
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | George Chaldakov | + 1643 word(s) | 1643 | 2021-04-23 05:49:49 | | | |
| 2 | Nora Tang | Meta information modification | 1643 | 2021-04-25 08:33:11 | | |
Video Upload Options
Abstract: Studies over the past 30 years have revealed that adipose tissue is the major endocrine and paracrine organ of the human body. Arguably, adiopobiology has taken its reasonable place in studying obesity and related cardiometabolic diseases (CMDs), including Alzheimer’s disease (AD), which is viewed herein as a neurometabolic disorder. The pathogenesis and therapy of these diseases are multiplex at basic, clinical and translational levels. Our present goal is to describe new developments in cardiometabolic and neurometabolic adipobiology. Accordingly, we focus on adipose- and/or skeletal muscle-derived signaling proteins (adipsin, adiponectin, nerve growth factor, brain-derived neuroptrophic factor, neurotrophin-3, irisin, sirtuins, Klotho, neprilysin, follistatin-like protein-1, meteorin-like (metrnl), as well as growth differentiation factor 11) as examples of metabotrophic factors (MTFs) implicated in the pathogenesis and therapy of obesity and related CMDs. We argue that these pathologies are MTF-deficient diseases. In 1993 the “vascular hypothesis of AD” was published and in the present review we propose the “vasculometabolic hypothesis of AD.” We discuss how MTFs could bridge CMDs and neurodegenerative diseases, such as AD. Greater insights on how to manage the MTF network would provide benefits to the quality of human life.
1. Introduction
Life at both the local and systemic levels require nutritional, immune, neurotrophic and metabotrophic support. Any dysfunction or deficit in these support systems may lead to serious pathologies, such as cardiometabolic diseases (CMDs), which includes Alzheimer’s disease (AD). Today, CMDs are the number one cause of death globally with phenotypes that encompass atherosclerosis, hypertension, obesity, type 2 diabetes mellitus (T2DM), metabolic syndrome, metabolic-cognitive syndrome and, probably, AD. In fact, growing evidence supports a strong and most likely causal association between CMDs, as well as its risk factors, with incidences of cognitive decline and AD. Individuals with CMDs and subclinical cardiovascular diseases (CVDs) are at higher risk for dementia and AD. Several CMD manifestations, such as obesity, hypertension, high levels of LDL cholesterol, low levels of HDL cholesterol and T2DM are also risk factors for AD [1][2][3]. AD and CMDs are frequently associated. It became clear, through altered insulin/IGF-1 signaling, that impaired systemic insulin signaling and glucose metabolism play a direct role in normal synaptic activity and cognitive function [4]. However, it is necessary to understand the mechanistic effect of cardiometabolic risk factors and circulating mediators on AD in order to better comprehend its pathophysiology. In this short review, we will focus on adipose tissue- or skeletal muscle tissue-secreted metabotrophic factors (MTFs) as potential bridging mechanism between CMDs and AD.
2. Adipose Tissue
Adipose tissue (AT) is a very plastic and metabolically dynamic organ that is being constantly remodeled to compete with weight gain and weight loss. It is a cellular and extracellular matrix assembly composed of adipocytes, fibroblasts, immune cells and matrix components, and is also rich in sympathetic innervation and stem cells. In the body, there are two major types of AT present. However, clear-cut demarcations do not exist between white adipose tissue (WAT) and brown adipose tissue (BAT), as the infiltration of white adipocytes can be found in BAT (whitening of BAT, a pathogenic phenomenon) and brown adipocytes in WAT (browning of WAT, a sanogenic phenomenon), leading to the formation of brown-in-white AT (brite AT) as well as beige AT [5].
In humans, WAT is one of the major metabolic and secretory organs that synthesizes and releases a vast number of biologically active compounds that regulate metabolic and cognitive homeostasis (e.g., leptin, adiponectin, visfatin, nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), nitric oxide (NO), and hydrogen sulfide (H2S) [6][7]. WAT is partitioned into two large depots (visceral and subcutaneous), and many small depots associated with various organs, including the heart, blood vessels, major lymph nodes, pancreas, ovaries, bone marrow, eyes, prostate and mammary glands (Figure 1).
