
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prakash Nagabovanalli B | + 2433 word(s) | 2433 | 2021-04-06 11:58:18 | | | |
| 2 | Vivi Li | -9 word(s) | 2424 | 2021-04-07 03:54:59 | | |
Video Upload Options
Plants’ ability to take up silicon from the soil, accumulate it within their tissues and then reincorporate it into the soil through litter creates an intricate network of feedback mechanisms in ecosystems. Here, we provide a study of silicon’s roles in soil chemistry and physics and in plant physiology and ecology, focusing on the processes that form these feedback mechanisms.
1. Introduction
Silicon (Si) uptake and accumulation is a functional trait with multiple implications for plant biology and ecology [1][2]. Silicon’s manifold functions in plant biology include protection from a myriad of abiotic and biotic stresses and confers many benefits to plants that are capable of taking up and accumulating large amounts of it, ranging from practically zero in some taxa to 5% dry weight (and in extreme cases even more) in grasses [1][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17], and probably even to those plants that take up considerably smaller amounts [5][18]. Moreover, its uptake from the soil and eventual reincorporation into the soil through plant litter and herbivore feces also affects soil properties and Si cycling [19][20][21][22][23]. Thus, Si uptake, accumulation and cycling by plants is a key phenomenon in many ecosystems [12][21][24][25][26], with direct and indirect implications for ecosystem properties and processes [1]. Here, we review the existing knowledge of Si in the soil–plant continuum, its roles in plant biology and ecology, in ecosystem processes, and the possible implications for various ecosystem services.
2. Historical Overview
In 1787, Antoine Lavoisier predicted the existence of the element Si, to which Sir Humphry Davy proposed the name “silicon” in 1808. It was eventually isolated and formally discovered in 1823 by Jöns Jacob Berzelius. The discovery of the occurrence of Si within plants quickly followed, owing to the works of some prominent scholars, including microscopy pioneer Christian Gottfried Ehrenberg (who suggested the word “phytolitharia” to describe plant mineral components) [27] and Charles Darwin, who provided him with some samples [28][29]. Silicon effects on plant performance are known for more than 170 years, starting with Struve in 1835 [30], and shortly after Davy’s pioneering publication from 1846, who suggested that Si is present in the epidermis of grasses, where it strengthens the plants and makes them more resistant to attacks by insects and “parasitical plants” [31]. A surge of research soon followed. Sachs [32] showed in 1862 that Si-accumulating plants were a less preferred food than their conspecific plants that were grown hydroponically in Si-poor media. He further found that Si accumulation started in plant hairs and further advanced into the epidermis and near leaf vascular tissues. He also suggested that not all Si deposits in plant leaves are hard, but some may remain in a colloidal state. Miliarakis [33] found in 1884 that basal (younger) leaf parts of Si-accumulating plants had lower Si concentrations than older leaf tips and could not deter feeding. He also found that horsetail (Equisetum) and sedge (Carex and Scirpus) old leaf sheaths had a high Si concentration and protected the younger and less silicified plant tissues from herbivory. Furthermore, in 1884, Kreuzhage and Wolff [34] suggested the importance of Si for oat plants. Kerner von Marilaun [35] suggested in 1887 that the sharp leaf margin of sedges may be due to Si deposits. In 1888, Stahl [36] summarized other studies and concluded that silica deposits in horsetails impeded grazing by snails. He also mentioned that plant tissue silicification reduced the food quality of tropical grasses in Africa for domestic animals.
A second surge of studies started in the 1920s. Between 1922 and 1925, Lemmermann and colleagues [37][38][39] found an increase in the yield of rye grown under phosphorus deficiency upon Si fertilization. Sommer [40] concluded in the year 1926 that rice plants without Si fertilization suffered from early increased leaf wilting, guttation, and retarded panicle formation. In parallel, a surge of studies on Si effects on rice took place in Japan, starting in the 1910s and continuing into the 1940s, summarized thoroughly by Ma and Takahashi [41]. In the 1910s, Onodera [42] found that blast-infected rice leaves had a lower Si concentration than healthy leaves. Miyake et al. [43] also found in 1922 that Si concentration was higher in blast-resistant plants than nonresistant (surprisingly, this specific Japanese research was published in German). Other studies showed an increased blast-resistance of rice plants following Si application [44][45]. In the 1930s Ishibashi [46][47][48] showed reduced growth and yield of rice plants under Si deficiency and reduced blast after Si fertilization. Raleigh [49] showed in 1939 that Si was strongly improving the growth of beet plants. A year later, Wagner [50] showed that Si protects plants against powdery mildew by silicifying host plants’ cell walls, hence reducing penetration.
