
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sungjun Park | + 1322 word(s) | 1322 | 2021-04-27 09:53:40 | | | |
| 2 | Catherine Yang | Meta information modification | 1322 | 2021-04-27 10:52:33 | | |
Video Upload Options
The rapid advances in human-friendly and wearable photoplethysmography (PPG) sensors have facilitated the continuous and real-time monitoring of physiological conditions, enabling self-health care without being restricted by location.
1. Introduction
With an aging population, the demand for wearable electronic devices to monitor physiological conditions has increased exponentially in recent years [1][2][3][4]. The continuous and real-time measurement of physiological parameters using different sensing techniques plays an important role in diagnosing health conditions. For example, small wearable sensor systems have been developed for people who are at risk of heart attack, seizures, and stroke [5][6][7]. Wearable devices are also becoming widely used to gather information about an individual’s muscle activity, sleep quality, and other physiological activities in daily life [8][9].
Several options for wearable technology have emerged in the form of integrated clothing, accessories, and body attachments [10][11][12][13][14]. A classic example is a photoplethysmography (PPG) sensor which utilizes infrared light to noninvasively measure changes in pulsatile blood flows at the skin surface [15]. At present, the PPG can be applied to various aspects of cardiovascular monitoring, including the detection of arterial oxygen saturation (SpO2), heart rate, blood pressure, cardiac output, respiration, arterial aging, endothelial function, microvascular blood flow, and autonomic function [16][17][18][19][20][21][22][23][24].
By applying sensors and mobile computing devices directly onto the body surface, the PPG sensor could also be designed as a skin-on interface [25][26]. The skin-interfaced wearable devices have the advantage of allowing the continuous monitoring of various physiological data as comfortably as possible. Moreover, flexible and stretchable electronic devices can conformally be placed on the skin and detect various signals with extremely high sensitivity [27][28][29].
2. Photoplethysmography (PPG) Sensor and Its Application
2.1. Mechanism of PPG Sensor
A PPG sensor is an optically obtained plethysmogram to monitor blood volume changes in the microvascular system. Conventional PPG sensors comprise two main components: light-emitting diodes (LEDs) and photodetectors (PDs). In the device architecture, red and near-infrared light from LEDs has generally been used as the long wavelengths are suitable for measuring deep-tissue blood flow [30]. The PDs detect volumetric changes in blood from cardiac pressure by absorbing light illumination from LEDs through the skin. The noninvasive and optical measurement system enables a PPG to monitor breathing, hypovolemia, and other circulatory conditions [31].The PPG signal can be acquired in reflection or transmission mode [32]. In transmission mode, the light transmitted through the medium (tissue, bone, and/or blood vessels) is detected, while backscattered or reflected light is detected in the reflection mode. In the PPG sensors’ transmission mode, the sensing locations are limited owing to confined transillumination [33]. Therefore, the location of PDs in transmission mode is limited by the thinness of the subject, such as on the fingers, earlobes, and neonatal feet. Conversely, in the reflection mode, the LEDs and PDs are integrated into the same plane, possibly located on various spots, such as the forehead, forearm, abdomen, and legs (Figure 1a).