
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Julio Galvez | + 1724 word(s) | 1724 | 2021-03-24 07:30:03 | | | |
| 2 | Dean Liu | -10 word(s) | 1714 | 2021-04-06 11:18:55 | | | | |
| 3 | Dean Liu | -179 word(s) | 1535 | 2021-04-15 06:10:07 | | |
Video Upload Options
Limosilactobacillus fermentum CECT5716 has become one of the most promising probiotics and it has been described to possess potential beneficial effects on inflammatory processes and immunological alterations.
1. Introduction
The joint Food and Agriculture Organization (FAO) and World Health Organization (WHO) defined probiotics as “live microorganisms which when consumed in adequate amounts, confer a health effect on the host” [1]. Different characteristics are usually required to consider a microorganism as a probiotic, including: (i) must be taxonomically characterized, (ii) able to survive to the human intestinal environmental conditions, (iii) alive in sufficient numbers in the product at an efficacious dose throughout shelf life, (iv) supported by at least one positive human clinical trial conducted according to generally accepted scientific standards, and (v) safe for the intended use. Concerning the latter, most of the probiotics are categorized by Food and Drug Administration (FDA) as Generally Recognized as Safe (GRAS).
The microorganisms mostly considered as probiotics belong to the Lactobacillus and Bifidobacterium genera, but also to other lactic acid bacteria, such as Lactococcus spp. and Streptococcus thermophilus. Other probiotic strains include the genera Bacillus, Escherichia (E. coli Nissle 1917), and Propionibacterium or yeasts like Saccharomyces boulardii.
Probiotics exert their health-associated activities through some of the following general actions (Figure 1):
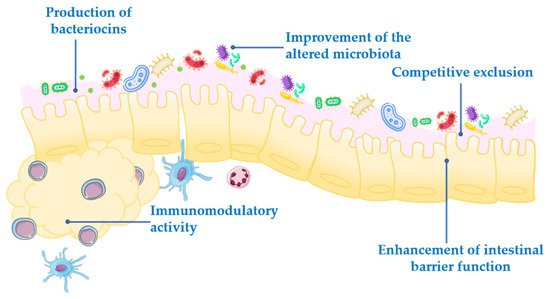
Figure 1. Mechanism of action of probiotics.
-
Competitive exclusion of pathogenic microorganisms. This occurs when one species of bacteria competes for receptor sites in the intestinal tract more actively than other species [2].
-
Enhancement of intestinal barrier function. The intestinal barrier function plays an important role in the absorption of nutrients from food and, at the same time, prevents the access of potentially harmful bacteria to the human body [3]. When the gut barrier is disrupted, food antigens and pathogenic microorganisms can develop intestinal disorders, mainly associated with a local inflammatory response [4]. It has been proposed that probiotics maintain the epithelial barrier function, through increased expression of junction proteins or mucins, and promote intestinal epithelial cell activation in response to bacterial infection [5][6].
-
Production of bacteriocins. These are antimicrobial peptides that prevent the proliferation of selected pathogens [7].
-
Improvement of the altered microbiota composition. In normal conditions, the gut is colonized by a large number of microorganisms in balance, to provide energy and nutrition, maintain the intestinal immune homeostasis and protect the intestinal structure [8]. This balance is altered in many diseases, leading to a situation known as dysbiosis [9].
-
Modulation of the immune response. Immunomodulation can be achieved by several mechanisms, including the modulation in the expression and/or production of anti- and pro-inflammatory cytokines [10] or increased production of immunoglobulins (Ig) [11].
In addition to these general actions exerted by the probiotics, it has been reported that other effects can also participate, which may be species- or even strain specific. However, the exact underlying mechanisms of action for each probiotic are still unclear, and the efficacy following their administration is quite different depending on the probiotic strain. Therefore, a deeper understanding of the mechanisms involved in the beneficial effects exerted by probiotics is especially relevant, and it should be considered that they must be characterized for each specific probiotic.