The study of silicon in plants continued shortly following the end of World War II, and especially since the 1960s. Engel [51] found in 1953 that the Si accumulated in rye culm was ~1/3 easily (hot water) extractable, suggesting no strong binding of this fraction to the plant cell walls. Holzapfel and Engel [52] found that Si accumulation in wheat increases over a study period of 30 days. In 1962, Yoshida [53] discovered the cuticle–Si double layer and suggested that this layer may be responsible for plant resistance against fungal diseases. There were several publications by Okuda and Takahashi, who found that Si improves plant resistance to metals [54] and rice growth and nutrition of [55]. Many of these studies were reviewed by Lewin and Reimann at the end of the 1960s [56]. During the same period, Jones and his colleagues (mainly Handreck) published some seminal papers on the occurrence, uptake, localization and functions of Si in oats [57][58][59][60] and clover [61]. At around the same time, a group that developed around Parry and his successor Sangster (both of which have passed away only in the past decade in their 90 s) further looked into the physiology of Si uptake and deposition [62][63][64][65][66][67][68][69][70][71]. Their early studies set the basis for a greater effort in Si plant and soil research, and a third surge that started in the 1980s and continues to these days, initiated by many important scientists and continuing to these days by the many who follow their footsteps.
3. Silicon Uptake by Plants
3.1. Active Uptake by Intrinsic Transporters
Plant taxa vary in the amounts of Si they take up and accumulate, a variability that is manifested through variations in Si contents, uptake mechanisms, forms and deposition locations. Traditionally, plant taxa have been divided into three major types based on their Si uptake capabilities, defined by the amounts of Si taken up by the plant (often measured as Si content in the xylem) relative to the amounts of available Si in the soil solution. If the amount of Si in the plant is considerably larger than that in the soil solution, the plant takes up Si actively; if the amount of Si in the plant is considerably lower than in the soil solution, the plant excludes Si; if the two contents are comparable, the plant takes up Si passively [72]. As straightforward as this division is, it is over-simplified and lacks mechanistic rigor.
For once, the reference to available Si content in the soil solution can be misleading since the great variability of soil Si pools implies that a species may take up Si both actively and passively, depending on the soil type and parent material. A dynamic approach is more appropriate since it indicates the plant’s response to varying soil Si availabilities and can furthermore point to the underlying internal (physiological) drivers of such responses [7][16]. For example, some species seem to increase their active Si uptake when Si availability in the soil is lower, suggesting an active response to fulfill plant internal Si demands when passive uptake is insufficient [73][74]. In some cases, this is achieved by increasing the expression of Si transporter genes and the density of these transporters under low Si availability conditions [16], indicating a truly active uptake that does not only rely on active uptake mechanisms but also on physiological responses of these mechanisms. Furthermore, Si uptake also depends on transpiration rates, with some species demonstrating passive (transpiration-driven) Si uptake in addition to active (transporter-governed) Si uptake [73][74][75]. The modes and drivers of Si uptake and accumulation and its variability among species are, therefore, not as simple as an active/passive/exclusion division implies.
Several transporters and genes that are involved in Si uptake and accumulation have been studied so far. Although the study of Si transporters focuses on rice and other grasses (as is commonly the case in plant Si research [5][18]), the first plant gene to regulate Si accumulation was discovered in the gourd Cucurbita (Cucurbitaceae), regulating Si and phytolith formation in the fruit rind [76]. Shortly after, a surge of discoveries of the physiology and genetics of Si uptake in grasses has arisen, revolving around the four Lsi transporters, all belonging to the NIP aquaporin family. The first transporter to be discovered was the influx transporter Lsi1, located in the distal plasma membrane of root exodermis and endodermis cells [77]. An efflux transporter on the proximal plasma membrane of the same cells, Lsi2, transports Si from the exodermis to the cortex and further loads it from the endodermis onto the xylem [78]. A third transporter, Lsi6, exists in the shoots and is responsible for xylem offloading [79]. In grass shoot nodes, Lsi6 and Lsi3 (previously thought to be Lsi2 due to structural similarities) are involved in distributing Si among branches [80][81][82][83]. Together, these transporters constitute an elaborate cooperative system of Si uptake and distribution in grasses, with some variations in the details of where exactly each transporter is localized within each species [16][80][84] (Figure 1).
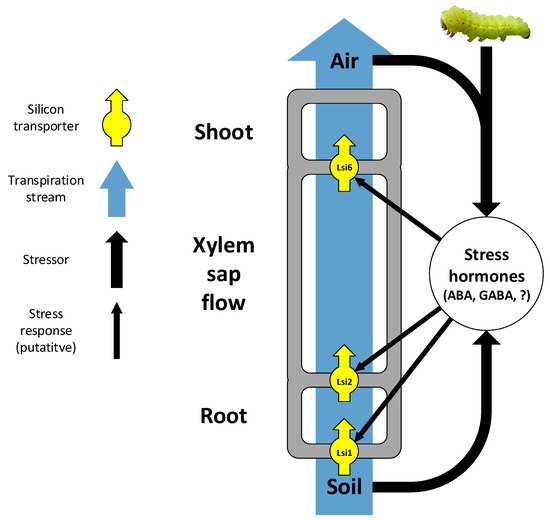
Figure 1. A simplified model of Si uptake from the soil to the shoot through the transpiration stream, including main transporters and responses to external factors.
