Plants’ ability to take up silicon from the soil, accumulate it within their tissues and then reincorporate it into the soil through litter creates an intricate network of feedback mechanisms in ecosystems. Here, we provide a concise review of silicon’s roles in soil chemistry and physics and in plant physiology and ecology, focusing on the processes that form these feedback mechanisms.
- silicon
- soil
- plants
- cycling
- ecosystem
- services
- feedback
1. Introduction
Silicon (Si) uptake and accumulation is a functional trait with multiple implications for plant biology and ecology [1,2]. Silicon’s manifold functions in plant biology include protection from a myriad of abiotic and biotic stresses and confers many benefits to plants that are capable of taking up and accumulating large amounts of it, ranging from practically zero in some taxa to 5% dry weight (and in extreme cases even more) in grasses [1,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17], and probably even to those plants that take up considerably smaller amounts [5,18]. Moreover, its uptake from the soil and eventual reincorporation into the soil through plant litter and herbivore feces also affects soil properties and Si cycling [19,20,21,22,23]. Thus, Si uptake, accumulation and cycling by plants is a key phenomenon in many ecosystems [12,21,24,25,26], with direct and indirect implications for ecosystem properties and processes [1]. Here, we review the existing knowledge of Si in the soil–plant continuum, its roles in plant biology and ecology, in ecosystem processes, and the possible implications for various ecosystem services.
2. Historical Overview
In 1787, Antoine Lavoisier predicted the existence of the element Si, to which Sir Humphry Davy proposed the name “silicon” in 1808. It was eventually isolated and formally discovered in 1823 by Jöns Jacob Berzelius. The discovery of the occurrence of Si within plants quickly followed, owing to the works of some prominent scholars, including microscopy pioneer Christian Gottfried Ehrenberg (who suggested the word “phytolitharia” to describe plant mineral components) [27] and Charles Darwin, who provided him with some samples [28,29]. Silicon effects on plant performance are known for more than 170 years, starting with Struve in 1835 [30], and shortly after Davy’s pioneering publication from 1846, who suggested that Si is present in the epidermis of grasses, where it strengthens the plants and makes them more resistant to attacks by insects and “parasitical plants” [31]. A surge of research soon followed. Sachs [32] showed in 1862 that Si-accumulating plants were a less preferred food than their conspecific plants that were grown hydroponically in Si-poor media. He further found that Si accumulation started in plant hairs and further advanced into the epidermis and near leaf vascular tissues. He also suggested that not all Si deposits in plant leaves are hard, but some may remain in a colloidal state. Miliarakis [33] found in 1884 that basal (younger) leaf parts of Si-accumulating plants had lower Si concentrations than older leaf tips and could not deter feeding. He also found that horsetail (Equisetum) and sedge (Carex and Scirpus) old leaf sheaths had a high Si concentration and protected the younger and less silicified plant tissues from herbivory. Furthermore, in 1884, Kreuzhage and Wolff [34] suggested the importance of Si for oat plants. Kerner von Marilaun [35] suggested in 1887 that the sharp leaf margin of sedges may be due to Si deposits. In 1888, Stahl [36] summarized other studies and concluded that silica deposits in horsetails impeded grazing by snails. He also mentioned that plant tissue silicification reduced the food quality of tropical grasses in Africa for domestic animals.
A second surge of studies started in the 1920s. Between 1922 and 1925, Lemmermann and colleagues [37,38,39] found an increase in the yield of rye grown under phosphorus deficiency upon Si fertilization. Sommer [40] concluded in the year 1926 that rice plants without Si fertilization suffered from early increased leaf wilting, guttation, and retarded panicle formation. In parallel, a surge of studies on Si effects on rice took place in Japan, starting in the 1910s and continuing into the 1940s, summarized thoroughly by Ma and Takahashi [41]. In the 1910s, Onodera [42] found that blast-infected rice leaves had a lower Si concentration than healthy leaves. Miyake et al. [43] also found in 1922 that Si concentration was higher in blast-resistant plants than nonresistant (surprisingly, this specific Japanese research was published in German). Other studies showed an increased blast-resistance of rice plants following Si application [44,45]. In the 1930s Ishibashi [46,47,48] showed reduced growth and yield of rice plants under Si deficiency and reduced blast after Si fertilization. Raleigh [49] showed in 1939 that Si was strongly improving the growth of beet plants. A year later, Wagner [50] showed that Si protects plants against powdery mildew by silicifying host plants’ cell walls, hence reducing penetration.
The study of silicon in plants continued shortly following the end of World War II, and especially since the 1960s. Engel [51] found in 1953 that the Si accumulated in rye culm was ~1/3 easily (hot water) extractable, suggesting no strong binding of this fraction to the plant cell walls. Holzapfel and Engel [52] found that Si accumulation in wheat increases over a study period of 30 days. In 1962, Yoshida [53] discovered the cuticle–Si double layer and suggested that this layer may be responsible for plant resistance against fungal diseases. There were several publications by Okuda and Takahashi, who found that Si improves plant resistance to metals [54] and rice growth and nutrition of [55]. Many of these studies were reviewed by Lewin and Reimann at the end of the 1960s [56]. During the same period, Jones and his colleagues (mainly Handreck) published some seminal papers on the occurrence, uptake, localization and functions of Si in oats [57,58,59,60] and clover [61]. At around the same time, a group that developed around Parry and his successor Sangster (both of which have passed away only in the past decade in their 90 s) further looked into the physiology of Si uptake and deposition [62,63,64,65,66,67,68,69,70,71]. Their early studies set the basis for a greater effort in Si plant and soil research, and a third surge that started in the 1980s and continues to these days, initiated by many important scientists and continuing to these days by the many who follow their footsteps.
