
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juan Larrubia | + 2878 word(s) | 2878 | 2021-04-20 10:12:21 | | | |
| 2 | Vivi Li | Meta information modification | 2878 | 2021-04-28 04:22:54 | | |
Video Upload Options
Thirty to fifty percent of hepatocellular carcinomas (HCC) display an immune class genetic signature. In this type of tumor, HCC-specific CD8 T cells carry out a key role in HCC control. Those potential reactive HCC-specific CD8 T cells recognize either HCC immunogenic neoantigens or aberrantly expressed host’s antigens, but they become progressively exhausted or deleted. These cells express the negative immunoregulatory checkpoint programmed cell death protein 1 (PD-1) which impairs T cell receptor signaling by blocking the CD28 positive co-stimulatory signal. The pool of CD8 cells sensitive to anti-PD-1/PD-L1 treatment is the PD-1dim memory-like precursor pool that gives rise to the effector subset involved in HCC control.
1. Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death worldwide and has an incidence of approximately 850,000 new cases per year. HCC accounts for approximately 90% of malignant liver tumors and is one of the most aggressive types of cancer, with few and unsatisfactory therapeutic options [1]. Since current therapies are limited and recurrence of HCC is common in patients treated with the standard therapeutic arsenal, immunotherapy presents itself as a promising possible response to the real need for new treatments [2]. The new emergence of treatments based on the modulation of inhibitory immune checkpoint (IC) opens new opportunities for the control of hepatocarcinoma [3][4][5]. Blockade of the programmed cell death protein 1 (PD-1)/PD-ligand(L)1 pathway is the paradigm of this type of treatment to counteract T cell response exhaustion [6][7], but we still do not have predictive variables to forecast a favorable response, and in many cases the initial response disappears [8][9]. The HCC-specific CD8 T cell response can be essential in HCC control due to its ability to recognize tumor cells and destroy them by cytolytic and no cytolytic mechanisms [10]. Nevertheless, these cells become exhausted during cancer progression [11][12] but can be temporally restored by immune checkpoint inhibitors (ICI). To enhance and extend the effect of this approach, the synergy of this therapy with other strategies acting at different levels (blocking other negative ICs, triggering positive co-stimulatory pathways, blocking vascular endothelial growth factor (VEGF) pathway, reprogramming mitochondrial metabolism, or adding loco-regional therapies) is being explored [13]. This work revises the types of HCC potentially sensitive to immunotherapy, the role of PD-1 expressing CD8 T cells in HCC treatment, the target population for CD8 T cell immunotherapy and the effect of PD-1 based treatment combinations on the restauration of HCC-specific cytotoxic T cell response.
2. Types of HCC According to Potential Sensitivity to PD-1 Based Immunotherapy
HCC is an inflammation-driven disease induced by chronic viral hepatitis (hepatitis B (HBV) and C (HCV) viruses) and non-viral “sterile” inflammation (alcohol abuse, non-alcoholic steatohepatitis (NASH) and other rare metabolic disorders, such as hemochromatosis or α-1 antitrypsin deficiency) [14]. Despite the different etiologies, the immune compositions are similar between HBV/HCV infected and virus negative tumors, apart from activated M2 macrophages that are observed mainly in virus-related HCC [15]. Nevertheless, the HCC-specific CD8 T cell response could be weaker in NASH-related HCC than in HCC associated with hepatitis virus infection [16], which could impact on immunotherapeutic strategies.
Previous extensive immunogenomic analysis of different type of tumors have shown diverse immune signatures. Based on these clusters, HCC can be classified according to the features of the induced immune response. Those non-immunogenic tumors are characterized by lymphocyte depletion or being immunologically quiet. Immunotolerant cancers are featured by the phenotypes “wound healing”, inflammatory and tumor growth factor (TGF)-β1 dominant. The immunogenic tumors comprise those cases that are interferon (IFN)-γ dominant [17]. The lymphocyte depleted cluster includes around 40–55% of HCC cases and are featured by M2 macrophage infiltration, moderate genomic heterogenicity, and high level of mutations in the catenin–β1 gene (CTNNB1). The “wound healing” HCCs are around 5–15% of cases and have a balance between macrophages and lymphocytes, high genomic heterogenicity, and mutations in the tumor suppressor gene TP53. The IFNγ dominant HCCs comprise around 10–20% of cases and are rich in CD8+ T cells and M1 macrophages with high PD-1 expression and T cell receptor (TCR) diversity. The HCC inflammatory type includes around 15–30% of cases and is featured by T helper (Th)17 infiltration, high Th1 response, low genomic heterogenicity, and high PD-L1 expression. Finally, the TGF-β1 dominant and the immunologically quiet clusters are poorly represented in HCC [17][18]. These different phenotypes should be considered to adapt the strategies to boost the immune response, as will be discussed at the end of this review (Figure 1). The detection of immunogenic HCC could be a key step to select those cases prone to respond to ICI. In those non-immune cases, strategies to induce a CD8+ T cell tumoral infiltration should be taken into consideration.
