
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raffaella Nenna | + 1308 word(s) | 1308 | 2021-04-07 13:11:17 | | | |
| 2 | Catherine Yang | Meta information modification | 1308 | 2021-04-08 02:50:47 | | |
Video Upload Options
Lung ultrasound has become increasingly used in both adult and pediatric populations, allowing the rapid evaluation of many lung and pleura diseases.
1. Introduction
The management of pediatric lung diseases has always been challenging for clinicians because of the variable clinical manifestations and often overlapping symptoms and signs. Traditionally, chest X-ray (CXR) has played a crucial role in the diagnosis of respiratory diseases. However, the potentially harmful effects of radiation exposure due to CXR reduce its applications, especially in children [1].
In recent years, a new imaging application of sonography has spread in emergency departments and clinical practice: lung ultrasound (LUS) [2]. It is quick, portable, repeatable, lacks ionizing radiation and, therefore, finds application in many different settings, both inpatient and outpatient, in both acute and chronic conditions [3]. In particular, growing evidence highlights the effectiveness of this method as a diagnostic tool in different pediatric diseases [4][5]. Infants and children are considered ideal candidates for this type of exam due to their thinner chest and smaller lung volumes. These features help to make any lesions better visible because, in smaller lungs, it is more likely that such lesions reach the pleura and make the linear probe able to complete the exam [6].
2. Lung Ultrasound Findings in Healthy Subjects
The first layers of the chest consist of subcutaneous tissue and muscles. In longitudinal sections, the ribs appear as curved structures with a posterior acoustic shadow. In axial sections, the intercostal acoustic window is used to study tissues located deep into the skin and muscle [7]. In a normally aerated lung, the only detectable structure is the pleura. It appears as a continuous hyperechogenic line that moves back and forth with the breaths. This movement is also known as “lung sliding” [8].
Below the pleural line, the lung is filled with air. This does not allow the direct visualization of the normal pulmonary parenchyma, but some artifacts and echographic findings remain describable.
“A-lines” represent some of these artifacts that can be found in a completely healthy lung (Figure 1) [7]. They are horizontal echogenic lines equidistant and parallel to each other and the pleura, representing reverberations of the pleura itself that arise when the ultrasound beam reflects off of the pleura and partially reflects off of the probe face back to the pleura again before getting back to the machine instead of entering the probe. They are caused by the large difference in acoustic impedance between the pleura and the air contained in the lungs. The distance from each other is related to the distance between the pleural line and the skin surface, and their position does not change with respiratory acts.
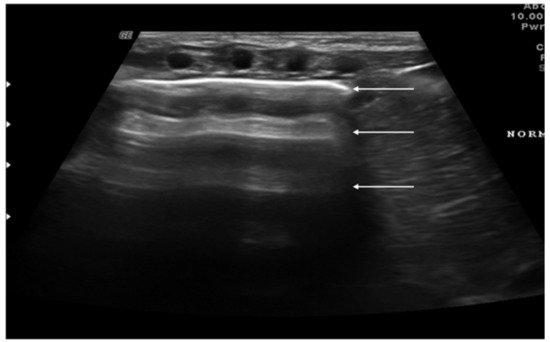
Figure 1. Appearance of a healthy lung. White arrows show pleural and A-lines.
Another sonographic artifact is represented by the “Z-lines”. These artifacts are formed by reverberation echoes from irregularities on the lung surface and appear as vertical echogenic lines (but less echogenic than a pleural line) originating from the pleura that does not erase the A-lines and do not move with lung sliding (Figure 2) [9]. These should be distinguished from B-lines that are similar to Z-lines but usually indicate the presence of fluid in the interstitial compartment or, in general, an abnormality in the alveolar or interstitial compartment. B-lines appear as a vertical line arising from the pleura, often erasing the A-lines and moving with the lung sliding (Figure 3) [9].
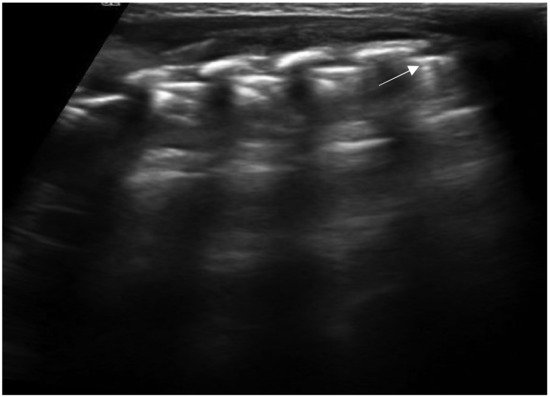
Figure 2. Isolated Z-lines (white arrow).