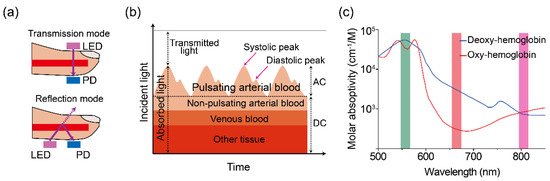 Figure 1. (a) Illustration of a two-mode (transmission and reflection) PPG sensor. (b) Variation in tissue light attenuation. (c) Molar absorptivity of oxygenated (blue line) and deoxygenated (red line) hemoglobin in arterial blood as of wavelength. Reproduced with permission from [34][35], ELSEVIER (1997) and SPIE (1999).The PDs can detect changes in light intensity from transmitted and/or reflected light in response to volumetric changes in veins and capillaries. As shown in Figure 1b, the typical PPG waveform consists of direct current (DC) and alternating current (AC) components. The DC component is caused by the absorption of nonpulsating arterial blood and scattering in all tissues, while the AC component is attributed to the volumetric change of arterial blood between systolic and diastolic phases in cardiac cycles [36][37]. The height of the AC signal fluctuates over time due to arterial blood pulsed by the heartbeat.Pulse oximetry is a revolutionary method for evaluating oxygen saturation levels (SpO2) using PPG devices. In a theoretical estimation, the oxygen saturation of the arterial blood can be expressed as the amount of oxygenated hemoglobin against the full quantity of hemoglobin: % SpO2=HbO2HbO2+Hb×100 %
Figure 1. (a) Illustration of a two-mode (transmission and reflection) PPG sensor. (b) Variation in tissue light attenuation. (c) Molar absorptivity of oxygenated (blue line) and deoxygenated (red line) hemoglobin in arterial blood as of wavelength. Reproduced with permission from [34][35], ELSEVIER (1997) and SPIE (1999).The PDs can detect changes in light intensity from transmitted and/or reflected light in response to volumetric changes in veins and capillaries. As shown in Figure 1b, the typical PPG waveform consists of direct current (DC) and alternating current (AC) components. The DC component is caused by the absorption of nonpulsating arterial blood and scattering in all tissues, while the AC component is attributed to the volumetric change of arterial blood between systolic and diastolic phases in cardiac cycles [36][37]. The height of the AC signal fluctuates over time due to arterial blood pulsed by the heartbeat.Pulse oximetry is a revolutionary method for evaluating oxygen saturation levels (SpO2) using PPG devices. In a theoretical estimation, the oxygen saturation of the arterial blood can be expressed as the amount of oxygenated hemoglobin against the full quantity of hemoglobin: % SpO2=HbO2HbO2+Hb×100 %
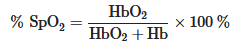
where HbO2 and Hb are the concentrations of oxy and deoxygenated hemoglobin, respectively. Typically, a pulse oximeter utilizes two different wavelengths of light. One is red and the other is infrared with wavelengths of 660 and 940 nm, respectively. The absorption intensity of light differs significantly depending on the oxygenated condition of hemoglobin. The iron-containing hemoglobin in the red blood cells can bind and transport oxygen during internal respiration. As shown in Figure 1c, the absorption spectra of hemoglobin varied under oxy/deoxygenated conditions. Significantly lower absorption in red (660 nm) light and slightly higher absorption in infrared (940 nm) are observed for oxyhemoglobin compared to deoxyhemoglobin [38]. The red/infrared modulation ratio (R), double-ratio of pulsatile (AC), and nonpulsatile (DC) components in red and infrared light can be used to calibrate the SpO2 level as follows:
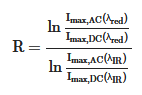
where A is absorbance. The calculated values of the modulation ratio R correspond to the SpO2 level. Considering low oxygenated conditions, for example, the amplitude of the AC component in the red-light region decreases compared to its behavior in the IR light region owing to the relatively higher absorption coefficient of deoxyhemoglobin at 660 nm (Figure 1c), resulting in a low R-value.
2.2. Applications of PPG Sensor
In clinical practice, the accurate measurement of the clinically relevant hemoglobin derivatives and bilirubin levels can provide useful diagnostic information. Figure 2 shows the absorption spectra of hemoglobin derivatives and bilirubin that can be further studied using skin-compatible PPG sensors.
