
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hao Wei | + 2278 word(s) | 2278 | 2021-04-21 05:31:47 | | | |
| 2 | Catherine Yang | Meta information modification | 2278 | 2021-04-21 08:59:17 | | |
Video Upload Options
Transcytosis of polymeric IgA and IgM from the basolateral surface to the apical side of the epithelium and subsequent secretion into mucosal fluids are mediated by the polymeric immunoglobulin receptor (pIgR).
1. Introduction
The mucosa is an extensive layer of protection for the respiratory, gastrointestinal and urogenital tracts and other secretory glands such as the mammary glands. Separating the internal and external environments, the mucosa is constantly exposed to a wide variety of microorganisms and extrinsic molecules including bacteria, viruses, fungi and toxins. In human beings, the total surface area of the epithelial barrier is about 400 m2 [1]. Protection of the mucosal epithelium is provided by a vast network of proteins, molecules and cells, which are collectively termed as mucosal immunity [2].
Among a myriad of effectors in mucosal immunity, polymeric immunoglobulins IgA and IgM are of particular importance. Immunoglobulin (Ig) is the antigen-recognition molecule derived from B cells, while antibodies are secreted versions of immunoglobulin. An antibody is formed by two identical pairs of heavy and light chains joined together by disulfide bonds. There are five main classes of antibodies: IgA, IgD, IgE, IgG and IgM, which can be distinguished by their heavy chains. Only IgA and IgM can polymerize. The formation of IgA dimers and IgM pentamers is mediated by the joining chain (J chain), while IgM hexamers can be formed in the absence of J chain. Polymerized IgA, and to a lesser extent IgM, protect the mucosal surfaces from infection. In addition, small amounts of IgD are secreted into the mucosal surfaces of oral, nasopharyngeal and lachrymal areas [3]. Daily production of IgA in humans reaches 40 to 60 mg per kg of bodyweight, which is higher than that of all of the other immunoglobulin isotypes combined [4].
Delivery of antibodies to the mucosal surfaces and secretion in milk requires transport across epithelial layers. The polymeric immunoglobulin receptor (pIgR) recognizes the J chain region of polymerized IgA and IgM and transports the antibodies across the epithelial cell. Following proteolytic cleavage of pIgR, polymerized Ig is secreted and released into the luminal space. Since its function was first discovered in the 1980s, J chain, along with the molecular details of Ig polymerization, has largely been overlooked in the field of immunological research. It was only in the last decade when researchers started to take notice of marginal zone B and B1 cell-specific protein (MZB1) [5][6][7], a novel regulator of J chain-mediated Ig polymerization that precedes pIgR-mediated transcytosis.
In this review, we present an overview of how pIgR mediates transcytosis and the consequences of pIgR deficiency. We also expand on the molecular details of J chain binding to Ig polymers and recognition by pIgR based on results from recently published structural studies. We further highlight the proposed roles of MZB1 in the polymerization of IgA and IgM and briefly summarize the latest reports that have implicated MZB1 in human diseases.
2. Polymeric Immunoglobulin Receptor (pIgR)
2.1. Structure and Expression of pIgR
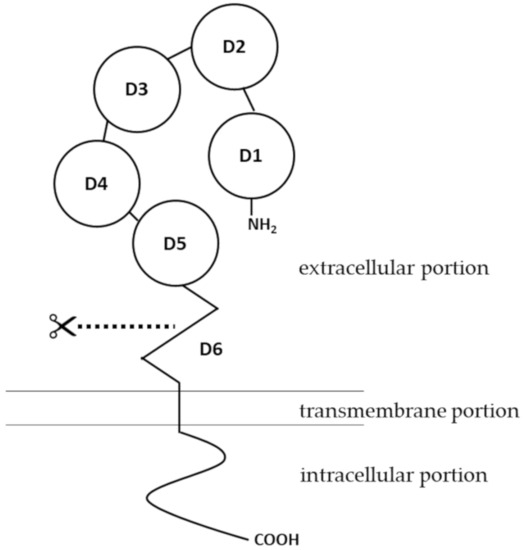
Human pIgR is a type I membrane protein with high glycosylation levels. It has a molecular weight of about 83 kDa [8]. Structural insights of pIgR were first obtained when the cDNA encoding the receptor was cloned and sequenced. As a typical type I transmembrane protein, the structure of pIgR can be categorized into three portions: an extracellular portion (620 amino acids, a.a.), a transmembrane portion (23 a.a.), and a cytoplasmic portion (103 a.a.) [9]. The extracellular portion of pIgR contains six domains [10]. Domains 1 to 5 are five tandem immunoglobulin-like domains that are involved in binding to IgA dimers or IgM pentamers. The cysteine residues in the extracellular portion, from which disulfide bonds may form, are conserved among human, mouse, rat and a few other mammalian species [11]. Domain 6, which is closest to the transmembrane portion, contains a highly conserved proteolytic cleavage site [12]. Compared to the extracellular portion, the intracellular portion of pIgR is more directly involved in intracellular sorting, endocytosis and transcytosis [13] (Figure 1). For structures of pIgR in vertebrates other than mammals, there have been several excellent articles covering the expression, structure and functions of pIgR in amphibians [14][15], fish [16][17][18], birds [19] and reptiles [20][21].