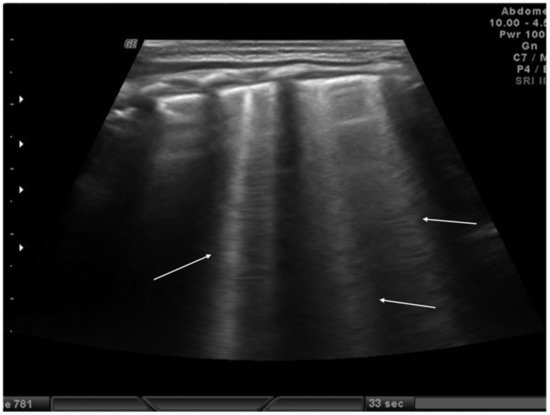
Figure 3. Isolated B-lines (white arrows).
Although these artifacts are not present in a normal lung, they may be observed in the infant’s lung in the first 48 h of life because of the possible delay in lung fluid resorption [10].
Until 2020, pediatric studies of lung ultrasound were based on the assumption that the ultrasound appearance of a healthy adult lung was the same as that of a child, and no studies had ever been conducted to describe the appearance of the healthy lung in children, particularly infants. Recently, Buonsenso et al. described the normal ultrasound appearance of the lung in infants during the first 6 months of life by performing an ultrasound at 10 days and 1, 3 and 6 months. The study showed that in the first months of life the healthy infant’s lung is characterized by a B-pattern with multiple vertical artifacts that tend to normalize toward the normal A-pattern typical of the adults at six months of age. No consolidations, pleural line abnormalities or pleural effusion were observed in healthy infants [11].
3. Lung and Chest Wall Ultrasound Applications in Children
The main indications for lung and chest wall ultrasound in the pediatric population include:
-
Diagnosis and follow-up of neonatal lung diseases;
-
Confirm antenatal diagnosis of lung malformations;
-
Diagnosis and follow-up of pediatric lung infectious diseases (namely bronchiolitis and pneumonia) and lung complications (such as pneumothorax, pleural effusion and lung abscess);
-
Diagnosis and follow-up of pulmonary edema;
-
Diagnosis of thoracic trauma and early detection of signs of child abuse;
-
Follow-up in children undergoing cardiac surgery;
-
Diaphragm ultrasound.
4. Neonatal Lung Diseases
Many lung diseases, such as respiratory distress syndrome, transient tachypnea, pneumonia, atelectasis and pneumothorax, can cause respiratory distress in newborns, especially among premature infants. The differential diagnosis of these diseases is often difficult, but LUS can help to discriminate these diseases [12][13].
Neonatal respiratory distress syndrome (NRDS) is characterized by a functional and structural immaturity of the lung resulting in respiratory distress that appears at birth. The ultrasound findings of NRDS are represented by coalescent B-lines, diffuse and symmetrically distributed in both lungs. These lines are due to the presence of fluid in the interstitial or alveolar compartment, and generally, their number correlates with the severity of the disease, up to “white lung” in the most severe cases. The pleural line appears irregular, poorly defined and thickened. In addition, small areas of hypoechogenic subpleural consolidation can often be observed, especially in the posterior lung fields (Figure 4) [14].
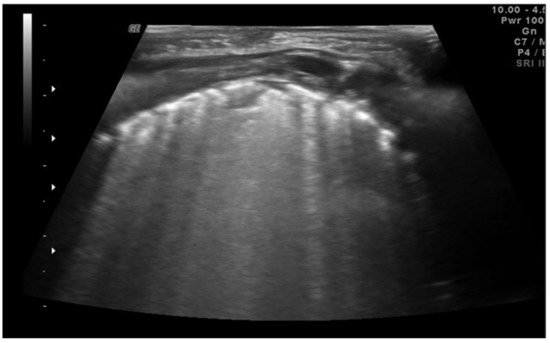
Figure 4. Neonatal respiratory distress syndrome (NRDS). Coalescent B-lines, irregularity of the pleural line and subpleural consolidation are visible.
The 2019 European consensus guidelines on the management of respiratory distress syndrome assert that lung ultrasound could be a useful tool for clinical decision making, and it seems able to differentiate NRDS from other common respiratory disorders of the newborn, reducing exposure to ionizing radiation [15]. In addition, some recent evidence has underlined how ultrasound performed in the first 12 h of life can be useful in identifying patients most likely to require surfactant therapy or mechanical ventilation, even before the oxygenation criteria [16].
Transient tachypnea of the newborn (TTN), also known as “wet lung,” is caused by a failure in the reabsorption of fluid from the fetal lung. It is typical of term or post-term infants in the case of rapid delivery or cesarean section [17]. Infants with TTN present compact B-lines in the lower lung fields and fewer and less compact B-lines in the upper fields in one or both lungs. These signs, also known as double-lung points, appear because of the greater involvement of the lower lung fields in the disease and are characterized by a sharp ultrasound demarcation line between the upper and lower lung fields of both lungs (Figure 5) [10]. The pleural line is regular, with normal echogenicity and movement with respiratory acts. In contrast to NRDS, no subpleural consolidations are observed. Due to these typical ultrasonographic findings, much evidence has shown how pulmonary ultrasonography can be useful in the early diagnosis of TTN and differentiate TTN from NRDS already by the first hours of life [18].
