
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandra Gallo | + 3395 word(s) | 3395 | 2021-04-25 11:13:09 | | | |
| 2 | Peter Tang | Meta information modification | 3395 | 2021-04-29 10:24:34 | | |
Video Upload Options
The application of engineered nanomaterials (ENMs) in marine environmental remediation gained increasing attention. Due to their large surface area and high reactivity, ENMs offer the potential for the efficient removal of pollutants from environmental matrices with better performances compared to conventional techniques.
1. Introduction
Environmental pollution results from the rising of industrial activities and urbanization, which constantly discharge man-made wastes into the environment altering its equilibrium, integrity, and health. On daily basis, different pollutants are released into soil, atmosphere, lakes, groundwater and rivers, which in turn reach seas and oceans [1]. Consequently, as a final sink of anthropogenic pollutants, marine ecosystems are under threat and need to be restored to healthy conditions. In this scenario, nanotechnology is the science of the 21st Century that can offer the most promising devices to counteract with chemical pollution, including the marine environmental remediation. Combining physical and chemical laws, nanotechnology is able to manipulate matter generating particles on a scale of less than 100 nm, known as engineered nanomaterials (ENMs) or nanoparticles (NPs) [2].
NPs can be synthesized starting from a wide variety of raw materials by applying biological, physical, or chemical methods. Based on the size of raw material, these methods include two general approaches: (i) Top-down approach starts from bulk material to create correspondingly smaller structures using finer tools; and (ii) Bottom-up approach assembles materials from the nanoscopic scale, such as molecules and atoms, to form larger structures [3][4].
Protocols for ENMs/NPs synthesis are improving, promoting their environmental application that refers to “the reduction or removal of contaminants from polluted media restoring their original status” [5][6]. The use of ENMs for environmental remediation, known as nanoremediation, is more effective compared to conventional remediation approaches since nanometric materials show high reactivity, high surface-area-to-volume ratio, and a target-specific ability to capture toxic compounds [7]. Additionally, the latter approach allows a faster degradation and stabilization of contaminants by ENMs reducing the time frame and even the costs of the process [8][9][10]. Moreover, ENMs are more sustainable because they minimize the addition of chemicals, reduce the amount of material needed in the clean-up process [11], and potentially extend the range of available in situ remediation technologies [12][13]. Nano-sized particles are transferred into contaminated media such as soils, sediments, and aquifers by in situ remediation technology. This strategy is preferred over other approaches being more cost-effective. In most applications, zero-valent iron nanoparticles (nZVI) are successfully used to remediate groundwater, soil, wetland and river sediments [14][15][16][17]. Furthermore, the effectiveness of other ENMs for in situ remediation of soil, groundwater and sediments has been demonstrated [18][19][20].Therefore, application methods for in situ treatment with ENMs may also be suitable for the deployment in the marine environment.
To date, experimental studies established that ENMs can be employed to efficiently restore different marine environmental matrices [21][22][23], reducing the impact of toxic chemicals, thus, preserving marine biodiversity, ecosystem functioning and services. However, as such, they are applied as primary ENMs to the environment and the balance between benefits and risks associated with their use is still under debate [24][25]. Their potential release in the environment and the deriving effects on the ecosystem health become a matter of concern to be addressed. For this purpose, it is necessary to elucidate the fate and behavior of ENMs, which depend not only on their physical and chemical proprieties, but also on the characteristics of the receiving environment [26]. Of particular interest is the marine environment, which appears as a dynamic and extreme environment, due to the high ionic strength conditions, pH, and presence of a high amount of naturally occurring particulates. Indeed, upon entry into the marine environment, ENMs undergo diverse processes, such as dissolution, transformation, speciation, agglomerate/aggregate and sedimentation, which may influence their fate and dispersion and determine the bioavailability and toxicity. These research areas need further investigation to establish a proper environmental risk assessment following an in-depth understanding of the ENMs physico-chemical properties.
Moreover, in order to achieve a “green and sustainable remediation” (GSR), it is essential to assess the risks posed by the ENMs applied to marine environment remediation techniques [27]. As a future goal of the remediation industry, environmental safety represents the main challenge for ENMs employed in marine nanoremediation and can be achieved by using environmental risk assessment approaches [24]. In such a way, the ecotoxicological testing strategy represents a fundamental aspect since it adapts the standardized ecotoxicity tests, or newly developed ones, allowing the determination of the potential impact of ENM/Ps toward different levels of biological organization, thus providing suitable toxicity data. This information will help to identify the ENMs properties that mediate the interaction with living organisms and, consequently, their toxicity, leading to the selection of the best ecofriendly and ecological sustainable ENMs [28].
2. ENM/NPs and Environmental Safety
ENMs have been identified as innovative tools to deal with the global concern of marine pollution. The best performances and higher sustainability of the ENMs, compared to macro-sized materials, are driving the progressive transition from remediation to nanoremediation. Several studies demonstrated the efficacy of ENMs in the decontamination of the marine polluted sites. However, more efforts are needed to unravel any potential environmental implication deriving from their use due to the documented toxicological outcomes on marine biota [24][28][29]. Therefore, it is crucial to develop an ecosafety strategy to protect the marine biota before the authorization of applications for in situ nanoremediation of the marine environment. A key issue in designing ENMs regards their fate after release. This aspect acquires more relevance since, once released in seawater, ENMs undergo significant transformations, which affect their behavior and toxicity on marine biota. According to the Scientific Committee on Emerging and Newly Identified Health Risks [30], chemical-physical properties of ENMs (size, surface composition, shape, solubility, aggregation, chemical reactivity) are fundamental in the risk assessment. In fact, the intrinsic characteristics of ENMs, together with the properties of the environmental matrices, are among the factors that can induce transformations of ENMs, and, consequently, affect their potential risks for human and ecosystems health [31][32][33]. Marine environment is an alkaline medium characterized by a high ionic strength and a wide variety of natural organic matter (NOM) [26]. ENMs can undergo dissolution into an ionic form driven by the particle chemistry, but they also rapidly co-aggregate (homoaggregation) or assemble with non-homologous particles (heteroaggregation) due to the high ionic strength and the relatively high pH of sea water. The ENM aggregation increases their size; consequently, the aggregates are less mobile and tend to be deposited to the sediments, becoming less available to organisms in the water column [34]. ENMs in marine water can also interact with the inorganic and organic colloidal particles, resulting in a greater stabilization effect on NPs that can influence their aggregation dynamics and colloidal stability.