The human pIgR gene (NCBI gene ID: 5284) is located on the q32.1 region of chromosome 1. With a total of 11 exons, the human pIgR gene spans about 19 kb [22]. PIgR is expressed on epithelial cells of the gastrointestinal tract, respiratory tract and the skin, as well as on the glandular epithelial cells of the breast and liver [1][23][24][25]. A variety of immunological factors have been identified to upregulate expression of pIgR, including interleukin-1 (IL-1), interleukin-17 (IL-17), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) [26][27][28][29]. Early studies also pointed out that interleukin-4 (IL-4), when acting in synergy with IFN-γ, can upregulate the expression of pIgR [30][31]. The effects of these cytokines on pIgR expression are mediated by transcription factors such as nuclear factor-κ light chain enhancer of activated B cells (NF-κB) and interferon regulatory factor-1 (IRF-1) [32][33], binding sites of which are located in the 5′-flanking region and intron 1 of pIgR gene [34].
From a functional perspective, upregulation of pIgR expression levels has been associated with bacterial, viral and chlamydial infections, where the immune system is activated and antibodies are produced and trafficked to fight off the pathogens [35][36][37][38][39]. Some pathogens have evolved strategies to utilize or suppress pIgR expression for the benefit of their infection. Streptococcus pneumoniae, Candida albicans and Epstein-Barr virus (EBV) can bind to pIgR, which aids their attachment to epithelial cells [40][41][42]. Escherichia coli and simian immunodeficiency virus (SIV) have been reported to downregulate pIgR expression, thereby evading the mucosal immune response [43][44][45]. The commensal microbiome also modulates pIgR expression. It was first reported when colonization of germ-free mice with a commensal bacterial strain Bacteroides thetaiotaomicron stimulated pIgR expression [46]. Later it was found that pIgR expression could be stimulated in vitro in HT-29 cells, a human intestinal epithelial cell line, when these cells were co-cultured with commensal bacterial strains from the family Enterobacteriaceae [33][47]. It was then proposed that microbial-associated molecular patterns (MAMPs) secreted from the commensal microbiome stimulate epithelial Toll-like receptors (TLRs), which triggers transcription of the pIgR gene by activating MyD88-dependent signaling pathways [23][25].
Interestingly, studies in mice have linked increased pIgR expression levels in submandibular glands to body exercise and heat acclimatization. Both studies have attributed this phenomenon to mild physiological stress, which might trigger an immune response [48][49]. In the past decade, modulation of pIgR expression, either elevated or reduced, has been increasingly reported in patients of cancer and metastasis, especially in hepatocellular and pancreatic cases [50][51][52][53][54][55][56]. It is possible that regulation of pIgR expression extends beyond the immune response. Further studies are required to elucidate the underlying mechanisms linking pIgR expression to cancer.
2.2. Functions of pIgR in Transcytosis of IgA and IgM
Epithelial cells form a layer of protection and insulation between the external and internal environments, defining the lumen of secretory organs and mucosal surfaces. These epithelial cells are polarized, which means the basolateral and apical membranes are of different compositions and functions. In order to move across the epithelial barrier, large solutes such as immunoglobulins must undergo transcytosis or the transcellular endosomal pathway [57]. The major function of pIgR, as its name suggests, is to bind polymeric immunoglobulins, thereby facilitating their transport across the epithelium [9].
Expressed on the basolateral surface of epithelial cells, pIgR, via its extracellular portion, binds to polymeric forms of IgA or IgM that are produced by local highly-differentiated plasma cells. Unlike other classes of antibodies (IgD, IgE, IgG), monomers of IgA and IgM can polymerize. Primarily, IgA forms dimers [58], whereas IgM forms pentamers and sometimes polymers of even higher orders [59]. This process of immunoglobulin polymerization is mediated by a special protein called joining chain (J chain) that binds to heavy chains of IgA and IgM through disulfide bonds at their C-terminal tailpieces [60]. Details of this process are discussed later in this article.