Lsi1 and Lsi6 transporters were also identified in the soybean Glycine max [85]. In the Cucurbitaceae, a Si-accumulating dicotyledonous family, Lsi1 was also identified in all root cells of Cucurbita [86]. Wang et al. [87] identified two putative Si transporters in cucumber (Cucumis) of the same family. Together with the early-identified gene responsible for Si accumulation in Cucurbita rinds [76], these studies suggest that Si transport systems in grasses and dicotyledons share some similarities. The recent identification of a gene regulating Si uptake by the mangrove Rhizophora apiculate without identifying the transporter itself [88]. Multiple genes regulating Si uptake and accumulation were also found in the horsetail Equisetum arvense [89]. Finally, it appears that Lsi-like genes that govern Si uptake are common in many groups of land plants, suggesting that the origins of these mechanisms are as ancient as the origins of land plants [90]. These findings further suggest that the physiology and genetics of Si transporters in non-grass species are only beginning to reveal themselves.
Expression of the Lsi1 gene in rice is downregulated by Si supply, dehydration stress and abscisic acid (more strongly in Si-depleted plants), suggesting regulation of active Si uptake in response to changes in the transpiration stream and plant internal water balance [91]. Further studies have demonstrated how the expression of Lsi1, Lsi2 and Lsi6 genes is regulated by plant hormones [92] and internal Si and metal concentrations [93][94].
3.2. External Factors Affecting Silicon Uptake
In addition to intrinsic transporters, external factors also affect Si uptake and accumulation in plants. These include both passive uptake mechanisms driven by the transpiration stream (the soil–plant–air continuum; Figure 1) and active mechanisms induced or enhanced by biotic stressors. Since Si is taken up from the soil as monosilicic acid within the soil solution, passive Si uptake depends on the transpiration stream. Several studies have shown that plant Si content in grasses increases with soil water content and availability, most probably for the simple reason that the more water a plant absorbs, the more Si is taken up with it [95][96][97][98][99][100][101]. On the other hand, transpiration, acting as the motive force of water uptake, has also been shown to increase Si content in grasses, to the degree that Si content has been suggested to serve as an indicator to plant transpiration stress [81][101][102][103][104][105]. Hence, along large rainfall gradients, Si content tends to demonstrate a U-shaped curve (minimum Si content at approximately 200–300 mm mean annual rainfall), implying an interplay between water availability and transpiration motive force [102][104]. Nevertheless, high plant Si contents in extremely arid conditions may also occur because grasses under drought stress take up Si more actively for the benefit it confers in resisting drought or in herbivore deterrence [102][104][105]. Nevertheless, the failure to observe this positive correlation in other studies of grasses [106][107] suggests that other variables may confound the simple positive effect of water availability [102][104] (For Si effects on soil water availability co-appearing with Si availability in soils). In the Asteraceae family, an intermediate Si accumulator with mostly passive Si uptake (as far as we know, no attempts were made so far to identify Si transporters in this family), there appears to be no clear, consistent pattern, suggesting that Si uptake is not simply driven by the transpiration stream [102][104]. That the expression of the Lsi1 gene in rice is down-regulated by dehydration stress and abscisic acid [91] is a further indication for the complex effects of the transpiration stream on Si uptake.
Several studies have shown that ambient CO2 concentrations also affect plant Si uptake, content and form, but with contradictory results. Ambient CO2 concentrations had no effect on root and shoot Si contents in sugarcane plants [108]. In rice, increased ambient CO2 concentrations reduced husk Si deposition by as much as 60% [109]. Increased ambient CO2 concentrations alter the composition of phytolith assemblages in Phragmites and reduce mean phytolith size [110], suggesting an effect on Si allocation and distribution. Despite these studies being limited and equivocal regarding the regulatory role of CO2 on plant Si uptake and accumulation, they harness potential significance for our understanding of global Si–carbon relationships, namely the possibility of Si being a partial substitute for carbon in plants and Si’s role in regulating the carbon cycle.
Among the biotic stressors known to affect plant Si content, herbivory is the one that was studied the most [111]. Exposure to invertebrate [112][113][114][115] and vertebrate [112][116] herbivores induces Si uptake and accumulation in grasses. Comparable induction by artificial clipping [117][118][119] further supports that this induction is directly associated with biomass removal or damage. While such induction was sometimes not observed in controlled experiments [112][119][120][121][122][123], this is likely because these experiments did not incorporate sufficiently long exposure times to initiate a response [102][112]. In natural landscapes, higher grass Si contents are associated with larger densities of herbivorous rodents [122][124][125][126][127], but not of larger herbivores [105][116][128] or following clipping [119], which is most likely explained by the involvement of other environmental variables in natural ecosystems having stronger effects on Si uptake and accumulation [102]. Nevertheless, recent evidence for cyclic dynamics of vole densities and grass Si contents [125][127][129] provides further support to the induction of grass Si uptake by herbivory. Among the Asteraceae, the only non-grass family in which the possible effect of herbivory on Si has been widely studied, such an effect was rare and weak [105].