2. Limosilactobacillus Fermentum
Lactic acid bacteria (LAB) belong to the phylum Firmicutes, class Bacilli, order Lactobacillales. They are considered to play important roles in food production, nutritional supplementation, agriculture, as well as in veterinary and human medicine [12]. LAB are Gram-positive bacteria, generally without catalase activity [13] that are able to produce lactic acid as the main end-product after carbohydrate fermentation. Different genera are considered in this LAB group: Aerococcus, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus, and Weissella [14]. LAB are present in different ecological niches, and many studies have established differences when considering their genetic and physiology [15]. The genus Lactobacillus is certainly the most studied genus in the LAB group, and more than 240 species have been reported to be included [16], which can be found at different localizations in the human body, including the gastrointestinal tract, as well as the urinary and genital systems. As commented above, this genus is considered one of the most important representative groups of probiotics [17], and particularly, Lactobacillus fermentum has become one of the most promising probiotics. In fact, it is used as a standard reference species in comparative studies with other probiotics, due to their beneficial health properties [17].
Recently, whole genome studies have been performed, and the taxonomy of Lactobacillaceae has been newly evaluated. Thus, the previous name, Lactobacillus fermentum, has been changed by Limosilactobacillus fermentum [18].
L. fermentum is a species with many strains isolated from different environments, including fermenting plant materials [19], dairy products [20], bread [21], naturally fermented sausages [22], breast milk [23], saliva [24], and human feces [25]. Remarkably, several L. fermentum strains have been described to possess promising beneficial effects both in preclinical studies (in vitro and in vivo models) and in human trials, which, in fact, have resulted in the development of different probiotic preparations for medical application and food preservation processes [26]. Actually, it has been suggested a potential role of L. fermentum in inflammatory-related diseases including intestinal inflammation [27], respiratory tract infections [28] and hepatic injury [29].
Table 1. In vitro studies: mechanisms of action of L. fermentum CECT5716.
| Experimental Models | Mechanisms of Action | Cell Model | Reference |
|---|---|---|---|
| Epithelial cell lines | ↓ Expression of pro-inflammatory profile (Il-6) and ↑ the mucins in stimulated cells | CMT-93 | [30] |
| Restoration of miRNA-150, miRNA-155, and miRNA-375 expression | |||
| ↓ NO, IL-8, and IL-1β in stimulated cells | Caco-2 | [31] | |
| ↓ MAPK p42/44 ERK and p38 in stimulated cells | |||
| Immune cells | ↓ Pro-inflammatory mediators of stimulated cells (TNF-ɑ and IL-1β) and ↑ anti-inflammatory mediators (IL-10) | BMDM | [32] |
| Restoration of miRNA-150, miRNA-155, and miRNA-375 expression | [30] | ||
| ↓ IL-1β and NO production in stimulated cells | RAW-264.7 | [31] | |
| Enhanced immune responses: Induction of the production of cytokines (TNFα, IL-1β, IL-8, MIP-1α, MIP-1β, and GM-CSF), activation of NK and T cell subsets, expansion of Treg cells | PBMC | [33] |
Table 2. Mechanisms of action of L. fermentum CECT5716 in animal models.