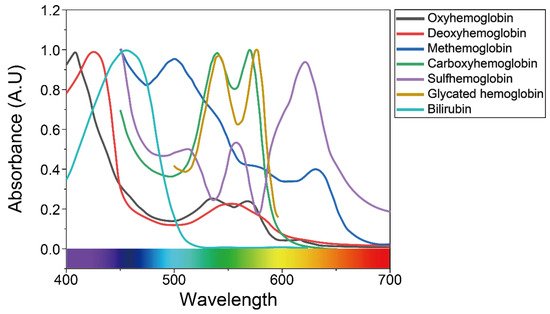 Figure 2. Absorbance of bilirubin and various types of hemoglobin in the visible light region, as mentioned in previous studies. Reproduced with permission from [39][40][41], ELSEVIER (1981), SPIE (2012), and ACS Publications (2016).
Figure 2. Absorbance of bilirubin and various types of hemoglobin in the visible light region, as mentioned in previous studies. Reproduced with permission from [39][40][41], ELSEVIER (1981), SPIE (2012), and ACS Publications (2016).
Methemoglobin (MetHb), along with carboxyhemoglobin (COHb) and sulfhemoglobin (SHb), represents a dyshemoglobin that does not bind O2. Normally, only small traces of MetHb and carboxyhemoglobin (COHb) are found in the blood, while sulfhemoglobin (SHb) is absent [42][43]. However, levels of MetHb and COHb are often elevated in severely ill patients, including clinical hypoxia [44], exposure to certain toxic agents [45], smokers [46], and the lack of genetic reductase [47].
Several observational studies reported the benefits of the continuous monitoring of MetHb and COHb levels to identify patients with carbon monoxide poisoning at triage in the hospital emergency departments [48][49][50]. Unlike MetHb, which is reversible with an antidote, methylene blue [51], SHb is an irreversible form of oxidized hemoglobin resulting from the incorporation of a sulfur atom in its porphyrin ring [52][53]. Currently, devices such as co-oximeters and blood gas analyzers are used to detect the abnormal presence of MetHb and SHb in blood samples [54][55]. However, the detection tests are not performed routinely in most intensive care units due to cost. The readings provided by those devices are not reliable, giving a false-positive result for methemoglobinemia [56]. Therefore, the noninvasive and low-cost approaches in the detection of MetHb and SHb at the point-of-care would be useful for the early detection of cyanotic conditions. Glycated hemoglobin (HbA1c), which is formed by the binding of hemoglobin, reflects the average blood glucose level over the preceding 60 days [57]. A range of studies [58][59][60][61] have demonstrated the benefits of point-of-care HbA1c testing, and the rapid availability of HbA1c testing has been shown to facilitate safe and effective diabetes management. Bilirubin levels are routinely monitored in cases of neonatal jaundice to identify hyperbilirubinemia and to prevent bilirubin-related neurotoxicity [62][63]. Many laboratory devices and methods are available to measure bilirubin levels in the blood. However, results are obtained 24 h after testing leading to delays in treatment. Thus, there is an important need for an accurate point-of-care system that allows rapid and appropriate treatment.
For the continuous and accurate evaluation of health status, securing stable adhesion between the light-responsive active components of PPG sensors and the target surfaces is crucial by minimizing motion artifacts [64]. Additionally, the devices percutaneously monitoring biometric signals require avoidance of toxicity, electric shock, electrolysis, and excessive thermogenesis [65]. To improve these problems, it is necessary to focus on the development of materials. In the next section, an innovative strategy for human skin-compatible PPG sensors depending on different types of light-absorbing semiconducting materials is highlighted.
References
- Ghamari, M. A Review on Wearable Photoplethysmography Sensors and Their Potential Future Applications in Health Care. Int. J. Biosens. Bioelectron. 2018, 4, 195–202.
- Rawassizadeh, R.; Price, B.A.; Petre, M. Wearables: Has the Age of Smartwatches Finally Arrived? Commun. ACM 2014, 58, 45–47.
- Wei, J. How Wearables Intersect with the Cloud and the Internet of Things: Considerations for the Developers of Wearables. IEEE Consum. Electron. Mag. 2014, 3, 53–56.