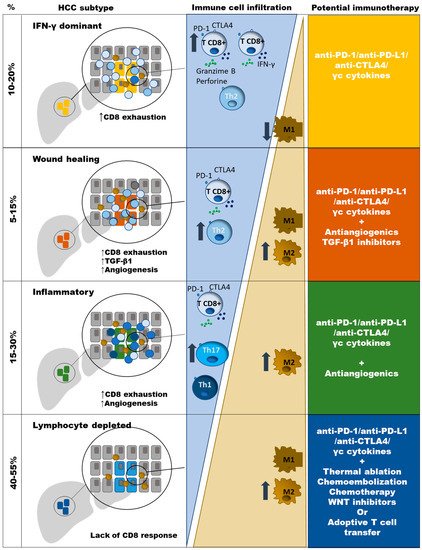
Figure 1. Type of anti-PD-1/PD-L1 based combination therapy, according to immune HCC subtypes. Progressive impairment in the balance between CD8 T cells and M2 TAM is observed in different HCC clusters. Neo-angiogenesis is induced in “Wound healing” and “Inflammatory” types. TGF-b1 genes are up-regulated in “Wound healing” HCC type. Absence of CD8 T cells in the “Lymphocyte depleted” type could be due to the lack of neoantigens or T cell deletion. TGF-β1 dominant and the immunologically quiet clusters are poorly represented in HCC.
The liver is an immunotolerant environment due to its need to deal with the exposure to many gut-derived exoantigens [19]. This special liver characteristic could facilitate the immune evasion in HCC, inducing either a defective T cell response or its physical deletion [20], linked to the induction of inhibitory IC such as PD-1 and its ligands [21][22]. Nevertheless, at HCC diagnosis not all tumors show data of complete HCC immune suppression. Trying to stratify the type of tumor that could respond to PD-1/PDL-1 manipulation, researchers have described another classification based on four scenarios according to PD-L1 expression. Type I tumors display tumor infiltrating lymphocytes (TIL) and PD-L1 expression on tumor cells. These features would suggest that an adaptive immune resistance is ongoing. Type II cases neither express PD-L1 nor have TIL, which indicates lack of immune response. Type III tumors express PD-L1 but without TIL, indicating an intrinsic induction of PD-L1. Finally, type IV tumors are PD-L1 negative but with TIL, suggesting that in these cases other mechanisms are involved in the development of intra-tumoral T cell exhaustion [23]. HCC patients with PD-L1 expression in the tumor have poorer overall survival (OS) than PD-L1 negative cases [24], but this can also be a marker of higher CD8 infiltration and higher survival after treatment [25]. In a recent metanalysis, PD-L1 positive patients had a risk of death higher than the double with respect to PD-L1 negative cases [26]. Around 20% of HCC expresses PD-L1 in more than 1% of tumor cells, and this correlates with a worse progression [27]. Fortunately, in the anti-PD-1 clinical trials with nivolumab and pembrolizumab (ChekMate-40 and KEYNOTE-224), those cases with higher PD-L1 expression had a higher response rate with a longer OS, but some patients with lower PD-L1 expression also responded to this treatment [28][29][30]. This response in negative cases could be due to biopsy sampling error because of the heterogenicity of PD-L1 expression in the HCC, since in resected HCC, PD-L1 detection has been reported in more than 80% of cases [31]. Consequently, this variable tested in a liver biopsy could not be useful to decide which patients should receive treatment to block the PD1/PD-L1 pathway, although it could be used as a prognosis marker. Consequently, high PD-L1 expression could be a marker of worse progression but also a prognosis factor for better treatment response. In any case, the role of PD-L1 expression to plot a therapy based on anti-PD-1 treatment is not clear yet and it must be clarified in future studies.
Another specific analysis of gene expression profiles from tumor, stromal, and immune cells in a big cohort of HCC showed that 25% of patients have a genetic signature related to the induction of an immune response [32]. These cases displayed a high level of PD-1 expression and markers of cytolytic activity. Within this “Immune Class” HCC, two different groups were described; those characterized by an effective response and those in whom there is an exhausted immune response that expressed many genes regulated by TGF-β1 [32]. These immune HCC types could correspond to the IFNγ dominant and the immunotolerant groups previously discussed in this section [17]. These distinct immunologic signatures correlate with different HCC outcomes. Those cases with an active immune response phenotype had a longer OS than those with an exhausted or non-detectable response [32]. The PD-1 up-regulation on this “Immune class” tumors could make them sensitive to ICI.