The interaction between ENMs and NOM in the aquatic matrices is emerging as an attractive research field. Recently, it has been suggested that NOM produced during the algal bloom may contribute stabilize ENMs, limiting the agglomeration process [35]. However, depending on the media proprieties, the bio-nano interaction can induce an opposite mechanism, promoting agglomeration [36][37].
In some condition, the interaction of NPs with dissolved biomolecules can favor the formation of NOM-related nanoscale coatings, analogous to protein corona in mammalian systems, potentially affecting aggregation and transport of the NPs, as well as bio-distribution, uptake and toxicity to marine species [38][39][40][41]. Alternatively, NPs can be adsorbed on the exterior surface of the organism driving surface inducing toxicity. The aggregation and adsorption processes can increase the ENM concentrations in the environmental matrices. Overall, once released in the marine environment, NPs may undergo rapid transformations due to their intrinsic and extrinsic properties, which drive NP fate and determine their ecotoxicity on the marine biota [38][42]. Despite the sign of progress in the research studies of the environmental fate and behavior and risk assessment of ENMs, to date, their life cycle is characterized by a regulatory gaps, from the design and synthesis, to their usage, until the final disposal. Specific international regulation for producing, labeling, and evaluating the environmental impact of ENMs is lacking [43]. Attempting to fill these gaps, in 2007, the European Commission introduced the ENMs in the register of chemical compounds REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) indicating that their safety assessment should follow the risk assessment methodology adopted for conventional chemicals. Based on the purpose of promoting green nanotechnology, which does not pose any risk for the environment and biota, progresses in nanoremediation are moving towards the design of eco-friendly nanosized devices with a low content of toxic substances, reduced material and energy requirements. Following this perspective, the United States Environmental Protection Agency published multiple documents as reference points, including the best management practice fact sheets for green and sustainable remediation [44]. Aiming to reduce the environmental impact of the ENMs and promote sustainable frontiers for nanoremediation, the “eco-design” approach (Figure 1) is gaining relevance.
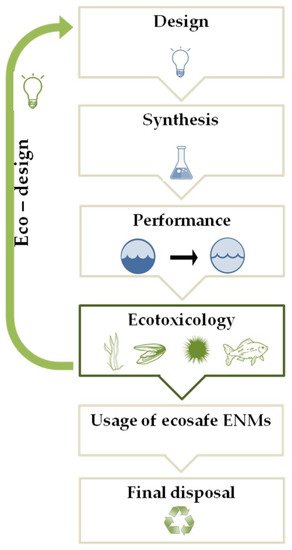
Figure 1. A schematic representation of the eco-design approach (see text for details).
This innovative approach is aimed to keep under control the environmental safety of new ENMs through the “safety-by-design” strategy (Figure 1) that results safe and sustainable in terms of ENM composition, production process, and performance. Therefore, ecotoxicology plays a key role in the “eco-design” approach in order to determine the potential risk of new synthesized ENMs for in situ nanoremediation in the marine environment [24][45]. The ecotoxicological assessment of ENMs can change the design and/or the composition of an ENM, realizing the eco-design of newly synthesized ENMs aimed at safeguarding the environment during the complete ENMs life-cycle, until their final disposal, when the nanoremediation process is over, producing recyclable and/or biodegradable ENMs, supporting the development of green nanotechnologies for marine remediation.
2.1. Ecotoxicological Assessment of ENMs
From the first evidence of ENM impact on aquatic species, nanoecotoxicology gained a relevant role in ecological risk assessment (ERA) of ENMs by recognizing how their transformations in sea water (i.e., size distribution, surface charges and bio-nano interactions) affected biological interactions and toxicological responses at population and ecosystem level [28][29][31][46]. Nanoscale dimension represents the main driver of cellular uptake, but exposure scenarios are affected by ENM transformations occurring in natural environments, which also identify potential target ecosystems (pelagic versus benthic) [47]. Linking exposure to the observed biological effects is a key aspect for proper ERA and bio-nano interactions are fundamental for the understanding of such complex natural exposure scenarios [28]. Eco-corona formation, as a results of particle physical-chemical interaction with dissolved biomolecules already existing in natural seawater, will affect particle uptake and related cellular pathways leading to toxicity [24][29][31][47]. To make regulatory references more suitable, a general agreement has been reached by the nanoecotox scientific community in using more realistic exposure scenarios for ERA of ENMs and in revising current standardized protocols based on bioassays [28][33][48][49]. Although effect-based tools including in vitro and in vivo bioassays have been successfully used to assess exposure and hazard for legacy and emerging marine pollutants, they present some limitations for ENMs [49][50][51]. Conversely, conventional biomarkers, such as those developed upon exposure to other toxicants (e.g., oxidative stress, lipid peroxidation, biotransformation and genotoxicity) have been successful to assess ENM effects at cellular level and identify common biological pathways, or determine toxicity [52][53]. Although not specifically modulated by ENM exposure, these biomarkers have been extremely helpful to identify cellular pathways affected by ions dissolution from NPs (i.e., AgNPs) or ENM interaction with biomolecules (i.e., DNA damage and neurotoxicity). More recently, autophagy, lysosomal dysfunction, and immunomodulation, all conserved mechanisms from invertebrates to mammals, are emerging as biomarkers for the early interactions between cells and ENMs [52][53][54][55]. Furthermore, the modern field of ecotoxicogenomics is promising in understanding the mode of action of ENMs and even in the recognition of adverse outcome pathways [56]. This will allow the identification of the first warning response upon ENM exposure and predict consequences on higher biological organization (i.e., from cell up to organs, organism and population) and marine taxa for an overall ecosystem assessment [57]. To this aim, a multi-biomarkers approach and integrate individual biomarker response indices has been proposed in order to limit any risk associated with over/underestimation of the observed biological effects [2][52][58][59][60][61].