References
- Katz, O. Silicon content is a plant functional trait: Implications in a changing world. Flora 2019, 254, 88–94.
- Cooke, J.; Leishman, M.R. Is plant ecology more siliceous than we realise? Trends Plant Sci. 2011, 16, 61–68.
- He, H.; Veneklaas, E.J.; Kuo, J.; Lambers, H. Physiological and ecological significance of biomineralization in plants. Trends Plant Sci. 2014, 19, 166–174.
- Epstein, E. The anomaly of silicon in plant biology. Proc. Natl. Acad. Sci. USA 1994, 91, 11–17.
- Putra, R.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Is it time to include legumes in plant silicon research? Funct. Ecol. 2020, 34, 1142–1157.
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160.
- Frew, A.; Weston, L.A.; Reynolds, O.L.; Gurr, G.M. The role of silicon in plant biology: A paradigm shift in research approach. Ann. Bot. 2018, 121, 1265–1273.
- Richmond, K.E.; Sussman, M. Got silicon? The non-essential beneficial plant nutrient. Curr. Opin. Plant Biol. 2003, 6, 268–272.
- Farooq, M.A.; Dietz, K.J. Silicon as versatile player in plant and human biology: Overlooked and poorly understood. Front. Plant Sci. 2015, 6, 994.
- Meharg, C.; Meharg, A.A. Silicon, the silver bullet for mitigating biotic and abiotic stress, and improving grain quality, in rice? Environ. Exp. Bot. 2015, 120, 8–17.
- Raven, J.A. The transport and function of silicon in plants. Biol. Rev. 1983, 58, 179–207.
- Schoelynck, J.; Struyf, E. Silicon in aquatic vegetation. Funct. Ecol. 2016, 30, 1323–1330.
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357.
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213.
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397.
- Ma, J.F.; Yamaji, N. Functions and transport of silicon in plants. Cell. Mol. Life Sci. 2008, 65, 3049–3057.
- Sahebi, M.; Hanafi, M.M.; Siti Nor Akmar, A.; Rafii, M.Y.; Azizi, P.; Tengoua, F.F.; Nurul Mayzaitul Azwa, J.; Shabanimofrad, M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010.
- Katz, O. Beyond grasses: The potential benefits of studying silicon accumulation in non-grass species. Front. Plant Sci. 2014, 5, 376.
- Frings, P.J.; Clymans, W.; Jeppesen, E.; Lauridsen, T.L.; Struyf, E.; Conley, D.J. Lack of steady-state in the global biogeochemical Si cycle: Emerging evidence from lake Si sequestration. Biogeochemistry 2014, 117, 255–277.
- Keller, C.; Guntzer, F.; Barboni, D.; Labreuche, J.; Meunier, J.D. Impact of agriculture on the Si biogeochemical cycle: Input from phytolith studies. C. R. Geosci. 2012, 344, 739–746.
- Carey, J.C.; Fulweiler, R.W. The terrestrial silica pump. PLoS ONE 2012, 7, e52932.
- Cornelis, J.T.; Delvaux, B. Soil processes drive the biological silicon feedback loop. Funct. Ecol. 2016, 30, 1298–1310.
- Katz, O. Silica phytoliths in angiosperms: Phylogeny and early evolutionary history. New Phytol. 2015, 208, 642–646.
- Cooke, J.; Degabriel, J.L. Plant silicon interactions between organisms and the implications for ecosystems. Front. Plant Sci. 2016, 7, 1001.
- Schoelynck, J.; Müller, F.; Vandevenne, F.; Bal, K.; Barão, L.; Smis, A.; Opdekamp, W.; Meire, P.; Struyf, E. Silicon-Vegetation interaction in multiple ecosystems: A review. J. Veg. Sci. 2014, 25, 301–313.
- Schaller, J.; Puppe, D.; Kaczorek, D.; Ellerbrock, R.; Sommer, M. Silicon cycling in soils revisited. Plants 2021, 10, 295.
- Ehrenberg, C.G. Mikrogeologie: Das Erden und Felsen Schaffende Wirken des Unsichtbar Kleinen Selbständigen Lebens auf der Erde; L. Voss: Leipzig, Germany, 1854.
- Powers, A.P. Historical review of European phytolith systematics. In Phytolith Systematics; Rapp, G.J., Mulholland, S.C., Eds.; Springer International Publishing: New York, NY, USA, 1992; pp. 15–35.
- Darwin, C. An account of the fine dust which often falls on vessels in the Atlantic Ocean. Q. J. Geol. Soc. Lond. 1846, 2, 26–30.
- Struve, G.A. De Silicia in Plantis Nonnullis; University of Berlin: Berlin, Germany, 1835.