An important issue in HCC-specific CD8 T cell response induction is the immunogenic capacity of the potential HCC antigens. The T cell response against aberrant expression of host’s antigen, such as glypican-3, NY-ESO-1, MAGE-A1, and MAGE-A3 are not commonly efficiently induced in HCC and subsequently cannot be considered the backbone for immunotherapy [33]. Nevertheless, intrahepatic T cells targeting immunogenic tumoral neo-antigens display an exhausted phenotype that suggests tumor recognition [34]. Therefore, immunotherapeutic rescue of T cells targeting tumor neo-antigens could be the best approach to improve tumor control [35]. HCC has an intermediate level of neo-antigen expression, which could be an advantage for immunotherapeutic strategies. Consequently, the existence of neo-antigens could be a premise for the presence of sensitive HCC-specific CD8 T cells to ICI. Therefore, tumor mutational burden (TMB) could be a predictive biomarker for the efficacy of ICI therapy [36]. Specifically, HCC develops regularly neoantigens although less frequently than other tumors, such as melanoma or lung cancer [37]. Nevertheless, in HCC the frequency of cases with high TMB is low and does not correlate with the rate of predicted neo-antigens [38]. Therefore, the role of TMB as predictor of response to ICI is not clear yet.
According to these data, the analysis of the immune response could be a predictive factor for ICI success. In fact, in those cases with a more immunosuppressive microenvironment such as in virus-associated HCC, a higher PD-1 expression is found, and this could impact on ICI response [39]. Moreover, in the nivolumab trial, those cases with higher T cell reactive liver inflammation correlated with better response and longer OS [32]. We could summarize that a good candidate to restore HCC-specific CD8 T cell response by PD-1/PD-L1 blockade could be considered those tumors with high grade of IFN-γ-secreting TIL, high PD-L1 expression, low M2 macrophage infiltration, and presence of HCC neo-antigens.
3. Role of PD-1-Expressing HCC-Specific CD8 T Cell Response in HCC Control
The tumor-associated antigen-specific CD8+ T cell response is essential for the control of solid tumors due to their ability to recognize tumor cells and to destroy them [40][41]. Exhausted CD8+ T cells are the main subset of TILs that perform anti-tumor effector functions [10][42]. Specifically, in HCC it is possible to find a high number of T cells infiltrating the tumor (Figure 2) and there is a correlation between the density of infiltrating lymphocytes and the prognosis of the disease [43]. Furthermore, after loco-regional treatment of HCC, a longer survival has been observed in those subjects in whom a specific CD8+ T response against HCC associated antigens is detected [44]. Therefore, large numbers of CD8+ TILs in HCC correlate with improved OS, longer relapse-free survival, and diminished disease progression [10][45][46]. HCC associated antigens comprise a heterogeneous set of autologous proteins that are rendered immunogenic in tumors by mutation [35] or aberrant expression [10][47] (Figure 2). The development of an effector cytotoxic T cell immune response against these antigens may provide a barrier to tumor progression. Nevertheless, most of the T cells targeting autologous aberrantly expressed epitopes are deleted or in a tolerogenic state [33][48], although some studies have shown a positive effect after PD-1 blockade in T cells targeting these kind of antigens [7]. Anyhow, the response to ICI could be more effective in T cells targeting HCC neo-antigens. A previous study has suggested that searching for host’s human leukocyte antigen (HLA) restricted tumoral neoantigens will permit to find T cells that after the appropriate treatment will exert tumor control [35]. HCC will commit mutations that will give rise to new epitopes that will be recognized as exogen antigens by T cells. Unfortunately, these cells will become exhausted during HCC progression, not being able to keep the tumor under control. These exhausted CD8+ T cells show a gene signature that is completely different from those of memory and effector T cells [49][50][51]. During the exhaustion process, T cell response progressively loses the effector functions and finally undergoes apoptosis [11][52]. The exhausted CD8 T cells are featured by the expression of different inhibitory IC, such as PD-1, cytotoxic T lymphocyte antigen-4 (CTLA-4), T cell immunoglobulin domain and mucin domain (Tim3), and lymphocyte-activation gene 3 (LAG3) that can be modulated to rescue T cell reactivity [21][53]. The ligands of these IC are expressed by resident liver cells. In fact, PD-L1 is expressed on hepatocytes [22], hepatic stellate cells [54], liver sinusoidal endothelial cell (LSEC) [55], and Kupffer cells [56][57], while PD-L2 expression is restricted to dendritic cells [58]. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients [59]. The in-vitro treatment of these T cells with antibodies against these myriad of inhibitory IC increases T cell proliferation and cytokine secretion.