Although standard test guidelines developed for conventional contaminants have been used to test ENMs, concerns have been raised on their appropriateness for addressing particle properties under different testing conditions and assessing the toxic effects. Due to the peculiar characteristics of ENMs, several issues must be taken into consideration including the behavior of ENMs in exposure media.
3. ENMs Employed for Marine Environment Remediation and Their Ecosafety
Marine environmental remediation can be achieved by different conventional methods and technologies, such as coagulation, precipitation, filtration, in situ burning of the oil spill, sediment-capping, and mechanical removal (ex situ treatments). Both the production process and the application of the traditional methods employed to clean polluted marine area need a huge amount of time, money, energy, and give rise to wastes that often cannot be regenerated. These issues can be overcome by the application of nano-based techniques, which offer more effective alternatives to traditional methods of seawater treatment. The ENM production processes can be simpler, limit the wasteful secondary reactions, and can reduce energy consumption with benefits for the environment and workers’ health [62].
Furthermore, ENMs can remove contaminants at lower concentrations compared to the traditional methods [63]. The higher performance of nanoremediation depends on the peculiar physical-chemical properties of ENMs. These can be grafted with functional groups increasing the sensitivity, the target selectivity, the timing, and the efficiency of the nanoremediation process [64][65]. The higher selectivity along with the few compounds employed during the production process causes a reduction of the wastes produced after remediation treatment and boosts the reuse or the recycling of the contaminant specifically removed [66][67]. However, despite the social, economic, and environmental benefits of nanoremediation, its application is scarce due to the lack of a comprehensive assessment of the environmental risks related to ENMs.
In fact, compared to the number of different ENMs synthesized for seawater decontamination (Table 1), studies assessing the toxicity of each single ENM are scarce (Table 2). Similarly, limited interdisciplinary investigations are present in the current literature on the remediation ability of new synthetized ENMs and the ecotoxicological impact on marine organisms. This stimulate a more efficient interaction between different research fields, such as chemistry, physics, engineering, and ecotoxicology, and improved research efforts on the ecotoxicological assessment of ENMs. To obtain safe ENMs, ecotoxicological tests should mimic the real conditions before and after the nanoremediation process, taking into consideration the peculiar characteristics of ENMs, such as different sizes, structures, and shapes that contribute to the interactions with the remediation media, affecting their behavior and toxicity.
Table 1. ENMs synthetized for the remediation of marine environment.
|
ENM |
Concentration |
Properties |
Target Contaminants |
Mechanism |
Media |
Remediation Efficiency |
Reference |
|---|---|---|---|---|---|---|---|
|
Graphene oxide sponge enriched with florin groups (USTC-6@GO@sponge) |
NF |
carbon-based ENM with microporosity and great hydrophobicity for the selective adsorption of organic compounds |
diesel oil, gasoline, soybean oil, light petroleum, n-hexane, bromobenzene, N’N-dimethylformamide (DMF), tetrahydrofuran, acetone, CCl4 methylbenzene |
adsorption |
seawater |
NF |
[68] |
|
Chitosan-grafted carbon nanotubes (CTS-g-CNTs) |
0.6 g L−1 |
external nanotube diameter of 30 nm and an inner diameter of 8.48 nm, stable in seawater |
Cs |
adsorption |
seawater |
NF |
[69] |
|
Graphene oxide functionalized with polyethyleneimine (GO-PEI) |
10 mg L−1 |
foam with three dimensional porous structures |
Hg |
adsorption |
seawater |
NF |
[70] |
|
Manganese-ferrite NPs (MnFe2O4) |
50 mg L−1 |
NP diameter of 75 ± 15 nm; magnetism |
As, Pb |
adsorption |
seawater |
NF |
|
|
Alginate and polyvinyl alcohol (PVA)-alginate entrapped nanoscale zero-valent iron (nZVI) |
1 g L−1 2 g L−1 |
Particles of powder average size 50 nm |
Cu, Zn, Cr, As |
adsorption |
saline wastewater |
Cu 84.2%; Cr 70.8%; Zn 31.2%; As 39.8% |
[74] |
|
nFe3O4/fly ash composite |
0.5 g in 25 mL of triphenyltinchloride (TPT) solution |
nFe3O4 size particles < 50 nm |
TPT |
adsorption |
seawater |
98.40% |
[75] |
|
Potasium copper hexacyanoferrate (KCuHCF) |
0.1 g L−1 |
NPs size of 10–17 nm |
Cs |
adsorption |
seawater |
99% |
[76] |
|
Zeolitic imidazolate framework-8 functionalized with ferrocyanide (ZIF-8-FC) |
V/m = 1000 mL g−1 |
cubic particles with a surface area of 589 m2 g−1 |
Cs |
adsorption |
seawater |
60% at 3 h 85% at 24 h |
[77] |
|
Magnetic multilayer core–shell (Fe3O4@SiO2@KTiFC) |
5 mg of Fe3O4@SiO2 @KTiFC particles added to 4 mL seawater |
microspheres with a magnetite core of 300 nm; magnetism |
Cs |
adsorption |
seawater |
97.7% |
[78] |
|
Prussian blue-embedded magnetic hydrogel beads (PB-MHBs) |
1 mg mL−1 |
average size of 33.8 mm; magnetism |
Cs |
adsorption |
seawater |
96.7% |
[23] |
|
Magnetic carbon microspheres (Fe3O4-CM) |
5 g L−1 |
diameter microspheres of ~30 μm; superparamagnetis |
polycyclic aromatic hydrocarbons (PAH) |