Transcytosis of IgA dimers and IgM pentamers is initiated once they bind to pIgR, and the Ig-pIgR complex is internalized into the cytoplasm of the epithelial cell via clathrin-mediated endocytosis, as was shown in an early in vitro study where pIgR was extrinsically expressed in MDCK cells [61]. Internalization of pIgR can occur even in the absence of its ligand [61]. The internalized Ig-pIgR complex travels along the endosomal pathway. The complex is first trafficked to the basolateral early endosome (EE), followed by transport to the common endosome (CE), before being sorted to the apical recycling endosome (ARE) that is localized beneath the apical epithelial membrane [62]. Sorting and targeting of pIgR throughout the endosomal transcytosis pathway is mediated by a signal of 17 membrane-proximal a.a. residues at the intracellular portion of the pIgR structure [63]. At the apical cell surface, the extracellular portion of pIgR, which binds to polymerized Ig molecules, undergoes endo-proteolytic cleavage at domain 6. The identity of the enzyme responsible for this cleavage remains obscure. The cleaved extracellular portion is referred to as the secretory component (SC). IgA dimers or IgM pentamers, which are originally bound by the SC, are released from the remaining transmembrane and intracellular portions of pIgR. The free, unbound SC-Ig polymer complexes are released as secretory Ig and diffuse into the mucus, where they act as an immunological barrier against infections by denying pathogens access to the epithelium [13][57][64]. This function of secretory Ig has been specifically termed as “immune exclusion” [65].
Apart from facilitating transport of the Ig polymers across the epithelium and their release into the mucus, SC, the cleaved extracellular portion of pIgR, has other critical functions. SC increases the stability of dimerized IgA, possibly by masking proteolytic cleavage sites within the IgA molecule, delaying the degradation of IgA by the host and bacterial enzymes in the mucus [66]. As SC is derived from pIgR, it is enriched in modifications such as N-glycosylation, so SC can help localize the secretory Ig complex in the mucus layer [67][68]. Even in the absence of Ig polymers, SC itself may bind and neutralize bacteria and toxins via its glycan moieties [1][69]. Cryo-EM structure of SC complexed with an IgA dimer showed that N65, N72, N168, N403, N451 and N481 are spatially away from any SC-IgA interaction surfaces, so glycosylation at these asparagine residues could be involved in the host and pathogen binding [70]. Functions of SC may be especially important for immunity in breast-fed infants, as the abundance of SC in both its free and Ig-bound forms has long been recorded in maternal milk [71][72][73].
2.3. Consequences of pIgR Deficiency
Studies on genetic knockout mice were pivotal in expanding our understanding of the functions of pIgR. The earliest studies of pIgR−/− mice were conducted in 1999. Epithelial transport of IgA was significantly reduced, although not ablated, in bile, feces and intestinal contents in pIgR−/− mice. Meanwhile, serum IgA levels were markedly increased in pIgR−/− mice [74][75]. These results demonstrated the essential roles of pIgR in transcytosis of IgA into the intestinal lumen, yet a small amount of IgA may be secreted via other pathways. Since the route for IgA transcytosis into the intestinal lumen is blocked in these genetically-deficient mice, IgA only has access to the blood. The increase in serum IgA levels might be further accounted for by increased numbers of plasma cells that secrete IgA, which was reported in both the lamina propria and the Peyer’s patch of pIgR−/− mice [76][77]. In addition, a lack of secretory IgA was observed in the pulmonary airways of pIgR−/− mice that developed signs similar to chronic obstructive pulmonary disease (COPD) [78][79][80]. These signs, which were caused by local infection and inflammation, were exacerbated by neutrophils of increased counts and activities [80].
Microbiota in the gut is altered as a result of pIgR deficiency. This was directly confirmed by comparing the intestinal microbiota between pIgR−/− and WT mice using 16s rRNA analysis [81]. Intestinal integrity was mildly compromised in pIgR−/− mice, which might be attributed to a slightly more severe bacterial insult in pIgR−/− mice [82][83]. This may explain the results from an early study, which showed that pIgR−/− mice were profoundly more sensitive to infection with Salmonella typhimurium via the fecal-oral route, and that bacteria excreted from pIgR−/− mice after S. typhimurium infection were more contagious for other mice [84], as the composition of the excreted bacterial population may differ between pIgR−/− and WT mice.
It is not surprising that pIgR deficiency has been extensively linked to inflammatory diseases in the gut, given the central role of secretory IgA in suppressing inflammation and maintaining homeostasis in the gut [85][86]. Dextran sulfate sodium (DSS)-induced colitis in mice is the most widely used animal model to study mechanisms of inflammatory bowel diseases (IBD) in humans, which mainly comprise ulcerative colitis and Crohn’s disease [87]. Morbidity and mortality of DSS-induced colitis were significantly enhanced in pIgR−/− mice [81]. Similarly, reduced levels of pIgR and secretory IgA in the gut, as a result of genetic deficiency in IL-17, were correlated with increased weight loss and more severe intestinal inflammation in mice following DSS administration [88]. Lower mRNA levels of pIgR in colonic mucosa have been proposed as a potential biomarker for the clinical diagnosis of IBD [89]. More recently, several cutting-edge studies compared whole-genome sequencing data from the colonic tissues of human IBD patients to those of healthy donors. Among these IBD patients, pIgR has been discovered as one of the most commonly shared sites of somatic mutations that are correlated with impaired protein functions, and these somatic mutations tend to accumulate with age [90][91][92]. Taken together, these data unequivocally illustrate the necessity of pIgR in transcytosis of polymeric immunoglobulins and thus in protection against inflammation.