- Riazul Islam, S.M.; Kwak, D.; Humaun Kabir, M.; Hossain, M.; Kwak, K.-S. The Internet of Things for Health Care: A Comprehensive Survey. IEEE Access 2015, 3, 678–708.
- Martins, A.F.; Santos, D.F.S.; Perkusich, A.; Almeida, H.O. UPnP and IEEE 11073: Integrating Personal Health Devices in Home Networks. In Proceedings of the 2014 IEEE 11th Consumer Communications and Networking Conference (CCNC), Las Vegas, NV, USA, 10–13 January 2014.
- Jeong, S.; Kim, S.; Kim, D.; Youn, C.-H.; Kim, Y.-W. A Personalized Healthcare System for Chronic Disease Care in Home-Hospital Cloud Environments. In Proceedings of the 2013 International Conference on ICT Convergence (ICTC), Jeju, Korea, 14–16 October 2013; pp. 371–376.
- Adamson, P.B.; Abraham, W.T.; Aaron, M.; Aranda, J.M.; Bourge, R.C.; Smith, A.; Stevenson, L.W.; Bauman, J.G.; Yadav, J.S. CHAMPION∗ Trial Rationale and Design: The Long-Term Safety and Clinical Efficacy of a Wireless Pulmonary Artery Pressure Monitoring System. J. Card. Fail. 2011, 17, 3–10.
- Mukhopadhyay, S.C. Wearable Sensors for Human Activity Monitoring: A Review. IEEE Sens. J. 2015, 15, 1321–1330.
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully Integrated Wearable Sensor Arrays for Multiplexed in Situ Perspiration Analysis. Nature 2016, 529, 509–514.
- Chan, M.; Estève, D.; Fourniols, J.-Y.; Escriba, C.; Campo, E. Smart Wearable Systems: Current Status and Future Chal lenges. Artif. Intell. Med. 2012, 56, 137–156.
- Yang, G.; Pang, G.; Pang, Z.; Gu, Y.; Mantysalo, M.; Yang, H. Non-Invasive Flexible and Stretchable Wearable Sensors with Nano-Based Enhancement for Chronic Disease Care. IEEE Rev. Biomed. Eng. 2019, 12, 34–71.
- Ghamari, M.; Janko, B.; Sherratt, R.; Harwin, W.; Piechockic, R.; Soltanpur, C. A Survey on Wireless Body Area Networks for EHealthcare Systems in Residential Environments. Sensors 2016, 16, 831.
- Park, S.; Jayaraman, S. Enhancing the Quality of Life through Wearable Technology. IEEE Eng. Med. Biol. Mag. 2003, 22, 41–48.
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors 2018, 18, 645.
- Parak, J.; Korhonen, I. Evaluation of Wearable Consumer Heart Rate Monitors Based on Photopletysmography. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3670–3673.
- McCombie, D.; Asada, H.; Reisner, A. Identification of Vascular Dynamics and Estimation of the Cardiac Output Waveform from Wearable PPG Sensors. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 3490–3493.
- Cha, J.; Choi, H.; Shin, J.; Lee, K. Unconstrained Respiration and Heart Rate Monitoring System Based on a PPG Pillow during Sleep. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 3224–3226.
- Rozi, R.M.; Usman, S.; Ali, M.A.M.; Reaz, M.B.I. Second Derivatives of Photoplethysmography (PPG) for Estimating Vascular Aging of Atherosclerotic Patients. In Proceedings of the 2012 IEEE-EMBS Conference on Biomedical Engineering and Sciences, Langkawi, Malaysia, 17–19 December 2012.
- Zakaria, H.; Mengko, T.L.R. Endothelial Dysfunction Assessment by Finger Photoplethysmogram. In Proceedings of the 2017 6th International Conference on Electrical Engineering and Informatics (ICEEI), Langkawi, Malaysia, 25–27 November 2017; pp. 1–4.