degradation |
marine sediments |
87% |
[79] |
|
Nano-hydroxyapatite particles (nHAp) |
0–10% nHAp/dry weight |
rod structure with dimensions of 20 nm (i.d.) × 200 nm (length); surface area of 130 m2 g−1 |
Pb, Cd |
sorption |
marine sediments |
NF |
[80] |
|
nZVI coated to polyacrylic acid (nanofer 25S) |
low (2, 3 and 4%) and high (5, 10 and 20%) dosages |
diameter of 50 nm; total iron content of 80–90 wt. %; surface area of 20–25 m2 g−1 |
Al, As, B, Ba, Co, Cu, Ni |
adsorption, reduction |
marine sediments slightly polluted by heavy metals |
at 3 g: Co 100%; at 4 g: Al 33.3%, As 76%, Cu 96.8%, B 0%; at 5 g: Al 71.4%, Cu 100%, As 62%; At 10 g: B 60.4%; at 20 g: Co 54.3% |
[81] |
|
Nanoscale zero valent iron (nZVI) |
0.01–1 g/L |
particle sizes < 100 nm |
polycyclic aromatic hydrocarbons (PAHs) |
oxidation |
PAHs contaminated sediments |
70.2% at 0.01 g/L, 78.3% at 0.1 g/L, 86.3% at 0.5 g/L, 78.0% at 1 g/L |
[55] |
|
polyvinylpyrrolidone-coated magnetic ENM (PVP-Fe3O4 NMs) |
167 mg/L |
median size of 11.2 nm |
Pb, Cr, Ni, Cd |
adsorption |
seawater |
Pb 100%; Cr 98.8%; Ni 60–70%; Cd 40–50% |
[82] |
|
375 ± 10 mg/L |
oil-water mixtures |
70% of lower-chain alkanes (C9–C22); 65% of higher-chain (C23–C26), |
[83] |
||||
|
Starch-based nanosponges |
12 mg in 15 mL |
citrate nanosponges with β-cyclodextrin (β-CD) or ®linecaps (®LC) scaffold |
Cu, Zn |
adsorption |
seawater |
Cu 80–84% Zn < 60% |
[84] |
|
pyromellitic nanosponges with β-cyclodextrin (β-CD) or ®linecaps (®LC) scaffold |
Cu 36–45%; Zn <60% |
||||||
|
Powder of Cellulose-Based Nanostructured Sponges (CNS) |
0.8 mg mL−1 |
particle size range 50 to 400 μm |
Zn, Cu, Cr, Cd |
adsorption |
seawater |
90% |
|
|
KCuHCF-cellulose hydrogel |
10 mg in 20 mL |
Cubic-shaped particles of 10–12 nm |
Cs |
adsorption |
seawater |
>90% |
[87] |
|
PB coating Fe3O4 NPs anchored to the surface of the GO sheets (PB/Fe3O4/GO) |
0.05 g of NPs in 30 mL |
average size of 17 nm;magnetism |
Cs |
adsorption |
seawater |
52.19% |
[88] |
NF: data not found.
Table 2. Ecotoxicological assessment of ENMs synthetized for the remediation of marine environment.
|
ENM |
Concentration |
Properties |
Experimental Conditions |
Species |
Effects |
Reference |
|---|---|---|---|---|---|---|
|
manganese-ferrite NPs (MnFe2O4 NPs) |
50 mg/L |
NPs size of 75 ± 15 nm |
24 h exposure in ASW (T 17.0 ± 1.0 °C; pH 8.0 ± 0.1; salinity 30 ± 1; photoperiod light/dark 12 h:12 h; continuous aeration) |
Mytilus galloprovincialis |
enhancement of antioxidant and biotransformation enzymes activities; lipids and protein damages; neurotoxicity |
[73] |
|
manganese-ferrite NPs (MnFe2O4 NPs) |
50 mg/L |
NPs size 75 ± 15 nm |
28 days’ exposure in ASW (T 17.0 ± 1.0 °C; pH 8.0 ± 0.1; salinity 30 ± 1; photoperiod light/dark 12 h:12 h; continuous aeration) |
Mytilus galloprovincialis |
depression of metabolic activity, oxidative stress, cellular membrane damage, neurotoxicity |
[72] |
|
GO-PEI |
10 mg/L |
foam with three dimensional porous structures |
28 days’ exposure in ASW (T 17.0 ± 1.0 °C; pH 8.0 ± 0.1; salinity 30 ± 1; photoperiod light/dark 12 h:12 h; continuous aeration) |
Mytilus galloprovincialis |
depression of metabolic activity, oxidative stress, cellular membrane damage, neurotoxicity necrosis and apoptosis in female gonads, cellular atrophy in digestive tubules |
[70] |
|
GO-PEI |
10 mg/L |
foam with three dimensional porous structures |
28 days exposure in ASW (T 17.0 ± 1.0 °C; pH 8.0 ± 0.1; salinity 30 ± 1; continuous aeration) |
Ruditapes philippinarum |
depression of metabolic activity, oxidative stress, cellular membrane damage; alteration in gills and in digestive tubules |
[71] |
|
CNS |
1.25 g/L |
powder of cellulose-based nanostructured sponges with particle size range of 50 to 400 μm |
48 h of exposure in ASW (T 18 ± 1 °C; pH 8 ± 0.1; salinity 40 ± 1) |
Mytilus galloprovincialis |
none in immune and gill cells and mantle |
[89] |
|
1.25 g/L serial diluitions (1:20, 1:10, 1:5, 1:2, undiluited) |
72 h of exposure in ASW initial density of 104 cells m/L |
Dunaliella tertiolecta |
algal growth inhibition with undiluited CNS |
[45] |
||
|
Nanofer25S |
0.01–100 mg/L |
commercial nanoscale zero-valent iron NPs with a size of 80–120 nm |
96 h of exposure in NSW (pH 8.1, 20 °C, salinity 34; light:dark cycle 14:10)initial density of 1–2 × 105 cells mL−1 |
Isochrysis galbana Dunaliella tertiolecta Thalassiosira pseudonana |
algal growth inhibition at: 3.1 mg/L for I. galbana; 1.3 mg/L for D. tertiolecta; 0.4 mg/L for T. pseudonana |
[90] |
|
1.8–10 mg/L |
commercial nanoscale zero-valent iron NPs size 80–120 nm |
2 h gamete exposure (T 0.5 °C, pH 8.1; salinity 35.1 ± 0.52, for sea urchins and mussels; T 17 ± 0.1 °C, salinity 36 ± 0.02, for sea squirts) egg: sperm ratio 1:1 × 106 |
Spermatozoa of Mytilus galloprovincialis, Ciona intestinalis and Psammechinus milliaris |
fertilization success decrease; embryo development delay |
[91] |
|
|
nano-Fe2O3 |
0; 100; 1000; 10,000 μg/L |
size of 50 nm |
NSW (T 15 ± 0.5 °C; pH 8.1; salinity 35.1 ± 0.52) |
Mytilus galloprovincialis |
None on embryo development |
[92] |
|
PVP-Fe3O4 NMs |
0–100 mg/L |
median size of 11.2 nm |
96 h exposure in ASW (T 25 ± 1 °C; salinity 30) |
Amphiascus tenuiremis |
None on copepod mortality up to 25 mg/L |
[93] |
NF: data not found; natural seawater (NSW); artificial seawater (ASW).