- Davy, H. Elements of Agricultural Chemistry; John J. Griffin and Company: Glasgow, UK, 1846.
- Sachs, J. Ergebnisse einiger neuerer untersuchungen uber die in pflanzen enthaltene Kieselsaure. Flora 1862, 33, 65–71.
- Miliarakis, S. Die Verkieselung lebender Elementarorgane bei den Pflanzen; University Würzburg: Würzburg, Germany, 1884.
- Kreuzhage, C.; Wolff, E. Bedeutung der kieselsäure für die entwicklung der haferpflanze. Landwirtsch. Versuchs-Stationen 1884, 30, 161–197.
- von Marilaun, A.K. Pflanzenleben: Bd. Gestalt und Legen der Pflanze; Verlag des Bibliographischen Institut: Leipzig, Germany, 1887.
- Stahl, E. Pflanzen und Schnecken: Eine Biologische Studie Über Die Schutzmittel der Pflanzen Gegen Schneckenfrass; G. Fischer: Jena, Germany, 1888.
- Lemmermann, O.; Wießmann, H. Die ertragssteigernde wirkung der kieselsäure bei unzureichender phosphorsäureernährung der pflanzen. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1922, 1, 185–246.
- Lemmermann, O.; Wießmann, H.; Lemmermann, O. Weitere versuche über die ertragssteigernde wirkung der kieselsäure bei unzureichender phosphorsäuredüngung. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1924, 3, 185–197.
- Wießmann, O.L.U.; Sammet, K.; Lemmermann, O. Untersuchungen über die ursache der ertragssteigernden wirkung der kieselsäure. Zeitschrift für Pflanzenernährung und Düngung A Wissenschaftlicher Teil 1925, 4, 265–315.
- Sommer, A.L. Studies Concerning the Essential Nature of Aluminum and Silicon for Plant Growth; University of California Press: Berkeley, CA, USA, 1926.
- Ma, J.F.; Takahashi, E. Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002.
- Onodera, I. Chemical studies on rice blast (1). J. Sci. Agric. Soc. 1917, 180, 606–617.
- Miyake, K.; Adachi, M. Chmische Untersucungen über die widerstandsfähigkeit der reisarten gegen die “imochi-krankheit”: Zweiter bericht. der einflusz der wasserstoffionenkonzentration auf das wachstum des pilzes. J. Biochem. 1922.
- Kawashima, R. Influence of silica on rice blast disease. Jpn. J. Soil Sci. Plant Nutr. 1927, 1, 86–91.
- Miyake, K.; Ikeda, M. Influence of silica application on rice blast. Jpn. J. Soil Sci. Plant Nutr. 1932, 6, 53–76.
- Ishibashi, H. Influence of silica on the growth of rice plant. Jpn. J. Soil Sci. Plant Nutr. 1936, 10, 244–256.
- Ishibashi, H. The effect of silica on the growth of cultivated plants. V. The effect of silica on the growth of rice plants growing on soils of various depth. J. Sci. Soil Manure 1937, 11, 535–549.
- Ishibashi, H. The eefct of silicic acid on the growth of rice plants. J. Sci. Soil Manure 1936, 10, 224–256.
- Raleigh, G.J. Evidence for the essentiality of silicon for the beet plant. Plant Physiol. 1939.
- Wagner, F. The importance of silicic acid for the growth of some cultivated plants, their metabolism, and their susceptibility to true mildew. Phytopathol. Zeitschrift 1940, 12, 427–479.
- Engel, W. Untersuchungen über die Kieselsäureverbindungen im Roggenhalm. Planta 1953, 41, 358–390.
- Holzapfel, L.; Engel, W. Beeinflussung der Kieselsäure-Aufnahme und-Abgabe bei Weizenpflanzen. Arch. Biochem. Biophys. 1959, 83, 268–274.
- Yoshida, S.; Ohnishi, Y.; Kitagishi, K. Histochemistry of silicon in rice plant: III. The presence of cuticle-silica double layer in the epidermal tissue. Soil Sci. Plant Nutr. 1962, 8, 1–5.
- Okuda, A.; Takahashi, E. Effects of silicon supply on the injuries due to excessive amount of Fe, Mn, Cu, As, Al, Co of barley and rice plants. Jpn. J. Soil Sci. Plant Nutr. 1962, 33, 1–8.
- Okuda, A.; Takahashi, E. Studies on the physiological role of silicon in crop plant. Part 3. Effect of various amount of silicon supply on the growth of rice plant and its nutrients uptake. J. Sci. Soil Manure 1961, 32, 533–537.
- Lewin, J.; Reimann, B.E.F. Silicon and plant growth. Annu. Rev. Plant Physiol. 1969, 20, 289–304.
- Jones, L.H.P.; Handreck, K.A. Studies of silica in the oat plant—III. Uptake of silica from soils by the plant. Plant Soil 1965, 23, 79–96.
- Jones, L.H.P.; Handreck, K.A. Silica in soils, plants, and animals. Adv. Agron. 1967, 19, 107–149.