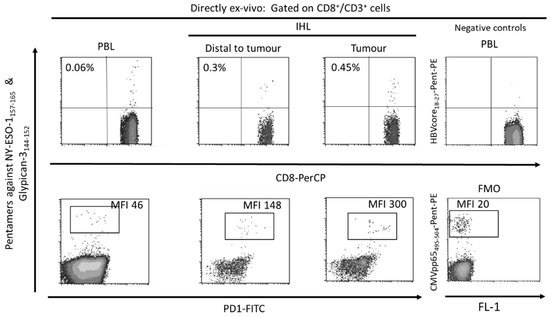
Figure 2. Flow-cytometric analysis of peripheral blood and intrahepatic lymphocytes in a patient with a Barcelona Clinic Liver Cancer Stage B hepatocellular carcinoma with hepatitis C cirrhosis, HBV negative, CMV positive. The ex-vivo CD8+ T cell response against the HLA-A2 restricted HCC epitopes Glypican-3144–152 and NY-ESO-1157–165 was tested by staining with CD8, CD3 and HLA-I pentameric complexes loaded with specific peptides. A tumoral sequestration of HCC-specific CD8 T cells and a PD-1 up-regulation gradient between peripheral blood and tumor was observed. PBL: peripheral blood lymphocytes, IHL: intrahepatic lymphocytes, MFI: mean fluorescence intensity, FMO: fluorescence minus one.
Nevertheless, these exhausted CD8+ cells develop epigenetic imprints that steadily maintain the functional impairment, which is transmitted to the progeny, making temporary the effects of immunomodulatory treatments [49]. Even in the context of a PD-1 knockdown HCC-specific CD8 T cell model, although a tumor killing enhancement is initially observed, this effect is limited by compensatory engagement of alternative co-inhibitory and senescence programs upon repetitive antigen stimulation [60]. These epigenetic imprints induce the expression of specific transcription factors, such as the thymocyte selection-associated high mobility group box protein (TOX) that is up-regulated on CD8+ T cells in HCC and promotes their exhaustion by regulating endocytic recycling of PD-1 [61]. Additionally, the up-regulation of the ligands of these negative IC is induced in the HCC microenvironment. The regulation of PD-L1 expression could be related with the level of M2 macrophages that are recruited by the tumor cell-intrinsic osteopontin secretion. In HCC animal models, the level of M2 macrophages decreases after osteopintin blockade, which correlates with an increased CD8 effector response to PD-L1 blockade [62]. M2-like macrophages favor an immunosuppressive landscape by depleting CD8 T cells and inducing CD4+ T regulatory cells (Tregs) [63]. Additionally, M1 macrophages can induce PD-L1 expression on hepatocarcinoma cells by IL-1β effect [64]. These data suggest an important role of macrophages in modulating CD8 T cell exhaustion in HCC by promoting a immunotolerant environment [65] and by up-regulating themselves both PD-L1 and PD-L2 expression [66]. However, PD-L1 expression on macrophages could also have a positive input because it correlates with CD8 T cell infiltration and increased OS after treatment [67], probably in the case of M1-like macrophage infiltration [17]. Moreover, HCC-specific CD8 T cell itself induces PD-L1 up-regulation on hepatocytes, linked to a subsequent impairment of IFN-γ secretion by T cells [68]. There is a gradient of PD-1 expression on intra-tumoral CD8+ cells, which probably defines the progenitor (PD-1dim) and effector (PD-1high) pools [69]. The PD-1high population can co-express positive co-stimulatory checkpoints, such as 4-1BB, that can be triggered to rescue these cells from exhaustion [70][71]. Nevertheless, to target the PD-1dim subset could be more operative to get a more efficient response to ICI. The PD-1 level of the intrahepatic resident CD8+ cells inversely correlates with the expression of the transcription factor T-bet [72], which has been correlated with a late dysfunctional phenotype in the PD-1high pool, since the effector late dysfunctional pool expresses the transcription factor Eomes and loses T-bet expression [73]. On the contrary, PD-1dim expression is a marker of the progenitor pool, which is the subset that provides the proliferative burst after anti-PD-1 treatment [69]. Therefore, T cell restoration should focus on PD-1dim CD8+ T cells. In order to improve both the precursor and the effector pools, searching for PD-1/PD-L1 based combinatory therapies, such as modulation of suppressive soluble mediators (interleukin (IL)-10, IL-17, TGF-β1), blocking suppressive cells (Tregs, myeloid derived suppressor cells (MDSC), M2 tumor associated macrophages (TAM)), triggering positive co-stimulation (tumor necrosis factor receptor superfamily member 9, 4-1BB), mitochondrial metabolic reprogramming, impairing neo-angiogenesis, inducing expression of HCC neo-antigens or epigenetic modulation by gamma-chain (γc) cytokines [11][12][14][74] could improve the response to PD-1/PD-L1 blocking monotherapy. Figure 3 summarizes the potential mechanisms involved in HCC-specific CD8 T cell impairment.