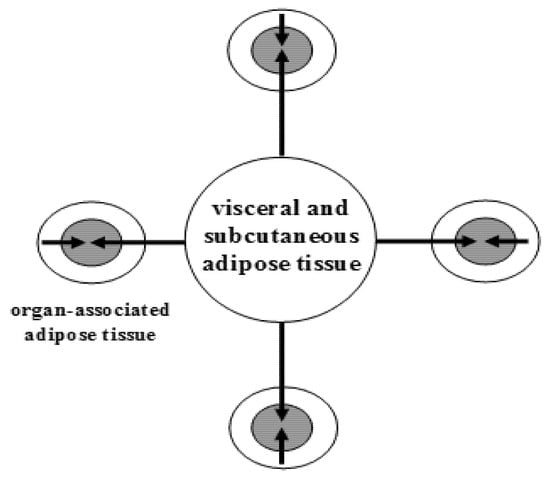
Figure 1. The topography of white adipose tissue. Schematic illustration of a large adipose depot (visceral and subcutaneous adipose tissue) and small adipose depots (organ-associated adipose tissue). Dual action of adipokines, via endocrine pathway (long arrows) and via paracrine pathways (short arrows), on adipose tissue-associated organs is depicted. From: [8] (reproduced with permission).
3. Brown, Brite and Beige Adipose Tissue
BAT can be visualized using 18F-fluorodeoxyglucose (FDG), an intravenously administered radioactive glucose analog, which is taken up but not metabolized (originally used to delineate metastatic cancers) and viewed using positron emission tomography (PET) scans (Table 1, for light microscopy view, see Figure 2).
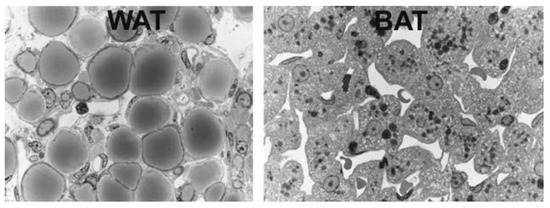
Figure 2. Light microscopy of murine white adipose tissue (WAT), left, and brown adipose tissue (BAT), right. White adipocytes are roundish with unilocular lipid droplets. Brown adipocytes are polyhedral with multilocular lipid droplets and a centrally positioned nucleus. From: [9] (reproduced with permission).
Table 1. Topography of brown adipose tissue. From: [10] (reproduced with permission).
| Visceral Brown Fat |
|---|
| Perivascular: aorta, common carotid artery, brachiocephalic artery, paracardial mediastinal fat, epicardial coronary artery and cardiac veins, internal mammary artery, and intercostal artery and vein |
| Periviscus: heart, trachea and major bronchi at lung hilum, esophagus, greater omentum, and transverse mesocolon |
| Around solid organs: thoracic paravertebral, pancreas, kidney, adrenal, liver, and hilum of spleen |
| Subcutaneous Brown Fat |
| Between anterior neck muscles and supraclavicular fossa |
| Under the clavicles |
| In the axilla |
| Anterior abdominal wall |
| Inguinal fossa |
4. Adipobiology: A Research Field Marked by Three Major Paradigm Shifts
One of biggest recent advances in studying obesity and related CMDs is associated with the “rediscovery” of a neglected tissue, the adipose tissue.
The discovery of leptin, an adipose-secreted protein hormone [11], marked a new period of revolutionary science (according to Thomas Kuhn’s 1962 book The Structure of Scientific Revolutions [12]) in studying AT. In effect, our understanding of this tissue has, at the epistemological level, undergone three major paradigm shifts in the last 27 years and it has taken the center stage when studying the pathobiology of a vast number of diseases.
The first paradigm shift revealed that AT, which had for a long time been considered as just an ordinary, inert mass of tissue that only passively stored lipids, was in fact the largest endocrine and paracrine organ of the human body (Table 2, Figure 3).