- Allen, J.; Frame, J.R.; Murray, A. Microvascular Blood Flow and Skin Temperature Changes in the Fingers Following a Deep Inspiratory Gasp. Physiol. Meas. 2002, 23, 365–373.
- Servati, A.; Zou, L.; Wang, Z.; Ko, F.; Servati, P. Novel Flexible Wearable Sensor Materials and Signal Processing for Vital Sign and Human Activity Monitoring. Sensors 2017, 17, 1622.
- Slapničar, G.; Luštrek, M.; Marinko, M. Continuous Blood Pressure Estimation from PPG Signal. Informatica 2018, 42, 10.
- Zhang, Q.; Kadefors, R. A Non-Invasive Measure of Changes in Blood flow in the Human Anterior Tibial Muscle. Eur. J. Appl. Physiol. 2001, 84, 448–452.
- Wu, H.-T.; Lee, C.-H.; Liu, A.-B. Assessment of Endothelial Function Using Arterial Pressure Signals. J. Signal Process. Syst. 2011, 64, 223–232.
- Matsumura, K.; Rolfe, P.; Lee, J.; Yamakoshi, T. IPhone 4s Photoplethysmography: Which Light Color Yields the Most Accurate Heart Rate and Normalized Pulse Volume Using the IPhysioMeter Application in the Presence of Motion Artifact? PLoS ONE 2014, 9, e91205.
- Asada, H.H.; Shaltis, P.; Reisner, A.; Rhee, S.; Hutchinson, R.C. Mobile Monitoring with Wearable Photoplethysmographic Biosensors. IEEE Eng. Med. Biol. Mag. 2003, 22, 28–40.
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft Microfluidic Assemblies of Sensors, Circuits, and Radios for the Skin. Science 2014, 344, 70–74.
- Huang, X.; Liu, Y.; Chen, K.; Shin, W.-J.; Lu, C.-J.; Kong, G.-W.; Patnaik, D.; Lee, S.-H.; Cortes, J.F.; Rogers, J.A. Stretchable, Wireless Sensors and Functional Substrates for Epidermal Characterization of Sweat. Small 2014, 10, 3083–3090.
- Kim, J.; Banks, A.; Cheng, H.; Xie, Z.; Xu, S.; Lee, J.W.; Liu, Z.; Gutruf, P.; Huang, X.; Wei, P.; et al. Epidermal Electronics with Advanced Capabilities in Near—Field Communication. Small 2015, 11, 906–912.
- Abay, T.Y. Reflectance Photoplethysmography as Noninvasive Monitoring of Tissue Blood Perfusion. IEEE Trans. Biomed. Eng. 2015, 62, 9.
- Allen, J. Photoplethysmography and Its Application in Clinical Physiological Measurement. Physiol. Meas. 2007, 28, R1-39.
- Dorlas, J.C.; Mahieu, H.F. Photoelectric Plethysmography-Some Fundamental Aspects of the Reflection and Transmission Method. Clin. Phys. Physiol. Meas. 1981, 2, 205–215.
- Ovadia-Blechman, Z.; Gino, O.; Dandeker, L.; Sheffer, N.; Baltaxe, E.; Aharonson, V. The Feasibility of Flat, Portable and Wireless Device for Non-Invasive Peripheral Oxygenation Measurement over the Entire Body. J. Biomed. Sci. Eng. 2016, 9, 147–159.
- Zijlstra, W.G.; Buursma, A. Spectrophotometry of Hemoglobin: Absorption Spectra of Bovine Oxyhemoglobin, Deoxyhemoglobin, Carboxyhemoglobin, and Methemoglobin. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 743–749.
- Roggan, A.; Friebel, M.; Dörschel, K.; Hahn, A.; Müller, G. Optical Properties of Circulating Human Blood in the Wavelength Range 400–2500 Nm. J. Biomed. Opt. 1999, 4, 36.