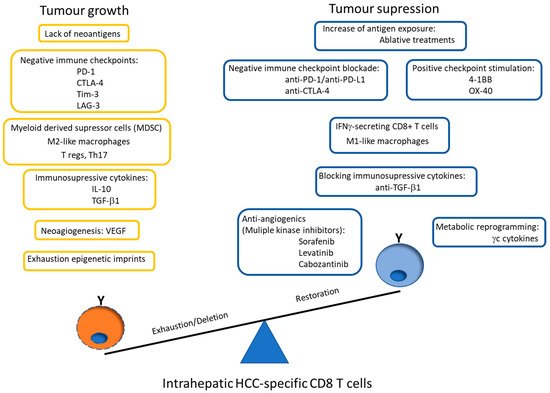
Figure 3. Scheme showing the potential mechanisms involved in HCC-specific CD8 T cell impairment. The graph also highlights the possible PD-1/PD-L1 blockade-based combination therapies to rescue effector and precursor HCC-specific cytotoxic T cell response. PD-1: programmed cell death protein 1, CTLA-4: cytotoxic T lymphocyte antigen-4, Tim-3: T cell immunoglobulin domain and mucin domain, LAG-3: lymphocyte-activation gene 3, T regs: CD4 T regulatory cells, Th: T helper, IL: interleukin, TFG: tumor growth factor, VEGF: vascular endothelial growth factor, 4-1BB: tumor necrosis factor receptor superfamily member 9, OX-40: Tumor necrosis factor receptor superfamily member 4, γc: gamma-chain.
References
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236.
- Hartke, J.; Johnson, M.; Ghabril, M. The diagnosis and treatment of hepatocellular carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159.
- Li, S.; Yang, F.; Ren, X. Immunotherapy for hepatocellular carcinoma. Drug Discov. Ther. 2015, 9, 363–371.
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489.
- El Dika, I.; Khalil, D.N.; Abou-Alfa, G.K. Immune checkpoint inhibitors for hepatocellular carcinoma. Cancer 2019, 125, 3312–3319.
- Freeman, G.J.; Wherry, E.J.; Ahmed, R.; Sharpe, A.H. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J. Exp. Med. 2006, 203, 2223–2227.
- Guo, X.; Jiang, H.; Shi, B.; Zhou, M.; Zhang, H.; Shi, Z.; Du, G.; Luo, H.; Wu, X.; Wang, Y.; et al. Disruption of PD-1 Enhanced the Anti-tumor Activity of Chimeric Antigen Receptor T Cells Against Hepatocellular Carcinoma. Front. Pharmacol. 2018, 9, 1118.
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465.
- Flecken, T.; Schmidt, N.; Hild, S.; Gostick, E.; Drognitz, O.; Zeiser, R.; Schemmer, P.; Bruns, H.; Eiermann, T.; Price, D.A.; et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8 + T-cell responses in hepatocellular carcinoma. Hepatology 2014, 59, 1415–1426.
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495.
- Zarour, H.M. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin. Cancer Res. 2016, 22, 1856–1864.
- Cheng, A.-L.; Hsu, C.; Chan, S.L.; Choo, S.-P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319.
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The immunology of hepatocellular carcinoma. Nat. Immunol. 2018, 19, 222–232.
- Cancer Genome Atlas Research Network. Electronic address wbe, Cancer Genome Atlas Research N. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.
- Inada, Y.; Mizukoshi, E.; Seike, T.; Tamai, T.; Iida, N.; Kitahara, M.; Yamashita, T.; Arai, K.; Terashima, T.; Fushimi, K.; et al. Characteristics of Immune Response to Tumor-Associated Antigens and Immune Cell Profile in Patients with Hepatocellular Carcinoma. Hepatology 2019, 69, 653–665.