- Handreck, K.A.; Jones, L.H.P. Studies of silica in the oat plant—IV. Silica content of plant parts in relation to stage of growth, supply of silica, and transpiration. Plant Soil 1968, 29, 449–459.
- Jones, L.H.P.P.; Milne, A.A.; Wadham, S.M. Studies of silica in the oat plant. Plant Soil 1963, 18, 358–371.
- Jones, L.H.P.; Handreck, K.A. Uptake of silica by Trifolium incarnatum in relation to the concentration in the external solution and to transpiration. Plant Soil 1969, 30, 71–80.
- Sangster, A.G. Characteristics of silica deposition in Digitaria sanguinalis (L.) scop. (Crabgrass). Ann. Bot. 1977, 41, 341–350.
- Sangster, A.G. Intracellular silica deposition in immature leaves in three species of the Gramineae. Ann. Bot. 1970, 34, 245–257.
- Parry, D.W.; Smithson, F. Silicification of bulliform cells in grasses. Nature 1958, 181, 1549–1550.
- Sangster, A.G.; Parry, D.W. Ultrastructure of silica deposits in higher plants. In Silicon and Siliceous Structures in Biological Systems; Simpson, T.L., Volcani, B.E., Eds.; Springer: New York, NY, USA, 1981; pp. 383–407.
- Parry, D.W.; Kelso, M. The distribution of silicon deposits in the roots of Molinia caerulea (L.) Moench. and Sorghum bicolor (L.) Moench. Ann. Bot. 1975, 39, 995–1001.
- Sangster, A.G.; Parry, D.W. Endodermal silicon deposits and their linear distribution in developing roots of Sorghum bicolor (L.) Moench. Ann. Bot. 1976, 40, 361–371.
- Blackman, E.; Parry, D.W. Opaline silica deposition in rye (Secale cereale L.). Ann. Bot. 1968, 32, 199–206.
- Blackman, E. Observations on the development of the silica cells of the leaf sheath of wheat (Triticum aestivum). Can. J. Bot. 1969.
- Blackman, E. The pattern and sequence of opaline silica deposition in rye (Secale cereale L.). Ann. Bot. 1968, 32, 207–218.
- Blackman, E. Opaline silica bodies in the range grasses of southern Alberta. Can. J. Bot. 1971, 49, 769–781.
- Liang, Y.; Si, J.; Römheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804.
- Henriet, C.; Draye, X.; Oppitz, I.; Swennen, R.; Delvaux, B. Effects, distribution and uptake of silicon in banana (Musa spp.) under controlled conditions. Plant Soil 2006, 287, 359–374.
- Faisal, S.; Callis, K.L.; Slot, M.; Kitajima, K. Transpiration-dependent passive silica accumulation in cucumber (Cucumis sativus) under varying soil silicon availability. Botany 2012, 90, 1058–1064.
- Mitani, N.; Ma, J.F. Uptake system of silicon in different plant species. J. Exp. Bot. 2005, 56, 1255–1261.
- Piperno, D.R.; Holst, I.; Wessel-Beaver, L.; Andres, T.C. Evidence for the control of phytolith formation in Cucurbita fruits by the hard rind (Hr) genetic locus: Archaeological and ecological implications. Proc. Natl. Acad. Sci. USA 2002, 99, 10923–10928.
- Ma, J.F.; Tamai, K.; Yamaji, N.; Mitani, N.; Konishi, S.; Katsuhara, M.; Ishiguro, M.; Murata, Y.; Yano, M. A silicon transporter in rice. Nature 2006, 440, 688–691.
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212.
- Yamaji, N.; Mitani, N.; Ma, J.F. A transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389.
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442.
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563.
- Yamaji, N.; Sakurai, G.; Mitani-Ueno, N.; Ma, J.F. Orchestration of three transporters and distinct vascular structures in node for intervascular transfer of silicon in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 11401–11406.
- Yan, G.; Fan, X.; Tan, L.; Yin, C.; Li, T.; Liang, Y. Root silicon deposition and its resultant reduction of sodium bypass flow is modulated by OsLsi1 and OsLsi2 in rice. Plant Physiol. Biochem. 2021, 158, 219–227.
- Yan, G.C.; Nikolic, M.; Ye, M.J.; Xiao, Z.X.; Liang, Y.C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150.
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315.
- Mitani, N.; Yamaji, N.; Ago, Y.; Iwasaki, K.; Ma, J.F. Isolation and functional characterization of an influx silicon transporter in two pumpkin cultivars contrasting in silicon accumulation. Plant J. 2011, 66, 231–240.
- Wang, H.S.; Yu, C.; Fan, P.P.; Bao, B.F.; Li, T.; Zhu, Z.J. Identification of two cucumber putative silicon transporter genes in cucumis sativus. J. Plant Growth Regul. 2015, 34, 332–338.