References
- Kaetzel, C.S. The Polymeric Immunoglobulin Receptor: Bridging Innate and Adaptive Immune Responses at Mucosal Surfaces. Immunol. Rev. 2005, 206, 83–99.
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal Immunity to Pathogenic Intestinal Bacteria. Nat. Rev. Immunol. 2016, 16, 135–148.
- Gutzeit, C.; Chen, K.; Cerutti, A. The Enigmatic Function of IgD: Some Answers at Last. Eur. J. Immunol. 2018, 48, 1101–1113.
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254.
- Van Anken, E.; Pena, F.; Hafkemeijer, N.; Christis, C.; Romijn, E.P.; Grauschopf, U.; Oorschot, V.M.J.; Pertel, T.; Engels, S.; Ora, A.; et al. Efficient IgM Assembly and Secretion Require the Plasma Cell Induced Endoplasmic Reticulum Protein PERp1. Proc. Natl. Acad. Sci. USA 2009, 106, 17019–17024.
- Shimizu, Y.; Meunier, L.; Hendershot, L.M. PERp1 Is Significantly Up-Regulated during Plasma Cell Differentiation and Contributes to the Oxidative Folding of Immunoglobulin. Proc. Natl. Acad. Sci. USA 2009, 106, 17013–17018.
- Flach, H.; Rosenbaum, M.; Duchniewicz, M.; Kim, S.; Zhang, S.L.; Cahalan, M.D.; Mittler, G.; Grosschedl, R. Mzb1 Protein Regulates Calcium Homeostasis, Antibody Secretion, and Integrin Activation in Innate-like B Cells. Immunity 2010, 33, 723–735.
- Zhou, M.; Liu, C.; Cao, G.; Gao, H.; Zhang, Z. Expression of Polymeric Immunoglobulin Receptor and Its Biological Function in Endometrial Adenocarcinoma. J. Cancer Res. Ther. 2019, 15, 420–425.
- Mostov, K.E.; Friedlander, M.; Blobel, G. The Receptor for Transepithelial Transport of IgA and IgM Contains Multiple Immunoglobulin-like Domains. Nature 1984, 308, 37–43.
- Stadtmueller, B.M.; Huey-Tubman, K.E.; López, C.J.; Yang, Z.; Hubbell, W.L.; Bjorkman, P.J. The Structure and Dynamics of Secretory Component and Its Interactions with Polymeric Immunoglobulins. eLife 2016, 5.
- Piskurich, J.F.; Blanchard, M.H.; Youngman, K.R.; France, J.A.; Kaetzel, C.S. Molecular Cloning of the Mouse Polymeric Ig Receptor. Functional Regions of the Molecule Are Conserved among Five Mammalian Species. J. Immunol. Baltim. Md 1950 1995, 154, 1735–1747.
- He, T.; Siwy, J.; Metzger, J.; Mullen, W.; Mischak, H.; Schanstra, J.P.; Zürbig, P.; Jankowski, V. Associations of Urinary Polymeric Immunoglobulin Receptor Peptides in the Context of Cardio-Renal Syndrome. Sci. Rep. 2020, 10, 8291.
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237.
- Braathen, R.; Hohman, V.S.; Brandtzaeg, P.; Johansen, F.-E. Secretory Antibody Formation: Conserved Binding Interactions between J Chain and Polymeric Ig Receptor from Humans and Amphibians. J. Immunol. 2007, 178, 1589–1597.
- Wcisel, D.J.; Yoder, J.A. The Confounding Complexity of Innate Immune Receptors within and between Teleost Species. Fish Shellfish Immunol. 2016, 53, 24–34.
- Wang, L.; Zhang, J.; Kong, X.; Pei, C.; Zhao, X.; Li, L. Molecular Characterization of Polymeric Immunoglobulin Receptor and Expression Response to Aeromonas Hydrophila Challenge in Carassius Auratus. Fish Shellfish Immunol. 2017, 70, 372–380.
- Kong, X.; Wang, L.; Pei, C.; Zhang, J.; Zhao, X.; Li, L. Comparison of Polymeric Immunoglobulin Receptor between Fish and Mammals. Vet. Immunol. Immunopathol. 2018, 202, 63–69.
- Parra, D.; Korytář, T.; Takizawa, F.; Sunyer, J.O. B Cells and Their Role in the Teleost Gut. Dev. Comp. Immunol. 2016, 64, 150–166.
- Stadtmueller, B.M.; Yang, Z.; Huey-Tubman, K.E.; Roberts-Mataric, H.; Hubbell, W.L.; Bjorkman, P.J. Biophysical and Biochemical Characterization of Avian Secretory Component Provides Structural Insights into the Evolution of the Polymeric Ig Receptor. J. Immunol. Baltim. 2016, 197, 1408–1414.