- Thorsson, V.; Gibbs, D.L.; Brown, S.; Wolf, D.; Bortone, D.S.; Ouyang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14.
- Hilmi, M.; Neuzillet, C.; Calderaro, J.; Lafdil, F.; Pawlotsky, J.-M.; Rousseau, B. Angiogenesis and immune checkpoint inhibitors as therapies for hepatocellular carcinoma: Current knowledge and future research directions. J. Immunother. Cancer 2019, 7, 1–13.
- Roth, G.S.; Decaens, T. Liver immunotolerance and hepatocellular carcinoma: Patho-physiological mechanisms and therapeutic perspectives. Eur. J. Cancer 2017, 87, 101–112.
- Jiang, Y.; Li, Y.; Zhu, B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015, 6, e1792.
- Kim, H.-D.; Song, G.-W.; Park, S.; Jung, M.K.; Kim, M.H.; Kang, H.J.; Yoo, C.; Yi, K.; Kim, K.H.; Eo, S.; et al. Association between expression level of PD1 by tumor-infiltrating CD8+ T cells and features of hepatocellular carcinoma. Gastroenterology 2018, 155, 1936.e17–1950.e17.
- Mühlbauer, M.; Fleck, M.; Schütz, C.; Weiss, T.; Froh, M.; Blank, C.; Schölmerich, J.; Hellerbrand, C. PD-L1 is induced in hepatocytes by viral infection and by interferon-α and -γ and mediates T cell apoptosis. J. Hepatol. 2006, 45, 520–528.
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145.
- Gao, Q.; Wang, X.-Y.; Qiu, S.-J.; Yamato, I.; Sho, M.; Nakajima, Y.; Zhou, J.; Li, B.-Z.; Shi, Y.-H.; Xiao, Y.-S.; et al. Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res. 2009, 15, 971–979.
- Gabrielson, A.; Wu, Y.; Wang, H.; Jiang, J.; Kallakury, B.; Gatalica, Z.; Reddy, S.; Kleiner, D.; Fishbein, T.; Johnson, L.; et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol. Res. 2016, 4, 419–430.
- Gao, X.-S.; Gu, X.; Xiong, W.; Guo, W.; Han, L.; Bai, Y.; Peng, C.; Cui, M.; Xie, M. Increased programmed death ligand-1 expression predicts poor prognosis in hepatocellular carcinoma patients. OncoTargets Ther. 2016, 9, 4805–4813.
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. 2016, 100, 88–98.
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502.
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952.
- Sangro, B.; Melero, I.; Wadhawan, S.; Finn, R.S.; Abou-Alfa, G.K.; Cheng, A.-L.; Yau, T.; Furuse, J.; Park, J.-W.; Boyd, Z.; et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2020, 73, 1460–1469.
- Yarchoan, M.; Xing, D.; Luan, L.; Xu, H.; Sharma, R.B.; Popovic, A.; Pawlik, T.M.; Kim, A.K.; Zhu, Q.; Jaffee, E.M.; et al. Characterization of the Immune Microenvironment in Hepatocellular Carcinoma. Clin. Cancer Res. 2017, 23, 7333–7339.
- Sia, D.; Jiao, Y.; Martinez-Quetglas, I.; Kuchuk, O.; Villacorta-Martin, C.; de Moura, M.C.; Putra, J.; Camprecios, G.; Bassaganyas, L.; Akers, N.; et al. Identification of an Immune-specific Class of Hepatocellular Carcinoma, Based on Molecular Features. Gastroenterology 2017, 153, 812–826.
- Tauber, C.; Schultheiss, M.; De Luca, R.; Buettner, N.; Llewellyn-Lacey, S.; Emmerich, F.; Zehe, S.; Price, D.A.; Neumann-Haefelin, C.; Schmitt-Graeff, A.; et al. Inefficient induction of circulating TAA-specific CD8+ T-cell responses in hepatocellular carcinoma. Oncotarget 2019, 10, 5194–5206.
- Ma, J.; Zheng, B.; Goswami, S.; Meng, L.; Zhang, D.; Cao, C.; Li, T.; Zhu, F.; Ma, L.; Zhang, Z.; et al. PD1Hi CD8+ T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J. Immunother. Cancer 2019, 7, 1–15.
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.-C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer Immunotherapy Based on Mutation-Specific CD4+ T Cells in a Patient with Epithelial Cancer. Science 2014, 344, 641–645.
- Chan, T.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56.
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.J.R.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.-L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421.
- Luchini, C.; Bibeau, F.; Ligtenberg, M.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243.