- Sahebi, M.; Hanafi, M.M.; Abdullah, S.N.A.; Rafii, M.Y.; Azizi, P.; Nejat, N.; Idris, A.S. Isolation and expression analysis of novel silicon absorption gene from roots of mangrove (Rhizophora apiculata) via suppression subtractive hybridization. BioMed Res. Int. 2014, 2014, 971985.
- Grégoire, C.; Rémus-Borel, W.; Vivancos, J.; Labbé, C.; Belzile, F.; Bélanger, R.R. Discovery of a multigene family of aquaporin silicon transporters in the primitive plant Equisetum arvense. Plant J. 2012, 72, 320–330.
- Trembath-Reichert, E.; Wilson, J.P.; McGlynn, S.E.; Fischer, W.W. Four hundred million years of silica biomineralization in land plants. Proc. Natl. Acad. Sci. USA 2015, 112, 5449–5454.
- Yamaji, N.; Ma, J.F. Spatial distribution and temporal variation of the rice silicon transporter Lsi1. Plant Physiol. 2007, 143, 1306–1313.
- Kumar, N.; Dubey, A.K.; Upadhyay, A.K.; Gautam, A.; Ranjan, R.; Srikishna, S.; Sahu, N.; Behera, S.K.; Mallick, S. GABA accretion reduces Lsi-1 and Lsi-2 gene expressions and modulates physiological responses in Oryza sativa to provide tolerance towards arsenic. Sci. Rep. 2017, 7, 8786.
- Chaiwong, N.; Bouain, N.; Prom-u-thai, C.; Rouached, H. Interplay between silicon and iron signaling pathways to regulate silicon transporter Lsi1 expression in rice. Front. Plant Sci. 2020, 11, 1065.
- Bokor, B.; Bokorová, S.; Ondoš, S.; Švubová, R.; Lukačová, Z.; Hýblová, M.; Szemes, T.; Lux, A. Ionome and expression level of Si transporter genes (Lsi1, Lsi2, and Lsi6) affected by Zn and Si interaction in maize. Environ. Sci. Pollut. Res. 2015, 22, 6800–6811.
- Lux, A.; Luxová, M.; Abe, J.; Tanimoto, E.; Hattori, T.; Inanaga, S. The dynamics of silicon deposition in the sorghum root endodermis. New Phytol. 2003, 158, 437–441.
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466.
- de Melo, S.P.; Korndörfer, G.H.; Korndörfer, C.M.; Lana, R.M.Q.; de Santana, D.G. Silicon accumulation and water deficit tolerance in Brachiaria grasses. Sci. Agric. 2003, 60, 755–759.
- Mayland, H.F.; Wright, J.L.; Sojka, R.E. Silicon accumulation and water uptake by wheat. Plant Soil 1991, 137, 191–199.
- Jenkins, E.; Jamjoum, K.; Nuimat, S.; Stafford, R.; Nortcliff, S.; Mithen, S. Identifying ancient water availability through phytolith analysis: An experimental approach. J. Archaeol. Sci. 2016, 73, 82–93.
- Jenkins, E.; Jamjoum, K.; Al Nuimat, S. Irrigation and phytolith formation: An experimental study. Water Life Civilis. 2011, 347–372.
- Rosen, A.M.; Weiner, S. Identifying ancient irrigation: A new method using opaline phytoliths from emmer wheat. J. Archaeol. Sci. 1994, 21, 125–132.
- Katz, O.; Lev-Yadun, S.; Bar (Kutiel), P. Plant silicon and phytolith contents as affected by water availability and herbivory: Integrating laboratory experimentation and natural habitat studies. Silicon 2018, 10, 2387–2389.
- Euliss, K.W.; Dorsey, B.L.; Benke, K.C.; Banks, M.K.; Schwab, A.P. The use of plant tissue silica content for estimating transpiration. Ecol. Eng. 2005, 25, 343–348.
- Katz, O.; Lev-Yadun, S.; Pua Bar, K. Plasticity and variability in the patterns of phytolith formation in Asteraceae species along a large rainfall gradient in Israel. Flora 2013, 208, 438–444.
- Katz, O.; Lev-Yadun, S.; Bar, P. Do phytoliths play an antiherbivory role in southwest Asian Asteraceae species and to what extent? Flora 2014, 209, 349–358.
- Johnston, A.; Bezeau, L.M.; Smoliak, S. Variation in silica content of range grasses. Can. J. Plant Sci. 1967, 47, 65–71.
- Webb, E.A.; Longstaffe, F.J. The relationship between phytolith- and plant-water δ 18O values in grasses. Geochim. Cosmochim. Acta 2003, 67, 1437–1449.
- Frew, A.; Allsopp, P.G.; Gherlenda, A.N.; Johnson, S.N. Increased root herbivory under elevated atmospheric carbon dioxide concentrations is reversed by silicon-based plant defences. J. Appl. Ecol. 2017, 54, 1310–1319.