| Experimental Models | Mechanisms of Action | Animal Model | Reference |
|---|---|---|---|
| Experimental colitis | ↓ Immune response: - Tnf-ɑ, iNos, and Il-6 expression. - LTB4 and TNF-ɑ protein levels. - MPO activity. |
Rat | [31][34] |
| ↑ Antioxidant activity: GSH content. | |||
| Induced growth of Lactobacilli species and increased more than doubled the production of the SCFAs (acetate, butyrate, and propionate) | |||
| Amelioration of the weight decrease in a 20% and amelioration of diarrhea incidence and gut dysbiosis | Mouse | [27][30] | |
| ↓ Tnf-ɑ, Il-1β, iNOS and Mmp-9 expression | |||
| Restoration of miR-155 and miR-223 expression | |||
| Microbiota restoration: increase microbial diversity and restore the F/B ratio. | |||
| Metabolic syndrome | Prevent liver steatosis and inflammatory status | Rat | [35] |
| ↓ Glucose and insulin levels in plasma | |||
| Gut dysbiosis restoration by preventing the increase in Bacteroidetes and the reduction in Firmicutes. Increase the levels of Akkermansia muciniphila |
|||
| Prevention of hypertriglyceridemia and hyperleptinemia | |||
| ↓ Body weight gain in 15–20% | Mouse | [36] | |
| Amelioration of glucose and lipid metabolism | |||
| ↑ Glut-4 expression | |||
| ↓ Tnf-α and Il-1β expression and inhibition of NADPH activity in aortic tissue | |||
| Restoration of impaired endothelial disfunction | |||
| Anti-inflammatory properties: ↓ Il-6, Tnf-α, Mcp-1, and Jnk-1 expression in liver and fat | |||
| Amelioration of obesity-associated dysbiosis: - ↑ Richness and diversity - Restore F/B ratio, decreasing it. - Restore levels of Verrumicrobia, Akkermansia and Bacteroides. - ↑ Lactate- and acetate-producing genera. |
|||
| Enhanced intestinal epithelial integrity (↑ occludin levels) and ↓ LPS plasma level |
|||
| Systemic lupus erythematosus | Prevention gut dysbiosis: - Restore F/B ratio. - ↑ Bifidobacterium and Parabacteroides genera. - ↓ Blautia and Lachnospira. |
Mouse | [37] |
| ↓ Pro-inflammatory cytokine (Tnf-α and Il-1β expression) /plasma levels of LPS | |||
| Intestinal integrity amelioration (↑ Zo-1 and Occludin expression) |
|||
| ↓ Hypertension | |||
| Prevention of the endothelial dysfunction (↑ acetylcholine-induced vasodilation) | |||
| ↓ NAPDH oxidase activity | |||
| Prevention of the altered T-cell polarization | |||
| Pregnancy and lactation stage | ↓ Cytotoxic T cells | Rat | [38] |
As expected, the mechanisms involved in the proposed beneficial effects reported for these probiotics include the general mechanisms described above [39][40]. Among these, the immunomodulatory properties have been proposed to have a key role in many strains of L. fermentum, since they are able to interact with immune cells, like macrophages and dendritic cells, as well as to regulate the synthesis and release of different cytokines [41][42]. In addition, L. fermentum strains have been proposed to exert bacteriostatic effects against a variety of pathogenic bacteria and fungi, including Staphylococcus aureus [43], Candida albicans [44], Helicobacter pylori [45], Campylobacter jejuni [46], and Aspergillus parasiticus [47], derived from their ability to produce organic acids (primarily lactic and acetic acids) and/or antimicrobial peptides [26][48]. Furthermore, it has been reported that some L. fermentum strains possess a complete glutathione-associated system, including the synthesis, transport, uptake, and redox cycling of this antioxidant peptide [49][50], thus providing protection against oxidative stress.
However, of all strains identified from L. fermentum, L. fermentum CECT5716 is one of the probiotics with more potential. Different studies have reported its possible beneficial effects in different pathologies [37][30]. However, the precise mechanisms underlying remain unknown. Therefore, it is still necessary to conduct more investigations to identify its mechanisms of action and possible interactions with the host. Here, we will summarize and provide updated information on its effect on host health, mechanisms, and therapeutic insights.
2.1. Limosilactobacillus fermentum CECT5716
L. fermentum CECT5716 is a probiotic strain initially isolated from the human breast milk of healthy mothers, and for over 15 years, it has been included in nutrition supplements and fermented milk products [23]. The application of whole-genome shotgun strategies provided the identification of its genome using L. fermentum IFO 3956 as reference. Both strains are highly similar, with the exception of 16 protein encoding genes that are not present in IFO 3965 [51]. Thus, the genome of L. fermentum CECT5716 is composed of 2100449 bp and contains 1109 protein encoding genes, 54 tRNA encoding genes, and 20 rRNA encoding genes. It is a circular chromosome with a CG content of 51.49%, with no plasmid, and includes putative enzymes with an important role in the metabolism of purines (allantoinase, guanosine monophosphate (GMP) oxidoreductase, GMP synthase), amino acids (serine-pyruvate transaminase, 3 glutamate synthases), lipids (acyltransferase), and carbohydrates (mannose-6-phosphate isomerase) (GenBank/EMBL under accession no. CP002033) [51].