- Lim, C.J.; Lee, Y.H.; Pan, L.; Lai, L.; Chua, C.; Wasser, M.; Lim, T.K.H.; Yeong, J.; Toh, H.C.; Lee, S.Y.; et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019, 68, 916–927.
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056.
- Eynde, B.J.V.D.; van der Bruggen, P. T cell defined tumor antigens. Curr. Opin. Immunol. 1997, 9, 684–693.
- Zheng, C.; Zheng, L.; Yoo, J.-K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16.
- Wada, Y.; Nakashima, O.; Kutami, R.; Yamamoto, O.; Kojiro, M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 1998, 27, 407–414.
- Mizukoshi, E.; Yamashita, T.; Arai, K.; Sunagozaka, H.; Ueda, T.; Arihara, F.; Kagaya, T.; Yamashita, T.; Fushimi, K.; Kaneko, S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 2013, 57, 1448–1457.
- Kang, T.-W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551.
- Chang, B.; Shen, L.; Wang, K.; Jin, J.; Huang, T.; Chen, Q.; Li, W.; Wu, P. High number of PD-1 positive intratumoural lymphocytes predicts survival benefit of cytokine-induced killer cells for hepatocellular carcinoma patients. Liver Int. 2018, 38, 1449–1458.
- Schmidt, N.; Flecken, T.; Thimme, R. Tumor-associated antigen specific CD8+T cells in hepatocellular carcinoma-a promising target for immunotherapy. OncoImmunology 2014, 3, e954919.
- Gehring, A.J.; Ho, Z.Z.; Tan, A.T.; Aung, M.O.; Lee, K.H.; Tan, K.C.; Lim, S.G.; Bertoletti, A. Profile of Tumor Antigen-Specific CD8 T Cells in Patients with Hepatitis B Virus-Related Hepatocellular Carcinoma. Gastroenterology 2009, 137, 682–690.
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016, 354, 1160–1165.
- Jadhav, R.R.; Im, S.J.; Hu, B.; Hashimoto, M.; Li, P.; Lin, J.-X.; Leonard, W.J.; Greenleaf, W.J.; Ahmed, R.; Goronzy, J.J. Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc. Natl. Acad. Sci. USA 2019, 116, 14113–14118.
- Sen, D.R.; Kaminski, J.; Barnitz, R.A.; Kurachi, M.; Gerdemann, U.; Yates, K.B.; Tsao, H.-W.; Godec, J.; LaFleur, M.W.; Brown, F.D.; et al. The epigenetic landscape of T cell exhaustion. Science 2016, 354, 1165–1169.
- Wherry, E.J.; Blattman, J.N.; Murali-Krishna, K.; Van Der Most, R.; Ahmed, R. Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment. J. Virol. 2003, 77, 4911–4927.
- Zhou, G.; Sprengers, D.; Boor, P.P.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119.e10.
- Yu, M.-C.; Chen, C.-H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 2004, 40, 1312–1321.
- Diehl, L.; Schurich, A.; Grochtmann, R.; Hegenbarth, S.; Chen, L.; Knolle, P.A. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology 2007, 47, 296–305.
- Kassel, R.; Cruise, M.W.; Iezzoni, J.C.; Taylor, N.A.; Pruett, T.L.; Hahn, Y.S. Chronically inflamed livers up-regulate expression of inhibitory B7 family members. Hepatology 2009, 50, 1625–1637.
- Wu, K.; Kryczek, I.; Chen, L.; Zou, W.; Welling, T.H. Kupffer Cell Suppression of CD8+ T Cells in Human Hepatocellular Carcinoma Is Mediated by B7-H1/Programmed Death-1 Interactions. Cancer Res. 2009, 69, 8067–8075.
- Mocan, T.; Sparchez, Z.; Craciun, R.; Bora, C.N.; Leucuta, D.C. Programmed cell death protein-1 (PD-1)/programmed death-ligand-1 (PD-L1) axis in hepatocellular carcinoma: Prognostic and therapeutic perspectives. Clin. Transl. Oncol. 2019, 21, 702–712.
- Shi, F.; Shi, M.; Zeng, Z.; Qi, R.-Z.; Liu, Z.-W.; Zhang, J.-Y.; Yang, Y.-P.; Tien, P.; Wang, F.-S. PD-1 and PD-L1 upregulation promotes CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 2010, 128, 887–896.