Based on their main chemical compositions, ENMs used for marine environment nanoremediation can be broadly grouped as: (i) Metal oxides based nanomaterials (the most abundant class, 37%); (ii) magnetic-core nanocomposites (21%); (iii) carbon-based and polysaccharides-based nanostructured materials employed at the same percentage (16%); (iv) hybrid nanomaterials, the less employed for marine clean-up (10%) (Figure 2).
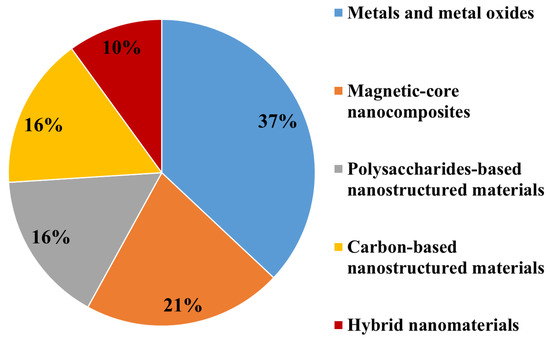
Figure 2. Classes of ENMs employed for marine environment nanoremediation.
References
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Poll. Bull. 2016, 112, 17–38.
- Blaise, C.; Gagné, F.; Ferard, J.; Eullaffroy, P. Ecotoxicity of selected nano-materials to aquatic organisms. Environ. Toxicol. 2008, 23, 591–598.
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27.
- Hobbs, R.G.; Petkov, N.; Holmes, J.D. Semiconductor nanowire fabrication by bottom-up and top-down paradigms. Chem. Mater. 2012, 24, 1975–1991.
- Bardos, P.; Merly, C.; Kvapil, P.; Koschitzky, H.P. Status of nanoremediation and its potential for future deployment: Risk-benefit and benchmarking appraisals. Remed. J. 2018, 28, 43–56.
- Lee, C.-C.; Lien, H.-L.; Wu, S.-C.; Doong, R.-A.; Chao, C.-C. Reduction of priority pollutants by nanoscale zerovalent iron in subsurface environments. In Aquananotechnology: Global Prospects; Reisner, D.E., Pradeep, T., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2014; Volume 63.
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for environmental remediation: Materials and applications. Molecules 2018, 23, 1760.
- U.S. EPA. National Priorities List (NPL) Sites—By State. 2009. Available online: (accessed on 11 January 2021).
- Mueller, N.C.; Nowack, B. Nanoparticles for remediation: Solving big problems with little particles. Elements 2010, 6, 395–400.
- The Project on Emerging Nanotechnologies (PEN). Nanoremediation Map. 2015. Available online: (accessed on 15 January 2021).
- Masciangioli, T.; Zhang, W.-X. Peer reviewed: Environmental technologies at the nanoscale. Environ. Sci. Technol. 2003, 37, 102–108.
- Bardos, P.; Bone, B.; Elliott, D.; Hartog, N.; Henstock, J.; Nathanail, P. A Risk/Benefit Approach to the Application of Iron Nanoparticles for the Remediation of Contaminated Sites in the Environment; Department for Environment, Food & Rural Affairs: Carlisle, UK, 2011.
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122.
- Chapman, E.E.V.; Moore, C.; Campbell, L.M. Evaluation of a nanoscale zero-valent iron amendment as a potential tool to reduce mobility, toxicity, and bioaccumulation of arsenic and mercury from wetland sediments. Environ. Sci. Pollut. Res. 2020, 27, 18757–18772.
- Jing, R.; Fusi, S.; Kjellerup, B.V. Remediation of polychlorinated biphenyls (PCBs) in contaminated soils and sediment: State of knowledge and perspectives. Front. Environ. Sci. 2018, 6, 79–96.
- Pak, T.; de Lima Luz, L.F.; Tosco, T.; Costa, G.S.R.; Rosa, P.R.R.; Archilha, N.L. Pore-scale investigation of the use of reactive nanoparticles for in situ remediation of contaminated groundwater source. Proc. Natl. Acad. Sci. USA 2020, 117, 13366–13373.
- Pilipović, D.T.; Kerkez, D.; Dalmacija, B.; Slijepčević, N.; Krčmar, D.; Rađenović, D.; Bečelić-Tomin, M. Remediation of toxic metal contaminated sediment using three types of nZVI supported materials. Bull. Environ. Contam. Toxicol. 2018, 101, 725–731.
- Ibrahim, R.K.; Hayyan, M.; AlSaadi, M.A.; Hayyan, A.; Ibrahim, S. Environmental application of nanotechnology: Air, soil, and water. Environ. Sci. Pollut. Res. 2016, 23, 13754–13788.
- Liang, Q.; Zhao, D. Immobilization of arsenate in a sandy loam soil using starch-stabilized magnetite nanoparticles. J. Hazard. Mater. 2014, 271, 16–23.
- Wang, T.; Liu, Y.; Wang, J.; Wang, X.; Liu, B.; Wang, Y. In-situ remediation of hexavalent chromium contaminated groundwater and saturated soil using stabilized iron sulfide nanoparticles. J. Environ. Manag. 2019, 231, 679–686.