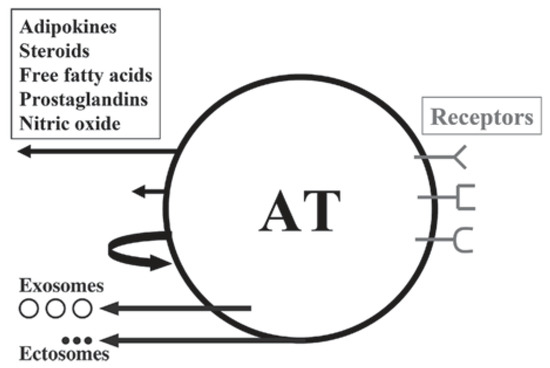
Figure 3. Schematic illustration of white adipose tissue (AT) as a multicrine organ. AT consists of adipocytes, fibroblasts, mast cells, macrophages and other immune cells.The arrows, left from up-to-down, indicate endocrine, paracrine and autocrine pathways; the other two arrows show the extracellular vesicles: exosomes and ectosomes. Depicted on the right are the adipose cell receptors for various ligands. From: [13] (reproduced with permission).
Table 2. The paradigm shifts in adipobiology.
| From |
| Adipose tissue is involved in lipid and energy storage in relation to obesity |
| To |
| Adipose tissue is an endocrine, paracrine, steroidogenic and immune organIt is a source of and target for inflammatory mediatorsIt produces all components of the rennin-angiotensin systemIt is thus involved in numerous diseases beyond obesity |
The second paradigm shift was derived from the studies of Jimmy Bell, head of the Molecular Imaging Group at Hammersmith Hospital in London, UK, who, together with his colleagues, scanned nearly 800 people via magnetic resonance imaging (MRI) to obtain a map of WAT. They demonstrated that as many as 45 percent of women and nearly 60 percent of men who had normal body mass index (BMI) scores (20–25 kg/m2) and appeared thin on the outside (TO) actually had excessive levels of internal AT, i.e., they were fat inside (FI). Therefore, they had the TOFI (thin outside, fat inside) phenotype of body fat, which may be considered an “invisible” (hidden) expression of Homo obesus (Table 3). Of note, such an adipotopography can be visualized by using current imaging technologies, such as echography, computed tomography, MRI and proton magnetic resonance spectroscopy. These results showed that “being thin does not automatically mean you are not fat,” quoting Dr. Bell. The concept of TOFI holds that small adipose depots, when enlarged and activated (by inflammatory, over-nutritional or other stimuli), may, via a paracrine way, exert disease-promoting actions over AT-associated organ(s) (see Figure 1). Thus, the traditional diagnostic significance of BMI, as well as other anthropometric criteria (waist and hip circumference alike), should be re-evaluated in obesity and its related CMDs. Importantly, dieting may be enough to keep one being thin outside, whereas physical activity prevents the accumulation of internal fat. Thus, the TOFI phenotype is a Trojan horse, a pathological phenomenon, whereas TOTI (thin outside, thin inside) is a healthy adipose phenotype. Briefly, slim or obese, a person should get their AT mapped to evaluate their health status.
Table 3. Adipotopography: localization of adipose tissue in the human body.
| Phenotype | Quality | |
|---|---|---|
| TOFI | ** | Thin Outside, Fat Inside |
| TOTI | ***** | Thin Outside, Thin Inside |
| FOFI | * | Fat Outside, Fat Inside |
| FOTI | *** | Fat Outside, Thin Inside |
A higher number of asterisks means better quality of health.
The third paradigm shift featured the increasing significance of BAT in both health and disease status. Of note, BAT, with its characteristic small multilocular adipocytes and central nuclei (as opposed to larger unilocular white adipocytes with nuclei at their peripheral rims), as well as granular cytoplasmic appearance on haematoxylin and eosin (H&E) staining, was demonstrated as a component of epicardial AT (EAT) surrounding coronary arteries. Uncoupling protein-1 (UCP-1) is unique to BAT as it uncouples mitochondrial oxidative phosphorylation, thus producing heat instead of ATP by non-shivering thermogenesis, and mRNAUCP-1 is highly expressed in human EAT compared with subcutaneous AT (Figure 4). The amount of BAT identified histologically at autopsy decreases with age and changes its morphology, which is due to the conversion of multilocular brown to unilocular white adipocytes, a process termed adipocyte transdifferentiation [10][14][15][16][17].