- Akula, S.; Mohammadamin, S.; Hellman, L. Fc Receptors for Immunoglobulins and Their Appearance during Vertebrate Evolution. PLoS ONE 2014, 9, e96903.
- Akula, S.; Hellman, L. The Appearance and Diversification of Receptors for IgM During Vertebrate Evolution. Curr. Top. Microbiol. Immunol. 2017, 408, 1–23.
- Krajci, P.; Kvale, D.; Taskén, K.; Brandtzaeg, P. Molecular Cloning and Exon-Intron Mapping of the Gene Encoding Human Transmembrane Secretory Component (the Poly-Ig Receptor). Eur. J. Immunol. 1992, 22, 2309–2315.
- Kaetzel, C.S. Cooperativity among Secretory IgA, the Polymeric Immunoglobulin Receptor, and the Gut Microbiota Promotes Host-Microbial Mutualism. Immunol. Lett. 2014, 162, 10–21.
- Hamuro, K.; Suetake, H.; Saha, N.R.; Kikuchi, K.; Suzuki, Y. A Teleost Polymeric Ig Receptor Exhibiting Two Ig-like Domains Transports Tetrameric IgM into the Skin. J. Immunol. 2007, 178, 5682–5689.
- Johansen, F.-E.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor and IgA Transport: New Advances in Environmental Factors That Stimulate PIgR Expression and Its Role in Mucosal Immunity. Mucosal Immunol. 2011, 4, 598–602.
- Hayashi, M.; Takenouchi, N.; Asano, M.; Kato, M.; Tsurumachi, T.; Saito, T.; Moro, I. The Polymeric Immunoglobulin Receptor (Secretory Component) in a Human Intestinal Epithelial Cell Line Is up-Regulated by Interleukin-1. Immunology 1997, 92, 220–225.
- Kumar, P.; Monin, L.; Castillo, P.; Elsegeiny, W.; Horne, W.; Eddens, T.; Vikram, A.; Good, M.; Schoenborn, A.A.; Bibby, K.; et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 2016, 44, 659–671.
- Moon, C.; VanDussen, K.L.; Miyoshi, H.; Stappenbeck, T.S. Development of a Primary Mouse Intestinal Epithelial Cell Monolayer Culture System to Evaluate Factors That Modulate IgA Transcytosis. Mucosal Immunol. 2014, 7, 818–828.
- Piskurich, J.F.; France, J.A.; Tamer, C.M.; Willmer, C.A.; Kaetzel, C.S.; Kaetzel, D.M. Interferon-Gamma Induces Polymeric Immunoglobulin Receptor MRNA in Human Intestinal Epithelial Cells by a Protein Synthesis Dependent Mechanism. Mol. Immunol. 1993, 30, 413–421.
- Sarkar, J.; Gangopadhyay, N.N.; Moldoveanu, Z.; Mestecky, J.; Stephensen, C.B. Vitamin A Is Required for Regulation of Polymeric Immunoglobulin Receptor (PIgR) Expression by Interleukin-4 and Interferon-Gamma in a Human Intestinal Epithelial Cell Line. J. Nutr. 1998, 128, 1063–1069.
- Ackermann, L.W.; Wollenweber, L.A.; Denning, G.M. IL-4 and IFN-Gamma Increase Steady State Levels of Polymeric Ig Receptor MRNA in Human Airway and Intestinal Epithelial Cells. J. Immunol. Baltim. Md 1950 1999, 162, 5112–5118.
- Blanch, V.J.; Piskurich, J.F.; Kaetzel, C.S. Cutting Edge: Coordinate Regulation of IFN Regulatory Factor-1 and the Polymeric Ig Receptor by Proinflammatory Cytokines. J. Immunol. Baltim. Md 1950 1999, 162, 1232–1235.
- Bruno, M.E.C.; Frantz, A.L.; Rogier, E.W.; Johansen, F.-E.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor by the Classical and Alternative NF-ΚB Pathways in Intestinal Epithelial Cells. Mucosal Immunol. 2011, 4, 468–478.
- Kushiro, A.; Sato, T. Polymeric Immunoglobulin Receptor Gene of Mouse: Sequence, Structure and Chromosomal Location. Gene 1997, 204, 277–282.
- Pal, K.; Kaetzel, C.S.; Brundage, K.; Cunningham, C.A.; Cuff, C.F. Regulation of Polymeric Immunoglobulin Receptor Expression by Reovirus. J. Gen. Virol. 2005, 86, 2347–2357.
- Deng, L.; Xu, H.; Liu, P.; Wu, S.; Shi, Y.; Lv, Y.; Chen, X. Prolonged Exposure to High Humidity and High Temperature Environment Can Aggravate Influenza Virus Infection through Intestinal Flora and Nod/RIP2/NF-ΚB Signaling Pathway. Vet. Microbiol. 2020, 251, 108896.