- Owoseni, O.; Nyankson, E.; Zhang, Y.; Adams, S.J.; He, J.; McPherson, G.L.; Bose, A.; Gupta, R.B.; John, V.T. Release of surfactant cargo from interfacially-active halloysite clay nanotubes for oil spill remediation. Langmuir 2014, 30, 13533–13541.
- Shen, Q.; Xu, S.J.; Xu, Z.L.; Zhang, H.Z.; Dong, Z.Q. Novel thin-film nanocomposite membrane with water-soluble polyhydroxylated fullerene for the separation of Mg2+/Li+ aqueous solution. J. Appl. Polym. Sci. 2019, 136, 48029–48043.
- Yang, H.-M.; Hwang, J.R.; Lee, D.Y.; Kim, K.B.; Park, C.W.; Kim, H.R.; Lee, K.-W. Eco-friendly one-pot synthesis of Prussian blue-embedded magnetic hydrogel beads for the removal of cesium from water. Sci. Rep. 2018, 8, 1–10.
- Corsi, I.; Fiorati, A.; Grassi, G.; Bartolozzi, I.; Daddi, T.; Melone, L.; Punta, C. Environmentally sustainable and ecosafe polysaccharide-based materials for water nano-treatment: An eco-design study. Materials 2018, 11, 1228.
- Patil, S.S.; Shedbalkar, U.U.; Truskewycz, A.; Chopade, B.A.; Ball, A.S. Nanoparticles for environmental clean-up: A review of potential risks and emerging solutions. Environ. Technol. Innov. 2016, 5, 10–21.
- Klaine, S.J.; Alvarez, P.J.; Batley, G.E.; Fernandes, T.F.; Handy, R.D.; Lyon, D.Y.; Mahendra, S.; McLaughlin, M.J.; Lead, J.R. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects. Environ. Toxicol. Chem. 2008, 27, 1825–1851.
- Martino, L.E.; Dona, C.L.; Dicerbo, J.; Hawkins, A.; Moore, B.; Horner, R. Green and sustainable remediation practices in Federal Agency cleanup programs. Environ. Earth Sci. 2016, 75, 1–13.
- Corsi, I.; Cherr, G.N.; Lenihan, H.S.; Labille, J.; Hassellov, M.; Canesi, L.; Dondero, F.; Frenzilli, G.; Hristozov, D.; Puntes, V. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano 2014, 8, 9694–9709.
- Matranga, V.; Corsi, I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar. Environ. Res. 2012, 76, 32–40.
- Ahlbom, A.; Bridges, J.; De Jong, W.; Jung, T.; Mattsson, O.; Pagès, J.M.; Rydzynski, K.; Stahl, D.; Thomsen, M.; Thomsen, M. Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR Risk Assessment of Products of Nanotechnologies. 2009. Available online: (accessed on 19 January 2021).
- Blasco, J.; Corsi, I.; Matranga, V. Particles in the oceans: Implication for a safe marine environment. Mar. Environ. Res. 2015, 111, 1–4.
- Handy, R.D.; Von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314.
- Scott-Fordsmand, J.J.; Peijnenburg, W.J.; Semenzin, E.; Nowack, B.; Hunt, N.; Hristozov, D.; Marcomini, A.; Irfan, M.A.; Jiménez, A.S.; Landsiedel, R. Environmental risk assessment strategy for nanomaterials. Int. J. Environ. Res. Public Health 2017, 14, 1251.
- Rana, S.; Kalaichelvan, P. Ecotoxicity of nanoparticles. ISRN Toxicol. 2013, 2013, 574648–574660.
- Gondikas, A.; Gallego-Urrea, J.; Halbach, M.; Derrien, N.; Hassellöv, M. Nanomaterial fate in seawater: A rapid sink or intermittent stabilization? Front. Environ. Sci. 2020, 8, 151–160.
- Danielsson, K.; Persson, P.; Gallego-Urrea, J.; Abbas, Z.; Rosenqvist, J.; Jonsson, C. Effects of the adsorption of NOM model molecules on the aggregation of TiO2 nanoparticles in aqueous suspensions. Nanoimpact 2018, 10, 177–187.
- Surette, M.C.; Nason, J.A. Effects of surface coating character and interactions with natural organic matter on the colloidal stability of gold nanoparticles. Environ. Sci. Nano 2016, 3, 1144–1152.
- He, X.; Fu, P.; Aker, W.G.; Hwang, H.-M. Toxicity of engineered nanomaterials mediated by nano-bio-eco interactions. J. Environ. Sci. Health C 2018, 36, 21–42.
- Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270.
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534.
- Nasser, F.; Constantinou, J.; Lynch, I. Nanomaterials in the Environment Acquire an “Eco-Corona” Impacting their Toxicity to Daphnia Magna—A Call for Updating Toxicity Testing Policies. Proteomics 2020, 20, e1800412.
- Corsi, I.; Fiorati, A.; Grassi, G.; Pedrazzo, A.R.; Caldera, F.; Trotta, F.; Punta, C. Ecosafe nanomaterials for environmental remediation. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Nederland, 2020; pp. 383–405.
- Upreti, G.; Dhingra, R.; Naidu, S.; Atuahene, I.; Sawhney, R. Life cycle assessment of nanomaterials. In Green Processes for Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 393–408.
- U.S. EPA. Incorporating Sustainable Practices into Site Remediation; EPA 542-R-08-002; U.S. Environmental Protection Agency: Washington, DC, USA, 2008.
- Fiorati, A.; Grassi, G.; Graziano, A.; Liberatori, G.; Pastori, N.; Melone, L.; Bonciani, L.; Pontorno, L.; Punta, C.; Corsi, I. Eco-design of nanostructured cellulose sponges for sea-water decontamination from heavy metal ions. J. Clean. Prod. 2020, 246, 119009–119020.
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 1–35.
- Corsi, I.; Bergami, E.; Grassi, G. Behavior and bio-interactions of anthropogenic particles in marine environment for a more realistic ecological risk assessment. Front. Environ. Sci. 2020, 8, 60–81.