- Buchs, A.; Slovik, Y.; Rapoport, M.; Rosenfeld, C.; Khanokh, B.; Nitzan, M. Right-Left Correlation of the Sympathetically Induced Fluctuations of Photoplethysmographic Signal in Diabetic and Non-Diabetic Subjects. Med. Biol. Eng. Comput. 2005, 43, 252–257.
- Allen, J.; Oates, C.P.; Lees, T.A.; Murray, A. Photoplethysmography Detection of Lower Limb Peripheral Arterial Occlusive Disease: A Comparison of Pulse Timing, Amplitude and Shape Characteristics. Physiol. Meas. 2005, 26, 811–821.
- Lochner, C.M.; Khan, Y.; Pierre, A.; Arias, A.C. All-Organic Optoelectronic Sensor for Pulse Oximetry. Nat. Commun. 2014, 5, 5745.
- Anderson, R.R.; Parrish, A. Optical Properties of Human Skin. J. Biomed. Opt. 2012, 17, 090901.
- Baranoski, G.V.G.; Chen, T.F.; Kimmel, B.W.; Miranda, E.; Yim, D. On the Noninvasive Optical Monitoring and Differentiation of Methemoglobinemia and Sulfhemoglobinemia. J. Biomed. Opt. 2012, 17, 15.
- Jo, E.-J.; Mun, H.; Kim, M.-G. Homogeneous Immunosensor Based on Luminescence Resonance Energy Transfer for Glycated Hemoglobin (HbA1c) Detection Using Upconversion Nanoparticles. Anal. Chem. 2016, 88, 2742–2746.
- Gharahbaghian, L.; Massoudian, B.; DiMassa, G. Methemoglobinemia and Sulfhemoglobinemia in Two Pediatric Patients after Ingestion of Hydroxylamine Sulfate. West. J. Emerg. Med. 2009, 10, 197–201.
- Yarynovska, I.H.; Bilyi, A.I. Absorption Spectra of Sulfhemoglobin Derivates of Human Blood; Coté, G.L., Priezzhev, A.V., Eds.; SPIE: San Jose, CA, USA, 2006; p. 60940G.
- Le, Q.-T.; Courter, D. Clinical Biomarkers for Hypoxia Targeting. Cancer Metastasis Rev. 2008, 27, 351–362.
- Wright, R.O.; Lewander, W.J.; Woolf, A.D. Methemoglobinemia: Etiology, Pharmacology, and Clinical Management. Ann. Emerg. Med. 1999, 34, 646–656.
- Scherer, G. Carboxyhemoglobin and Thiocyanate as Biomarkers of Exposure to Carbon Monoxide and Hydrogen Cyanide in Tobacco Smoke. Exp. Toxicol. Pathol. 2006, 58, 101–124.
- Faivre, B.; Menu, P.; Labrude, P.; Vigneron, C. Hemoglobin Autooxidation/Oxidation Mechanisms and Methemoglobin Prevention or Reduction Processes in the Bloodstream Literature Review and Outline of Autooxidation Reaction. Artif. Cells Blood Substit. Biotechnol. 1998, 26, 17–26.
- Suner, S.; Partridge, R.; Sucov, A.; Valente, J.; Chee, K.; Hughes, A.; Jay, G. Clinical Laboratory in Emergency Medicine. 10. Non-invasive pulse CO-oximetry screening in the emergency department identifies occult carbon monoxide toxicity. J. Emerg. Med. 2008, 34, 441–450.
- Chee, K.J.; Nilson, D.; Partridge, R.; Hughes, A.; Suner, S.; Sucov, A.; Jay, G. Finding Needles in a Haystack: A Case Series of Carbon Monoxide Poisoning Detected Using New Technology in the Emergency Department. Clin. Toxicol. 2008, 46, 461–469.
- Coulange, M.; Barthelemy, A.; Hug, F.; Thierry, A.L.; Haro, L.D. Reliability of New Pulse CO-Oximeter in Victims of Carbon Monoxide Poisoning. Undersea Hyperb. Med. 2008, 35, 107–111.