- Armitage, C.W.; O’Meara, C.P.; Beagley, K.W. Chlamydial Infection Enhances Expression of the Polymeric Immunoglobulin Receptor (PIgR) and Transcytosis of IgA. Am. J. Reprod. Immunol. 2017, 77.
- Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sánchez-Gómez, M.C.; Campos-Rodríguez, R. Modulation by Bovine Lactoferrin of Parameters Associated with the IgA Response in the Proximal and Distal Small Intestine of BALB/c Mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73.
- Godínez-Victoria, M.; Campos-Rodriguez, R.; Rivera-Aguilar, V.; Lara-Padilla, E.; Pacheco-Yepez, J.; Jarillo-Luna, R.A.; Drago-Serrano, M.E. Intermittent Fasting Promotes Bacterial Clearance and Intestinal IgA Production in Salmonella Typhimurium-Infected Mice. Scand. J. Immunol. 2014, 79, 315–324.
- Zhang, J.R.; Mostov, K.E.; Lamm, M.E.; Nanno, M.; Shimida, S.; Ohwaki, M.; Tuomanen, E. The Polymeric Immunoglobulin Receptor Translocates Pneumococci across Human Nasopharyngeal Epithelial Cells. Cell 2000, 102, 827–837.
- Van der Wielen, P.A.; Holmes, A.R.; Cannon, R.D. Secretory Component Mediates Candida Albicans Binding to Epithelial Cells. Oral Dis. 2016, 22, 69–74.
- Sixbey, J.W.; Yao, Q.Y. Immunoglobulin A-Induced Shift of Epstein-Barr Virus Tissue Tropism. Science 1992, 255, 1578–1580.
- Liu, G.; Ren, W.; Fang, J.; Hu, C.-A.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-Arginine Protect against Enterotoxigenic Escherichia Coli Infection via Intestinal Innate Immunity in Mice. Amino Acids 2017, 49, 1945–1954.
- Li, D.; Wang, F.-J.; Yu, L.; Yao, W.-R.; Cui, Y.-F.; Yang, G.-B. Expression of PIgR in the Tracheal Mucosa of SHIV/SIV-Infected Rhesus Macaques. Zool. Res. 2017, 38, 44–48.
- Wang, Y.; Yang, G.B. Alteration of Polymeric Immunoglobulin Receptor and Neonatal Fc Receptor Expression in the Gut Mucosa of Immunodeficiency Virus-Infected Rhesus Macaques. Scand. J. Immunol. 2016, 83, 235–243.
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science 2001, 291, 881–884.
- Bruno, M.E.C.; Rogier, E.W.; Frantz, A.L.; Stefka, A.T.; Thompson, S.N.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor in Intestinal Epithelial Cells by Enterobacteriaceae: Implications for Mucosal Homeostasis. Immunol. Invest. 2010, 39, 356–382.
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary Exercise Increases IgA Concentration and Polymeric Ig Receptor Expression in the Rat Submandibular Gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496.
- Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats. Int. J. Mol. Sci. 2020, 21, 815.
- Ohkuma, R.; Yada, E.; Ishikawa, S.; Komura, D.; Kubota, Y.; Hamada, K.; Horiike, A.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; et al. High Expression Levels of Polymeric Immunoglobulin Receptor Are Correlated with Chemoresistance and Poor Prognosis in Pancreatic Cancer. Oncol. Rep. 2020, 44, 252–262.
- Yue, X.; Ai, J.; Xu, Y.; Chen, Y.; Huang, M.; Yang, X.; Hu, B.; Zhang, H.; He, C.; Yang, X.; et al. Polymeric Immunoglobulin Receptor Promotes Tumor Growth in Hepatocellular Carcinoma. Hepatology 2017, 65, 1948–1962.
- Ai, J.; Tang, Q.; Wu, Y.; Xu, Y.; Feng, T.; Zhou, R.; Chen, Y.; Gao, X.; Zhu, Q.; Yue, X.; et al. The Role of Polymeric Immunoglobulin Receptor in Inflammation-Induced Tumor Metastasis of Human Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2011, 103, 1696–1712.
- Qi, X.; Li, X.; Sun, X. Reduced Expression of Polymeric Immunoglobulin Receptor (PIgR) in Nasopharyngeal Carcinoma and Its Correlation with Prognosis. Tumour Biol. 2016, 37, 11099–11104.
- Arumugam, P.; Bhattacharya, S.; Chin-Aleong, J.; Capasso, M.; Kocher, H.M. Expression of Polymeric Immunoglobulin Receptor and Stromal Activity in Pancreatic Ductal Adenocarcinoma. Pancreatology 2017, 17, 295–302.