- Kahru, A.; Dubourguier, H.-C. From ecotoxicology to nanoecotoxicology. Toxicology 2010, 269, 105–119.
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the aquatic environment: A European Union-United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067.
- Depledge, M.; Fossi, M. The role of biomarkers in environmental assessment (2). Invertebrates. Ecotoxicology 1994, 3, 161–172.
- Nowack, B.; Baalousha, M.; Bornhöft, N.; Chaudhry, Q.; Cornelis, G.; Cotterill, J.; Gondikas, A.; Hassellöv, M.; Lead, J.; Mitrano, D.M. Progress towards the validation of modeled environmental concentrations of engineered nanomaterials by analytical measurements. Environ. Sci. Nano 2015, 2, 421–428.
- Blaise, C. Microbiotesting: An expanding field in aquatic toxicology. Ecotoxicol. Environ. Saf. 1998, 40, 115–119.
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012, 9, 1–17.
- Châtel, A.; Mouneyrac, C. Signaling pathways involved in metal-based nanomaterial toxicity towards aquatic organisms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 196, 61–70.
- Chen, C.W.; Binh, N.T.; Chang, Y.K.; Hung, C.M.; Dong, C.D. Remediation of marine sediments contaminated with PAHs using sodium persulfate activated by temperature and nanoscale zero valent iron. Adv. Mater. Res. 2014, 1044–1045, 380–383.
- Snape, J.R.; Maund, S.J.; Pickford, D.B.; Hutchinson, T.H. Ecotoxicogenomics: The challenge of integrating genomics into aquatic and terrestrial ecotoxicology. Aquat. Toxicol. 2004, 67, 143–154.
- Revel, M.; Châtel, A.; Mouneyrac, C. Omics tools: New challenges in aquatic nanotoxicology? Aquat. Toxicol. 2017, 193, 72–85.
- Beliaeff, B.; Burgeot, T. Integrated biomarker response: A useful tool for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1316–1322.
- Breitholtz, M.; Rudén, C.; Hansson, S.O.; Bengtsson, B.-E. Ten challenges for improved ecotoxicological testing in environmental risk assessment. Ecotoxicol. Environ. Saf. 2006, 63, 324–335.
- Kim, W.-K.; Lee, S.-K.; Jung, J. Integrated assessment of biomarker responses in common carp (Cyprinus carpio) exposed to perfluorinated organic compounds. J. Hazard. Mater. 2010, 180, 395–400.
- Xia, B.; Zhu, L.; Han, Q.; Sun, X.; Chen, B.; Qu, K. Effects of TiO2 nanoparticles at predicted environmental relevant concentration on the marine scallop Chlamys farreri: An integrated biomarker approach. Environ. Toxicol. Pharmacol. 2017, 50, 128–135.
- Somorjai, G.A.; McCrea, K. Roadmap for catalysis science in the 21st century: A personal view of building the future on past and present accomplishments. Appl. Catal. A 2001, 222, 3–18.
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662.
- Singh, K.P.; Singh, A.K.; Gupta, S.; Rai, P. Modeling and optimization of reductive degradation of chloramphenicol in aqueous solution by zero-valent bimetallic nanoparticles. Environ. Sci. Poll. Res. 2012, 19, 2063–2078.
- Zhou, L.; Ji, L.; Ma, P.-C.; Shao, Y.; Zhang, H.; Gao, W.; Li, Y. Development of carbon nanotubes/CoFe2O4 magnetic hybrid material for removal of tetrabromobisphenol A and Pb (II). J. Hazard. Mater. 2014, 265, 104–114.
- Diallo, M.S.; Kotte, M.R.; Cho, M. Mining critical metals and elements from seawater: Opportunities and challenges. Environ. Sci. Technol. 2015, 49, 9390–9399.
- Su, C.; Puls, R.W.; Krug, T.A.; Watling, M.T.; O’Hara, S.K.; Quinn, J.W.; Ruiz, N.E. A two and half-year-performance evaluation of a field test on treatment of source zone tetrachloroethene and its chlorinated daughter products using emulsified zero valent iron nanoparticles. Water Res. 2012, 46, 5071–5084.
- Jiang, Z.-R.; Ge, J.; Zhou, Y.-X.; Wang, Z.U.; Chen, D.; Yu, S.-H.; Jiang, H.-L. Coating sponge with a hydrophobic porous coordination polymer containing a low-energy CF 3-decorated surface for continuous pumping recovery of an oil spill from water. NPG Asia Mater. 2016, 8, e253.
- Yang, S.; Shao, D.; Wang, X.; Hou, G.; Nagatsu, M.; Tan, X.; Ren, X.; Yu, J. Design of chitosan-grafted carbon nanotubes: Evaluation of how the–OH functional group affects Cs+ adsorption. Mar. Drugs 2015, 13, 3116–3131.
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.; Figueira, E.; Marques, P.A.; Polese, G.; Di Cosmo, A.; Pereira, E. Oxidative stress, metabolic and histopathological alterations in mussels exposed to remediated seawater by GO-PEI after contamination with mercury. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 243, 110674–110689.
- Coppola, F.; Bessa, A.; Henriques, B.; Russo, T.; Soares, A.M.; Figueira, E.; Pereira, E.; Marques, P.; Polese, G.; Freitas, R. The Role of Temperature on the Impact of Remediated Water towards Marine Organisms. Water 2020, 12, 2148.
- Coppola, F.; Tavares, D.S.; Henriques, B.; Monteiro, R.; Trindade, T.; Figueira, E.; Soares, A.M.; Pereira, E.; Freitas, R. Can water remediated by manganese spinel ferrite nanoparticles be safe for marine bivalves? Sci. Total Environ. 2020, 723, 137798–137812.
- Coppola, F.; Tavares, D.S.; Henriques, B.; Monteiro, R.; Trindade, T.; Soares, A.M.; Figueira, E.; Polese, G.; Pereira, E.; Freitas, R. Remediation of arsenic from contaminated seawater using manganese spinel ferrite nanoparticles: Ecotoxicological evaluation in Mytilus galloprovincialis. Environ. Res. 2019, 175, 200–212.