References
- Health and Nutritional Properties and Guidelines for Evaluation; FAO Food and Nutrition Paper; WHO: Geneva, Switzerland; FAO: Rome, Italy, 2006.
- Munoz-Quezada, S.; Bermudez-Brito, M.; Chenoll, E.; Genoves, S.; Gomez-Llorente, C.; Plaza-Diaz, J.; Matencio, E.; Bernal, M.J.; Romero, F.; Ramon, D.; et al. Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens. Br. J. Nutr. 2013, 109 (Suppl. 2), S63–S69.
- Ohland, C.L.; Macnaughton, W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G807–G819.
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512.
- Zhang, W.; Zhu, Y.H.; Yang, J.C.; Yang, G.Y.; Zhou, D.; Wang, J.F. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS ONE 2015, 10, e0125717.
- Hummel, S.; Veltman, K.; Cichon, C.; Sonnenborn, U.; Schmidt, M.A. Differential targeting of the E-Cadherin/beta-Catenin complex by gram-positive probiotic lactobacilli improves epithelial barrier function. Appl. Environ. Microbiol. 2012, 78, 1140–1147.
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic mechanisms of action. Ann. Nutr. Metab. 2012, 61, 160–174.
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic species in the modulation of gut microbiota: An overview. Biomed. Res. Int. 2018, 2018, 9478630.
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176.
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory effects of robiotics on cytokine profiles. Biomed. Res. Int. 2018, 2018, 8063647.
- Guarner, F.; Malagelada, J.R. Gut flora in health and disease. Lancet 2003, 361, 512–519.
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684.
- Engesser, D.M.; Hammes, W.P. Non-heme catalase activity of lactic acid bacteria. Syst. Appl. Microbiol. 1994, 17, 11–19.
- Stiles, M.E.; Holzapfel, W.H. Lactic acid bacteria of foods and their current taxonomy. Int. J. Food Microbiol. 1997, 36, 1–29.
- Ait Seddik, H.; Bendali, F.; Cudennec, B.; Drider, D. Anti-pathogenic and probiotic attributes of Lactobacillus salivarius and Lactobacillus plantarum strains isolated from feces of Algerian infants and adults. Res. Microbiol. 2017, 168, 244–254.
- Haakensen, M.; Dobson, C.M.; Hill, J.E.; Ziola, B. Reclassification of Pediococcus dextrinicus (Coster and White 1964) back 1978 (Approved Lists 1980) as Lactobacillus dextrinicus comb. nov., and emended description of the genus Lactobacillus. Int. J. Syst. Evol. Microbiol. 2009, 59, 615–621.
- Salvetti, E.; Torriani, S.; Felis, G.E. The genus Lactobacillus: A axonomic update. Probiotics Antimicrob. Proteins 2012, 4, 217–226.
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858.
- Nielsen, D.S.; Cho, G.S.; Hanak, A.; Huch, M.; Franz, C.M.; Arneborg, N. The effect of bacteriocin-producing Lactobacillus plantarum strains on the intracellular pH of sessile and planktonic Listeria monocytogenes single cells. Int. J. Food Microbiol. 2010, 141 (Suppl. 1), S53–S59.
- Coton, M.; Berthier, F.; Coton, E. Rapid identification of the three major species of dairy obligate heterofermenters Lactobacillus brevis, Lactobacillus fermentum and Lactobacillus parabuchneri by species-specific duplex PCR. FEMS Microbiol. Lett. 2008, 284, 150–157.
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Duenas, M.T.; Lopez, P.; Fiocco, D.; Spano, G. Riboflavin-overproducing strains of Lactobacillus fermentum for riboflavin-enriched bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700.
- Kaban, G.; Kaya, M. Identification of lactic acid bacteria and Gram-positive catalase-positive cocci isolated from naturally fermented sausage (sucuk). J. Food Sci. 2008, 73, M385–M388.
- Martin, R.; Langa, S.; Reviriego, C.; Jiminez, E.; Marin, M.L.; Xaus, J.; Fernandez, L.; Rodriguez, J.M. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 2003, 143, 754–758.