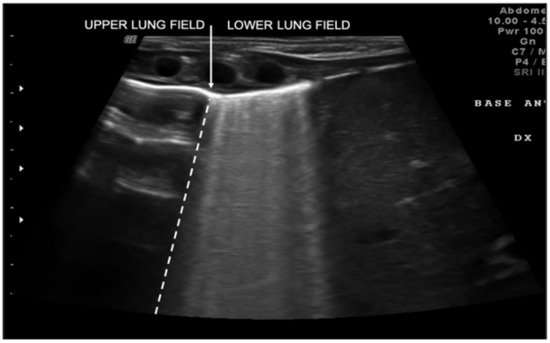
Figure 5. Transient tachypnea of the newborn. The dashed line shows the ultrasound demarcation line between the upper and lower lung fields: double-lung point (white arrow).
Meconium aspiration syndrome (MAS) refers to a multitude of respiratory symptoms caused by meconium aspiration during delivery. It is more frequent in post-term newborns. The ultrasound findings of MAS are as follows: B-pattern appearance, with coalescent or noncoalescent B-lines, several subpleural consolidations asymmetrically spread on both lungs with few spared areas and in severe cases of MAS a white lung pattern [19].
References
- Thomas, K.E.; Parnell-Parmley, J.E.; Haidar, S.; Moineddin, R.; Charkot, E.; Bendavid, G.; Krajewski, C. Assessment of radiation dose awareness among pediatricians. Pediatr. Radiol. 2006, 36, 823–832.
- Zechner, P.M.; Seibel, A.; Aichinger, G.; Steigerwald, M.; Dorr, K.; Scheiermann, P.; Schellhaas, S.; Cuca, C.; Breitkreutz, R.; Arbeitsgruppe des Moduls 5 in Anästhesie Fokussierte Sonographie der DGAI. Lung ultrasound in acute and critical care medicine. Anaesthesist 2012, 6, 608–617.
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591.
- Joshi, P.; Vasishta, A.; Gupta, M. Ultrasound of the pediatric chest. Br. J. Radiol. 2019, 92, 20190058.
- Cattarossi, L. Lung ultrasound: Its role in neonatology and pediatrics. Early Hum. Dev. 2013, 89.
- Copetti, R.; Cattarossi, L. Ultrasound diagnosis of pneumonia in children. Radiol. Med. 2008, 113, 190.
- Lobo, V.; Weingrow, D.; Perera, P.; Williams, S.R.; Gharahbaghian, L. Thoracic ultrasonography. Crit. Care Clin. 2014, 30, 93–117.
- Lichtenstein, D.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348.
- Lichtenstein, D.; Meziere, G.; Biderman, P.; Gepner, A.; Barré, O. The comet-tail artifact. An ultrasound sign of alveolar interstitial syndrome. Am. J. Respir. Crit. Care Med. 1997, 156, 1640–1646.
- Copetti, R.; Cattarossi, L. The “double lung point”: An ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology 2007, 91, 203–209.
- Buonsenso, D.; Soldati, G.; Curatola, A.; Morello, R.; De Rose, C.; Vacca, M.E.; Lazzareschi, I.; Musolino, A.M.; Valentini, P. Lung Ultrasound Pattern in Healthy Infants during the First 6 Months of Life. J. Ultrasound Med. 2020, 39, 2379–2388.
- Lichtenstein, D.; Mauriat, P. Lung ultrasound in the critically ill neonate. Curr. Pediatr. Rev. 2012, 8, 217–223.
- Sharma, D.; Farahbakhsh, N. Role of chest ultrasound in neonatal lung disease: A review of current evidences. J. Matern. Fetal Neonatal Med. 2019, 32, 310–316.
- Copetti, R.; Cattarossi, L.; Macagno, F.; Violino, M.; Furlan, R. Lung ultrasound in respiratory distress syndrome: A useful tool for early diagnosis. Neonatology 2008, 94.
- Sweet, D.G.; Carnielli, V.; Greisen, G.; Hallman, M.; Ozek, E.; Pas, A.T.; Plavka, R.; Roehr, C.C.; Saugstad, O.D.; Simeoni, U.; et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2019 Update. Neonatology 2019, 115, 432–450.
- Gregorio-Hernández, R.; Arriaga-Redondo, M.; Pérez-Pérez, A.; Ramos-Navarro, C.; Sánchez-Luna, M. Lung ultrasound in preterm infants with respiratory distress: Experience in a neonatal intensive care unit. Eur. J. Pediatrics 2020, 179, 81–89.
- Reuter, S.; Moser, C.; Baack, M. Respiratory distress in the newborn. Pediatr. Rev. 2014, 35, 417–428.
- Liu, J.; Wang, Y.; Fu, W.; Yang, C.S.; Huang, J.J. Diagnosis of neonatal transient tachypnea and its differentiation from respiratory distress syndrome using lung ultrasound. Medicine 2014, 93.
- Piastra, M.; Yousef, N.; Brat, R.; Manzoni, P.; Mokhtari, M.; De Luca, D. Lung ultrasound findings in meconium aspiration syndrome. Early Hum. Dev. 2014, 90.