- Mansouri, A.; Lurie, A.A. Methemoglobinemia. Am. J. Hematol. 1993, 42, 7–12.
- Aravindhan, N.; Chisholm, D.G. Sulfhemoglobinemia Presenting as Pulse Oximetry Desaturation. Anesthesiology 2000, 93, 883–884.
- Malone Rubright, S.L.; Pearce, L.L.; Peterson, J. Environmental Toxicology of Hydrogen Sulfide. Nitric Oxide 2017, 71, 1–13.
- Docherty, S.; Zmuidinaite, R.; Coulson, J.; Besser, M.; Iles, R. The Diagnosis of Sulfated Hemoglobin (SulfHb) Secondary to Sulfur Dioxide Poisoning Using Matrix-Assisted Laser Desorption Time-of-Flight Mass Spectrometry (MALDI-ToF MS)—A Novel Approach to an Unusual Clinical Problem. Diagnostics 2020, 10, 94.
- Schiemsky, T.; Penders, J.; Kieffer, D. Failing Blood Gas Measurement Due to Methemoglobin Forming Hemoglobin Variants: A Case Report and Review of the Literature. Acta Clin. Belg. 2016, 71, 167–170.
- Camp, N.E. Methemoglobinemia. J. Emerg. Nurs. 2007, 33, 172–174.
- Petersson, J.; Åkesson, K.; Sundberg, F.; Särnblad, S. Translating Glycated Hemoglobin A1c into Time Spent in Glucose Target Range: A Multicenter Study. Pediatr. Diabetes 2019, 20, 339–344.
- Schnell, O.; Crocker, J.B.; Weng, J. Impact of HbA1c Testing at Point of Care on Diabetes Management. J. Diabetes Sci. Technol. 2017, 11, 611–617.
- O’Connor, P.J.; Desai, J.R.; Butler, J.C.; Kharbanda, E.O.; Sperl-Hillen, J.M. Current Status and Future Prospects for Electronic Point-of-Care Clinical Decision Support in Diabetes Care. Curr. Diab. Rep. 2013, 13, 172–176.
- John, A.S.; Davis, T.M.E.; Goodall, I.; Townsend, M.A.; Price, C.P. Nurse-Based Evaluation of Point-of-Care Assays for Glycated Haemoglobin. Clin. Chim. Acta 2006, 365, 257–263.
- Knaebel, J.; Irvin, B.R.; Xie, C.Z. Accuracy and Clinical Utility of a Point-of-Care HbA1c Testing Device. Postgrad. Med. 2013, 125, 8.
- Ma, X.-L.; Chen, Z.; Zhu, J.-J.; Shen, X.-X.; Wu, M.-Y.; Shi, L.-P.; Du, L.-Z.; Fu, J.-F.; Shu, Q. Management Strategies of Neonatal Jaundice during the Coronavirus Disease 2019 Outbreak. World J. Pediatr. 2020, 16, 247–250.
- Shapiro, S.M.; Riordan, S.M. Review of Bilirubin Neurotoxicity II: Preventing and Treating Acute Bilirubin Encephalopathy and Kernicterus Spectrum Disorders. Pediatr. Res. 2020, 87, 332–337.
- Bent, B. Investigating Sources of Inaccuracy in Wearable Optical Heart Rate Sensors. NPJ Digit. Med. 2020, 3, 18.
- Wei, P.; Yang, X.; Cao, Z.; Guo, X.-L.; Jiang, H.; Chen, Y.; Morikado, M.; Qiu, X.; Yu, D. Flexible and Stretchable Electronic Skin with High Durability and Shock Resistance via Embedded 3D Printing Technology for Human Activity Monitoring and Personal Healthcare. Adv. Mater. Technol. 2019, 4, 1900315.