- Liu, F.; Ye, P.; Bi, T.; Teng, L.; Xiang, C.; Wang, H.; Li, Y.; Jin, K.; Mou, X. COLORECTAL Polymeric Immunoglobulin Receptor Expression Is Correlated with Hepatic Metastasis and Poor Prognosis in Colon Carcinoma Patients with Hepatic Metastasis. Hepatogastroenterology 2014, 61, 652–659.
- Dewdney, B.; Hebbard, L. A Novel Role for Polymeric Immunoglobulin Receptor in Tumour Development: Beyond Mucosal Immunity and into Hepatic Cancer Cell Transformation. Hepatobiliary Surg. Nutr. 2018, 7, 52–55.
- Garcia-Castillo, M.D.; Chinnapen, D.J.-F.; Lencer, W.I. Membrane Transport across Polarized Epithelia. Cold Spring Harb. Perspect. Biol. 2017, 9.
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057.
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53.
- Castro, C.D.; Flajnik, M.F. Putting J Chain Back on the Map: How Might Its Expression Define Plasma Cell Development? J. Immunol. 2014, 193, 3248–3255.
- Mostov, K.E.; Deitcher, D.L. Polymeric Immunoglobulin Receptor Expressed in MDCK Cells Transcytoses IgA. Cell 1986, 46, 613–621.
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic Pathways and Endosomal Trafficking: A Primer. Wien. Med. Wochenschr. 1946 2016, 166, 196–204.
- Mostov, K.E. Transepithelial Transport of Immunoglobulins. Annu. Rev. Immunol. 1994, 12, 63–84.
- Asano, M.; Komiyama, K. Polymeric Immunoglobulin Receptor. J. Oral Sci. 2011, 53, 147–156.
- Everett, M.L.; Palestrant, D.; Miller, S.E.; Bollinger, R.R.; Parker, W. Immune Exclusion and Immune Inclusion: A New Model of Host-Bacterial Interactions in the Gut. Clin. Appl. Immunol. Rev. 2004, 4, 321–332.
- Wallace, A.L.; Schneider, M.I.; Toomey, J.R.; Schneider, R.M.; Klempner, M.S.; Wang, Y.; Cavacini, L.A. IgA as a Potential Candidate for Enteric Monoclonal Antibody Therapeutics with Improved Gastrointestinal Stability. Vaccine 2020, 38, 7490–7497.
- Mathias, A.; Corthésy, B. N-Glycans on Secretory Component: Mediators of the Interaction between Secretory IgA and Gram-Positive Commensals Sustaining Intestinal Homeostasis. Gut Microbes 2011, 2, 287–293.
- Plomp, R.; de Haan, N.; Bondt, A.; Murli, J.; Dotz, V.; Wuhrer, M. Comparative Glycomics of Immunoglobulin A and G From Saliva and Plasma Reveals Biomarker Potential. Front. Immunol. 2018, 9.
- Corthésy, B. Role of Secretory Immunoglobulin A and Secretory Component in the Protection of Mucosal Surfaces. Future Microbiol. 2010, 5, 817–829.
- Kumar, N.; Arthur, C.P.; Ciferri, C.; Matsumoto, M.L. Structure of the Secretory Immunoglobulin A Core. Science 2020, 367, 1008–1014.
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Lessons from Mother: Long-Term Impact of Antibodies in Breast Milk on the Gut Microbiota and Intestinal Immune System of Breastfed Offspring. Gut Microbes 2014, 5, 663–668.
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631.
- Goldman, A.S.; Garza, C.; Nichols, B.L.; Goldblum, R.M. Immunologic Factors in Human Milk during the First Year of Lactation. J. Pediatr. 1982, 100, 563–567.
- Shimada, S.; Kawaguchi-Miyashita, M.; Kushiro, A.; Sato, T.; Nanno, M.; Sako, T.; Matsuoka, Y.; Sudo, K.; Tagawa, Y.; Iwakura, Y.; et al. Generation of Polymeric Immunoglobulin Receptor-Deficient Mouse with Marked Reduction of Secretory IgA. J. Immunol. 1999, 163, 5367–5373.
- Johansen, F.E.; Pekna, M.; Norderhaug, I.N.; Haneberg, B.; Hietala, M.A.; Krajci, P.; Betsholtz, C.; Brandtzaeg, P. Absence of Epithelial Immunoglobulin A Transport, with Increased Mucosal Leakiness, in Polymeric Immunoglobulin Receptor/Secretory Component-Deficient Mice. J. Exp. Med. 1999, 190, 915–922.
- Uren, T.K.; Johansen, F.-E.; Wijburg, O.L.C.; Koentgen, F.; Brandtzaeg, P.; Strugnell, R.A. Role of the Polymeric Ig Receptor in Mucosal B Cell Homeostasis. J. Immunol. 2003, 170, 2531–2539.