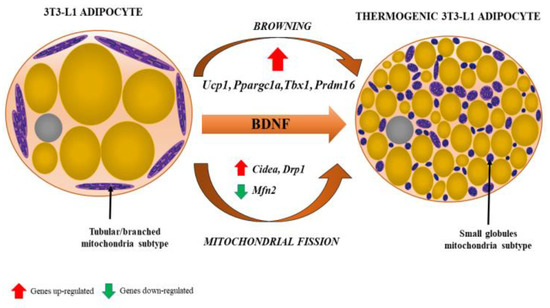
Figure 4. Browning of white adipocyte induced by brain-derived neurotrophic factor (BDNF). The phenotypic change from fat storing to thermogenic adipocytes is recognized by the presence of multilocular lipid droplets and fissed mitochondria that tend to surround lipid droplets, maximizing the efficiency of fatty acid release for thermogenesis. Various genes (their up- and downregulation) involved in the process of browning and mitochondrial fission are indicated by red and green arrows, respectively. From: [18] (reproduced with permission).
References
- Stampfer, M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006, 260, 211–223.
- Tini, G.; Scagliola, R.; Monacelli, F.; La Malfa, G.; Porto, I.; Brunelli, C.; Rosa, G.M. Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol. Res. Pr. 2020, 2020, 1–10.
- Santos, C.Y.; Snyder, P.J.; Wu, W.C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimers Dement (Amst.) 2017, 7, 69–87.
- Bhat, N.R. Linking cardiometabolic disorders to sporadic Alzheimer’s disease: A perspective on potential mechanisms and mediators. J. Neurochem. 2010, 115, 551–562.
- Blirando, K. Epigenetic Regulation of Adipocytes Phenotype: Implication for Perivascular Adipose Tissue Contribution to Cardiometabolic Diseases. Adipobiology 2017, 8, 19–34.
- Ottaviani, E.; Malagoli, D.; Franceschi, C. The evolution of the adipose tissue: A neglected enigma. Gen. Comp. Endocrinol. 2011, 174, 1–4.
- Coelho, M.; Oliveira, T.; Fernandes, R. State of the art paper Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200.
- Chaldakov, G.; Stankulov, I.; Hristova, M.; Ghenev, P. Adipobiology of Disease: Adipokines and Adipokine-Targeted Pharmacology. Curr. Pharm. Des. 2003, 9, 1023–1031.
- Cinti, S.; Vettor, R. The Adipose Organ, in Adipose Tissue and Infammation; Awad, A.B., Bradford, P.G., Eds.; Taylor and Francis Group: Abingdon, UK, 2010; pp. 1–21.
- Sacks, H.; Symonds, M.E. Anatomical Locations of Human Brown Adipose Tissue: Functional Relevance and Implications in Obesity and Type 2 Diabetes. Diabetes 2013, 62, 1783–1790.
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nat. Cell Biol. 1994, 372, 425–432.
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962; p. 172.
- Chaldakov, G.N.; Fiore, M. Human body as a multicrine gland. Adipobiology 2010, 2, 73.
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling Protein-1 and Related Messenger Ribonucleic Acids in Human Epicardial and Other Adipose Tissues: Epicardial Fat Functioning as Brown Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615.
- Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Di Gioia, C.R.T. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine 2005, 29, 251–255.
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinol. 2013, 154, 2992–3000.
- Frühbeck, G.; Becerril, S.; Sáinz, N.; Garrastachu, P.; García-Velloso, M.J. BAT: A new target for human obesity? Trends Pharm. Sci. 2009, 30, 387–396.
- Colitti, M.; Montanari, T. Brain-derived neurotrophic factor modulates mitochondrial dynamics and thermogenic phenotype on 3T3-L1 adipocytes. Tissue Cell 2020, 66, 101388.





