 46, 1404–1414.
- Foo, C.C.; Ting Poon, S.H.; Yiu Chiu, R.H.; Lam, W.Y.; Cheung, L.C.; Law, W.L. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction: A meta-analysis of randomised control trials. Surg. Endosc. 2019, 33, 293–302.
- Köhler, G.; Antoniou, S.A.; Lechner, M.; Mayer, F.; Mair, J.; Emmanuel, K. Stenting for Emergency Colorectal Obstruction: An Analysis of 204 Patients in Relation to Predictors of Failure and Complications. Scand. J. Surg. 2015, 104, 146–153.
- Avlund, T.H.; Erichsen, R.; Iversen, L.H. Sensitivity and positive predictive value of the registration of self-expanding metal stent treatment for obstructive colorectal cancer in two Danish nationwide registries. Clin. Epidemiol. 2018, 10, 1411–1415.
- Kim, H.J.; Choi, G.S.; Park, J.S.; Park, S.Y.; Jun, S.H. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int. J. Colorectal Dis. 2013, 28, 407–414.
- Kim, S.J.; Wook Kim, H.; Park, S.B.; Kang, D.K.; Choi, C.W.; Song, B.J.; Hong, J.B.; Kim, D.J.; Park, B.S.; Son, G.M. Colonic perforation either during or after stent insertion as a bridge to surgery for malignant colorectal obstruction increases the risk of peritoneal seeding. Surg. Endosc. 2015, 29, 3499–3506.
- Sabbagh, C.; Browet, F.; Diouf, M.; Cosse, C.; Brehant, O.; Bartoli, E.; Mauvais, F.; Chauffert, B.; Dupas, J.L.; Nguyen-Khan, E. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Annu. Surg. 2013, 258, 107–115.
- Maruthachalam, K.; Lash, G.E.; Shenton, B.K.; Horgan, A.F. Tumour cell dissemination following endoscopic stent insertion. Br. J. Surg. 2007, 94, 1151–1154.
- Kim, M.K.; Kye, B.H.; Lee, I.K.; Oh, S.T.; Ahn, C.H.; Lee, Y.S.; Lee, S.C.; Kang, W.K. Outcome of bridge to surgery stenting for obstructive left colon cancer. ANZ J. Surg. 2017, 87, E245–E250.
- Fiori, E.; Crocetti, D.; Lamazza, A.; DE Felice, F.; Tarallo, M.; Sterpetti, A.V.; Mingoli, A.; Sapienza, P.; DE Toma, G. Resection or Stenting in the Treatment of Symptomatic Advanced Metastatic Rectal Cancer: A Dilemma. Anticancer Res. 2019, 39, 6781–6786.
- Fiori, E.; Lamazza, A.; Sterpetti, A.V.; Crocetti, D.; DE Felice, F.; DI Muzio, M.; Mingoli, A.; Sapienza, P.; DE Toma, G. Quality of Life for Patients with Incurable Stage IV Colorectal Cancer: Randomized Controlled Trial Comparing Resection Versus Endoscopic Stenting. Vivo 2019, 33, 2065–2070.
- Fiori, E.; Crocetti, D.; Lamazza, A.; De Felice, F.; Scotti, G.B.; Sterpetti, A.V.; Mingoli, A.; Sapienza, P.; De Toma, G. Defecatory Dysfunction After Colon Cancer Resection: The Role of Inferior Mesenteric Artery Tie. Anticancer Res. 2020, 40, 2969–2974.
- Fiori, E.; Crocetti, D.; Lamazza, A.; De Felice, F.; Sterpetti, A.V.; Irace, L.; Mingoli, A.; Sapienza, P.; De Toma, G. Is Low Inferior Mesenteric Artery Ligation Worthwhile to Prevent Urinary and Sexual Dysfunction After Total Mesorectal Excision for Rectal Cancer? Anticancer Res. 2020, 40, 4223–4228.