- Otano, I.; Escors, D.; Schurich, A.; Singh, H.; Robertson, F.; Davidson, B.R.; Fusai, G.; Vargas, F.A.; Tan, Z.M.; Aw, J.Y.; et al. Molecular Recalibration of PD-1+ Antigen-Specific T Cells from Blood and Liver. Mol. Ther. 2018, 26, 2553–2566.
- Wang, X.; He, Q.; Shen, H.; Xia, A.; Tian, W.; Yu, W.; Sun, B. TOX promotes the exhaustion of antitumor CD8+ T cells by preventing PD1 degradation in hepatocellular carcinoma. J. Hepatol. 2019, 71, 731–741.
- Zhu, Y.; Yang, J.; Xu, D.; Gao, X.-M.; Zhang, Z.; Hsu, J.L.; Li, C.-W.; Lim, S.-O.; Sheng, Y.-Y.; Zhang, Y.; et al. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut 2019, 68, 1653–1666.
- Freitas-Lopes, M.A.; Mafra, K.; David, B.A.; Carvalho-Gontijo, R.; Menezes, G.B. Differential Location and Distribution of Hepatic Immune Cells. Cells 2017, 6, 48.
- Zong, Z.; Zou, J.; Mao, R.; Ma, C.; Li, N.; Wang, J.; Wang, X.; Zhou, H.; Zhang, L.; Shi, Y. M1 Macrophages Induce PD-L1 Expression in Hepatocellular Carcinoma Cells Through IL-1β Signaling. Front. Immunol. 2019, 10, 1643.
- Yun, J.; Yu, G.; Hu, P.; Chao, Y.; Li, X.; Chen, X.; Wei, Q.; Wang, J. PD-1 expression is elevated in monocytes from hepatocellular carcinoma patients and contributes to CD8 T cell suppression. Immunol. Res. 2020, 68, 436–444.
- Yasuoka, H.; Asai, A.; Ohama, H.; Tsuchimoto, Y.; Fukunishi, S.; Higuchi, K. Increased both PD–L1 and PD–L2 expressions on monocytes of patients with hepatocellular carcinoma was associated with a poor prognosis. Sci. Rep. 2020, 10, 10377.
- Liu, C.-Q.; Xu, J.; Zhou, Z.-G.; Jin, L.-L.; Yu, X.-J.; Xiao, G.; Lin, J.; Zhuang, S.-M.; Zhang, Y.-J.; Zheng, L. Expression patterns of programmed death ligand 1 correlate with different microenvironments and patient prognosis in hepatocellular carcinoma. Br. J. Cancer 2018, 119, 80–88.
- Huang, C.-Y.; Wang, Y.; Luo, G.-Y.; Han, F.; Li, Y.-Q.; Zhou, Z.-G.; Xu, G.-L. Relationship Between PD-L1 Expression and CD8+ T-cell Immune Responses in Hepatocellular Carcinoma. J. Immunother. 2017, 40, 323–333.
- Im, S.J.; Hashimoto, M.; Gerner, M.Y.; Lee, J.; Kissick, H.T.; Burger, M.C.; Shan, Q.; Hale, J.S.; Lee, J.; Nasti, T.H.; et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nat. Cell Biol. 2016, 537, 417–421.
- Kim, H.; Park, S.; Jeong, S.; Lee, Y.J.; Lee, H.; Kim, C.G.; Kim, K.H.; Hong, S.; Lee, J.; Kim, S.; et al. 4-1BB Delineates Distinct Activation Status of Exhausted Tumor-Infiltrating CD8 + T Cells in Hepatocellular Carcinoma. Hepatology 2020, 71, 955–971.
- Zhang, Y.; Zhang, H.; Wei, M.; Mou, T.; Shi, T.; Ma, Y.; Cai, X.; Li, Y.; Dong, J.; Wei, J. Recombinant Adenovirus Expressing a Soluble Fusion Protein PD-1/CD137L Subverts the Suppression of CD8+ T Cells in HCC. Mol. Ther. 2019, 27, 1906–1918.
- Chew, V.; Lai, L.; Pan, L.; Lim, C.J.; Li, J.; Chong, T.H.; Chua, C.; Leong, J.Y.; Lim, K.H.; Toh, H.C.; et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc. Natl. Acad. Sci. USA 2017, 114, E5900–E5909.
- Escobar, G.; Mangani, D.; Anderson, A.C. T cell factor 1: A master regulator of the T cell response in disease. Sci. Immunol. 2020, 5, eabb9726.
- Abdelsamed, H.A.; Zebley, C.C.; Youngblood, B. Epigenetic Maintenance of Acquired Gene Expression Programs during Memory CD8 T Cell Homeostasis. Front. Immunol. 2018, 9, 6.