- Takahashi, N.; Isogai, A.; Ling, P.P.; Kato, Y.; Kurata, K. Effects of elevated atmospheric carbon dioxide concentration on silica deposition in rice (Oryza sativa L.) panicle. Plant Prod. Sci. 2008, 11, 307–315.
- Li, N.N.; Jie, D.M.; Ge, Y.; Guo, J.X.; Liu, H.Y.; Liu, L.D.; Qiao, Z.H. Response of phytoliths in Phragmites communis to elevated CO2 concentration in Songnen Grassland, China. Quat. Int. 2014, 321, 97–104.
- Hartley, S.E.; DeGabriel, J.L. The ecology of herbivore-induced silicon defences in grasses. Funct. Ecol. 2016, 30, 1311–1322.
- Massey, F.P.; Roland Ennos, A.; Hartley, S.E. Herbivore specific induction of silica-based plant defences. Oecologia 2007, 152, 677–683.
- Kvedaras, O.L.; An, M.; Choi, Y.S.; Gurr, G.M. Silicon enhances natural enemy attraction and biological control through induced plant defences. Bull. Entomol. Res. 2010, 100, 367–371.
- Islam, T.; Moore, B.D.; Johnson, S.N. Novel evidence for systemic induction of silicon defences in cucumber following attack by a global insect herbivore. Ecol. Entomol. 2020, 45, 1373–1381.
- Johnson, S.N.; Reynolds, O.L.; Gurr, G.M.; Esveld, J.L.; Moore, B.D.; Tory, G.J.; Gherlenda, A.N. When resistance is futile, tolerate instead: Silicon promotes plant compensatory growth when attacked by above- and belowground herbivores. Biol. Lett. 2019, 15, 20190361.
- Soininen, E.M.; Bråthen, K.A.; Jusdado, J.G.H.; Reidinger, S.; Hartley, S.E. More than herbivory: Levels of silica-based defences in grasses vary with plant species, genotype and location. Oikos 2013, 122, 30–41.
- Massey, F.P.; Ennos, A.R.; Hartley, S.E. Grasses and the resource availability hypothesis: The importance of silica-based defences. J. Ecol. 2007, 95, 414–424.
- McNaughton, S.J.; Tarrants, J.L. Grass leaf silicification: Natural selection for an inducible defense against herbivores. Proc. Natl. Acad. Sci. USA 1983, 80, 790–791.
- Bañuelos, M.J.; Obeso, J.R. Effect of grazing history, experimental defoliation, and genotype on patterns of silicification in Agrostis tenuis Sibth. Ecoscience 2000, 7, 45–50.
- Garbuzov, M.; Reidinger, S.; Hartley, S.E. Interactive effects of plant-available soil silicon and herbivory on competition between two grass species. Ann. Bot. 2011, 108, 1355–1363.
- Quigley, K.M.; Anderson, T.M. Leaf silica concentration in Serengeti grasses increases with watering but not clipping: Insights from a common garden study and literature review. Front. Plant Sci. 2014, 5, 568.
- Cid, M.S.; Detling, J.K.; Brizuela, M.A.; Whicker, A.D. Patterns in grass silicification: Response to grazing history and defoliation. Oecologia 1989, 80, 268–271.
- Kindomihou, V.M.; Dagbénonbakin, G.D.; Bognonkpe, J.P.; Sinsin, B.A.; Meerts, P.J. Silica concentration is related to leaf traits but not to a specific anatomical tissue in tropical fodder grass species. Eur. J. Sci. Res. 2011, 62, 559–570.
- Brizuela, M.A.; Detling, J.K.; Cid, M.S. Silicon concentration of grasses growing in sites with different grazing histories. Ecology 1986, 67, 1098–1101.
- Massey, F.P.; Smith, M.J.; Lambin, X.; Hartley, S.E. Are silica defences in grasses driving vole population cycles? Biol. Lett. 2008, 4, 419–422.
- Huitu, O.; Forbes, K.M.; Helander, M.; Julkunen-Tiitto, R.; Lambin, X.; Saikkonen, K.; Stuart, P.; Sulkama, S.; Hartley, S. Silicon, endophytes and secondary metabolites as grass defenses against mammalian herbivores. Front. Plant Sci. 2014, 5, 478.
- Reynolds, J.J.H.; Lambin, X.; Massey, F.P.; Reidinger, S.; Sherratt, J.A.; Smith, M.J.; White, A.; Hartley, S.E. Delayed induced silica defences in grasses and their potential for destabilising herbivore population dynamics. Oecologia 2012, 170, 445–456.
- Melzer, S.E.; Knapp, A.K.; Kirkman, K.P.; Smith, M.D.; Blair, J.M.; Kelly, E.F. Fire and grazing impacts on silica production and storage in grass dominated ecosystems. Biogeochemistry 2010, 97, 263–278.
- Hartley, S.E. Round and round in cycles? Silicon-based plant defences and vole population dynamics. Funct. Ecol. 2015, 29, 151–153.