- Sun, Y.; Lei, C.; Khan, E.; Chen, S.S.; Tsang, D.C.; Ok, Y.S.; Lin, D.; Feng, Y.; Li, X.-d. Aging effects on chemical transformation and metal (loid) removal by entrapped nanoscale zero-valent iron for hydraulic fracturing wastewater treatment. Sci. Total Environ. 2018, 615, 498–507.
- Fatoki, O.S.; Ayanda, O.S.; Adekola, F.A.; Ximba, B.J. Sorption of Triphenyltin Chloride to n Fe3O4, Fly Ash, and n Fe3O4/Fly Ash Composite Material in Seawater. Clean-Soil Air Water 2014, 42, 472–479.
- Torad, N.L.; Takahashi, A.; Kawakami, M.; Kawamoto, T.; Tanaka, H. Decontamination of very dilute Cs in seawater by a coagulation-precipitation method using a nanoparticle slurry of copper hexacyanoferrate. Environ. Sci. Water Res. Technol. 2019, 5, 1328–1338.
- Le, Q.T.N.; Cho, K. Caesium adsorption on a zeolitic imidazolate framework (ZIF-8) functionalized by ferrocyanide. J. Colloid Interface Sci. 2021, 581, 741–750.
- Yi, R.; Ye, G.; Wu, F.; Wen, M.; Feng, X.; Chen, J. Highly efficient removal of 137 Cs in seawater by potassium titanium ferrocyanide functionalized magnetic microspheres with multilayer core-shell structure. RSC Adv. 2014, 4, 37600–37608.
- Dong, C.-D.; Lu, Y.-C.; Chang, J.-H.; Wang, T.-H.; Chen, C.-W.; Hung, C.-M. Enhanced persulfate degradation of PAH-contaminated sediments using magnetic carbon microspheres as the catalyst substrate. Process Saf. Environ. Prot. 2019, 125, 219–227.
- Zhang, Z.; Li, M.; Chen, W.; Zhu, S.; Liu, N.; Zhu, L. Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environ. Pollut. 2010, 158, 514–519.
- De Gisi, S.; Minetto, D.; Lofrano, G.; Libralato, G.; Conte, B.; Todaro, F.; Notarnicola, M. Nano-scale zero valent iron (nZVI) treatment of marine sediments slightly polluted by heavy metals. Chem. Eng. Trans 2017, 60, 139–144.
- Hong, J.; Xie, J.; Mirshahghassemi, S.; Lead, J. Metal (Cd, Cr, Ni, Pb) removal from environmentally relevant waters using polyvinylpyrrolidone-coated magnetite nanoparticles. RSC Adv. 2020, 10, 3266–3276.
- Alabresm, A.; Chen, Y.P.; Decho, A.W.; Lead, J. A novel method for the synergistic remediation of oil-water mixtures using nanoparticles and oil-degrading bacteria. Sci. Total Environ. 2018, 630, 1292–1297.
- Rubin Pedrazzo, A.; Smarra, A.; Caldera, F.; Musso, G.; Dhakar, N.K.; Cecone, C.; Hamedi, A.; Corsi, I.; Trotta, F. Eco-friendly β-cyclodextrin and linecaps polymers for the removal of heavy metals. Polymers 2019, 11, 1658.
- Melone, L.; Rossi, B.; Pastori, N.; Panzeri, W.; Mele, A.; Punta, C. TEMPO-oxidized cellulose cross-linked with branched polyethyleneimine: Nanostructured adsorbent sponges for water remediation. ChemPlusChem 2015, 80, 1408–1415.
- Paladini, G.; Venuti, V.; Almásy, L.; Melone, L.; Crupi, V.; Majolino, D.; Pastori, N.; Fiorati, A.; Punta, C. Cross-linked cellulose nano-sponges: A small angle neutron scattering (SANS) study. Cellulose 2019, 26, 9005–9019.
- Kim, Y.; Kim, Y.K.; Kim, S.; Harbottle, D.; Lee, J.W. Nanostructured potassium copper hexacyanoferrate-cellulose hydrogel for selective and rapid cesium adsorption. Chem. Eng. J. 2017, 313, 1042–1050.
- Yang, H.; Sun, L.; Zhai, J.; Li, H.; Zhao, Y.; Yu, H. In situ controllable synthesis of magnetic Prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water. J. Mater. Chem. 2014, 2, 326–332.
- Liberatori, G.; Grassi, G.; Guidi, P.; Bernardeschi, M.; Fiorati, A.; Scarcelli, V.; Genovese, M.; Faleri, C.; Protano, G.; Frenzilli, G. Effect-based approach to assess nanostructured cellulose sponge removal efficacy of zinc ions from seawater to prevent ecological risks. Nanomaterials 2020, 10, 1283.
- Keller, A.A.; Garner, K.; Miller, R.J.; Lenihan, H.S. Toxicity of nano-zero valent iron to freshwater and marine organisms. PLoS ONE 2012, 7, e43983.
- Kadar, E.; Dyson, O.; Handy, R.D.; Al-Subiai, S.N. Are reproduction impairments of free spawning marine invertebrates exposed to zero-valent nano-iron associated with dissolution of nanoparticles? Nanotoxicology 2013, 7, 135–143.
- Kadar, E.; Simmance, F.; Martin, O.; Voulvoulis, N.; Widdicombe, S.; Mitov, S.; Lead, J.; Readman, J. The influence of engineered Fe2O3 nanoparticles and soluble (FeCl3) iron on the developmental toxicity caused by CO2-induced seawater acidification. Environ. Pollut. 2010, 158, 3490–3497.
- Alabresm, A.; Mirshahghassemi, S.; Chandler, G.T.; Decho, A.W.; Lead, J. Use of PVP-coated magnetite nanoparticles to ameliorate oil toxicity to an estuarine meiobenthic copepod and stimulate the growth of oil-degrading bacteria. Environ. Sci. Nano 2017, 4, 1859–1865.