- Turula, H.; Bragazzi Cunha, J.; Mainou, B.A.; Ramakrishnan, S.K.; Wilke, C.A.; Gonzalez-Hernandez, M.B.; Pry, A.; Fava, J.; Bassis, C.M.; Edelman, J.; et al. Natural Secretory Immunoglobulins Promote Enteric Viral Infections. J. Virol. 2018, 92.
- Gohy, S.T.; Detry, B.R.; Lecocq, M.; Bouzin, C.; Weynand, B.A.; Amatngalim, G.D.; Sibille, Y.M.; Pilette, C. Polymeric Immunoglobulin Receptor Down-Regulation in Chronic Obstructive Pulmonary Disease. Persistence in the Cultured Epithelium and Role of Transforming Growth Factor-β. Am. J. Respir. Crit. Care Med. 2014, 190, 509–521.
- Richmond, B.W.; Brucker, R.M.; Han, W.; Du, R.-H.; Zhang, Y.; Cheng, D.-S.; Gleaves, L.; Abdolrasulnia, R.; Polosukhina, D.; Clark, P.E.; et al. Airway Bacteria Drive a Progressive COPD-like Phenotype in Mice with Polymeric Immunoglobulin Receptor Deficiency. Nat. Commun. 2016, 7, 11240.
- Richmond, B.W.; Du, R.-H.; Han, W.; Benjamin, J.T.; van der Meer, R.; Gleaves, L.; Guo, M.; McKissack, A.; Zhang, Y.; Cheng, D.-S.; et al. Bacterial-Derived Neutrophilic Inflammation Drives Lung Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2018, 58, 736–744.
- Reikvam, D.H.; Derrien, M.; Islam, R.; Erofeev, A.; Grcic, V.; Sandvik, A.; Gaustad, P.; Meza-Zepeda, L.A.; Jahnsen, F.L.; Smidt, H.; et al. Epithelial-Microbial Crosstalk in Polymeric Ig Receptor Deficient Mice. Eur. J. Immunol. 2012, 42, 2959–2970.
- Kato-Nagaoka, N.; Shimada, S.-I.; Yamakawa, Y.; Tsujibe, S.; Naito, T.; Setoyama, H.; Watanabe, Y.; Shida, K.; Matsumoto, S.; Nanno, M. Enhanced Differentiation of Intraepithelial Lymphocytes in the Intestine of Polymeric Immunoglobulin Receptor-Deficient Mice. Immunology 2015, 146, 59–69.
- Betz, K.J.; Maier, E.A.; Amarachintha, S.; Wu, D.; Karmele, E.P.; Kinder, J.M.; Steinbrecher, K.A.; McNeal, M.M.; Luzader, D.H.; Hogan, S.P.; et al. Enhanced Survival Following Oral and Systemic Salmonella Enterica Serovar Typhimurium Infection in Polymeric Immunoglobulin Receptor Knockout Mice. PLoS ONE 2018, 13, e0198434.
- Wijburg, O.L.C.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.-E.; Brandtzaeg, P.; Strugnell, R.A. Innate Secretory Antibodies Protect against Natural Salmonella Typhimurium Infection. J. Exp. Med. 2006, 203, 21–26.
- Suzuki, K. Diversified IgA-Bacteria Interaction in Gut Homeostasis. Adv. Exp. Med. Biol. 2020, 1254, 105–116.
- Okai, S.; Usui, F.; Ohta, M.; Mori, H.; Kurokawa, K.; Matsumoto, S.; Kato, T.; Miyauchi, E.; Ohno, H.; Shinkura, R. Intestinal IgA as a Modulator of the Gut Microbiota. Gut Microbes 2017, 8, 486–492.
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14.
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 Cells Upregulate Polymeric Ig Receptor and Intestinal IgA and Contribute to Intestinal Homeostasis. J. Immunol. 2012, 189, 4666–4673.
- Bruno, M.E.C.; Rogier, E.W.; Arsenescu, R.I.; Flomenhoft, D.R.; Kurkjian, C.J.; Ellis, G.I.; Kaetzel, C.S. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 2976–2984.
- Kakiuchi, N.; Yoshida, K.; Uchino, M.; Kihara, T.; Akaki, K.; Inoue, Y.; Kawada, K.; Nagayama, S.; Yokoyama, A.; Yamamoto, S.; et al. Frequent Mutations That Converge on the NFKBIZ Pathway in Ulcerative Colitis. Nature 2020, 577, 260–265.
- Olafsson, S.; McIntyre, R.E.; Coorens, T.; Butler, T.; Jung, H.; Robinson, P.S.; Lee-Six, H.; Sanders, M.A.; Arestang, K.; Dawson, C.; et al. Somatic Evolution in Non-Neoplastic IBD-Affected Colon. Cell 2020, 182, 672–684.e11.
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic Inflammatory Gene Mutations in Human Ulcerative Colitis Epithelium. Nature 2020, 577, 254–259.