3. Silicon Uptake by Plants
3.1. Active Uptake by Intrinsic Transporters
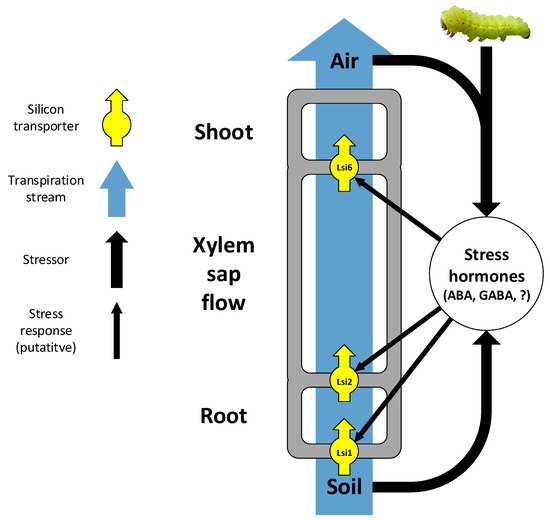
3.2. External Factors Affecting Silicon Uptake
In addition to intrinsic transporters, external factors also affect Si uptake and accumulation in plants. These include both passive uptake mechanisms driven by the transpiration stream (the soil–plant–air continuum; Figure 1) and active mechanisms induced or enhanced by biotic stressors. Since Si is taken up from the soil as monosilicic acid within the soil solution, passive Si uptake depends on the transpiration stream. Several studies have shown that plant Si content in grasses increases with soil water content and availability, most probably for the simple reason that the more water a plant absorbs, the more Si is taken up with it [127,128,129,130,131,132,133]. On the other hand, transpiration, acting as the motive force of water uptake, has also been shown to increase Si content in grasses, to the degree that Si content has been suggested to serve as an indicator to plant transpiration stress [113,133,134,135,136,137]. Hence, along large rainfall gradients, Si content tends to demonstrate a U-shaped curve (minimum Si content at approximately 200–300 mm mean annual rainfall), implying an interplay between water availability and transpiration motive force [134,136]. Nevertheless, high plant Si contents in extremely arid conditions may also occur because grasses under drought stress take up Si more actively for the benefit it confers in resisting drought or in herbivore deterrence (see Section 5 below) [134,136,137]. Nevertheless, the failure to observe this positive correlation in other studies of grasses [138,139] suggests that other variables may confound the simple positive effect of water availability [134,136] (For Si effects on soil water availability co-appearing with Si availability in soils, see Section 3.2 above). In the Asteraceae family, an intermediate Si accumulator with mostly passive Si uptake (as far as we know, no attempts were made so far to identify Si transporters in this family), there appears to be no clear, consistent pattern, suggesting that Si uptake is not simply driven by the transpiration stream [134,136]. That the expression of the Lsi1 gene in rice is down-regulated by dehydration stress and abscisic acid [123] is a further indication for the complex effects of the transpiration stream on Si uptake.
Several studies have shown that ambient CO2 concentrations also affect plant Si uptake, content and form, but with contradictory results. Ambient CO2 concentrations had no effect on root and shoot Si contents in sugarcane plants [140]. In rice, increased ambient CO2 concentrations reduced husk Si deposition by as much as 60% [141]. Increased ambient CO2 concentrations alter the composition of phytolith assemblages in Phragmites and reduce mean phytolith size [142], suggesting an effect on Si allocation and distribution. Despite these studies being limited and equivocal regarding the regulatory role of CO2 on plant Si uptake and accumulation, they harness potential significance for our understanding of global Si–carbon relationships, namely the possibility of Si being a partial substitute for carbon in plants (see Section 5 below) and Si’s role in regulating the carbon cycle (see Section 6 below).
Among the biotic stressors known to affect plant Si content, herbivory is the one that was studied the most [143]. Exposure to invertebrate [144,145,146,147] and vertebrate [144,148] herbivores induces Si uptake and accumulation in grasses. Comparable induction by artificial clipping [149,150,151] further supports that this induction is directly associated with biomass removal or damage. While such induction was sometimes not observed in controlled experiments [144,151,152,153,154,155], this is likely because these experiments did not incorporate sufficiently long exposure times to initiate a response [134,144]. In natural landscapes, higher grass Si contents are associated with larger densities of herbivorous rodents [154,156,157,158,159], but not of larger herbivores [137,148,160] or following clipping [151], which is most likely explained by the involvement of other environmental variables in natural ecosystems having stronger effects on Si uptake and accumulation [134]. Nevertheless, recent evidence for cyclic dynamics of vole densities and grass Si contents [157,159,161] provides further support to the induction of grass Si uptake by herbivory. Among the Asteraceae, the only non-grass family in which the possible effect of herbivory on Si has been widely studied, such an effect was rare and weak [137].
This entry is adapted from the peer-reviewed paper 10.3390/plants10040652