- Dal Bello, F.; Hertel, C. Oral cavity as natural reservoir for intestinal lactobacilli. Syst. Appl. Microbiol. 2006, 29, 69–76.
- Jang, Y.J.; Kim, W.K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711.
- Naghmouchi, K.; Belguesmia, Y.; Bendali, F.; Spano, G.; Seal, B.S.; Drider, D. Lactobacillus fermentum: A bacterial species with potential for food preservation and biomedical applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 3387–3399.
- Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Utrilla, M.P.; Chueca, N.; Garcia, F.; Olivares, M.; Rodriguez-Cabezas, M.E.; Galvez, J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: Impact on microRNAs expression and microbiota composition. Mol. Nutr. Food Res. 2017, 61, 1700144.
- Maldonado, J.; Canabate, F.; Sempere, L.; Vela, F.; Sanchez, A.R.; Narbona, E.; Lopez-Huertas, E.; Geerlings, A.; Valero, A.D.; Olivares, M.; et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 55–61.
- Nardone, G.; Compare, D.; Liguori, E.; Di Mauro, V.; Rocco, A.; Barone, M.; Napoli, A.; Lapi, D.; Iovene, M.R.; Colantuoni, A. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G669–G676.
- Algieri, F.; Garrido-Mesa, J.; Vezza, T.; Rodriguez-Sojo, M.J.; Rodriguez-Cabezas, M.E.; Olivares, M.; Garcia, F.; Galvez, J.; Moron, R.; Rodriguez-Nogales, A. Intestinal anti-inflammatory effects of probiotics in DNBS-colitis via modulation of gut microbiota and microRNAs. Eur. J. Nutr. 2020.
- Rodriguez-Nogales, A.; Algieri, F.; Vezza, T.; Garrido-Mesa, N.; Olivares, M.; Comalada, M.; Riccardi, C.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. The viability of Lactobacillus fermentum CECT5716 is not essential to exert intestinal anti-inflammatory properties. Food Funct. 2015, 6, 1176–1184.
- Diaz-Ropero, M.P.; Martin, R.; Sierra, S.; Lara-Villoslada, F.; Rodriguez, J.M.; Xaus, J.; Olivares, M. Two Lactobacillus strains, isolated from breast milk, differently modulate the immune response. J. Appl. Microbiol. 2007, 102, 337–343.
- Perez-Cano, F.J.; Dong, H.; Yaqoob, P. In vitrolee immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Immunobiology 2010, 215, 996–1004.
- Peran, L.; Camuesco, D.; Comalada, M.; Nieto, A.; Concha, A.; Adrio, J.L.; Olivares, M.; Xaus, J.; Zarzuelo, A.; Galvez, J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int. J. Colorectal Dis. 2006, 21, 737–746.
- Rivero-Gutierrez, B.; Gamez-Belmonte, R.; Suarez, M.D.; Lavin, J.L.; Aransay, A.M.; Olivares, M.; Martinez-Augustin, O.; Sanchez de Medina, F.; Zarzuelo, A. A synbiotic composed of Lactobacillus fermentum CECT5716 and FOS prevents the development of fatty acid liver and glycemic alterations in rats fed a high fructose diet associated with changes in the microbiota. Mol. Nutr. Food Res. 2017, 61, 1600622.
- Molina-Tijeras, J.A.; Diez-Echave, P.; Vezza, T.; Hidalgo-Garcia, L.; Ruiz-Malagon, A.J.; Rodriguez-Sojo, M.J.; Romero, M.; Robles-Vera, I.; Garcia, F.; Plaza-Diaz, J.; et al. Lactobacillus fermentum CECT5716 ameliorates high fat diet-induced obesity in mice through modulation of gut microbiota dysbiosis. Pharmacol. Res. 2021, 105471.
- Toral, M.; Robles-Vera, I.; Romero, M.; de la Visitacion, N.; Sanchez, M.; O’Valle, F.; Rodriguez-Nogales, A.; Galvez, J.; Duarte, J.; Jimenez, R. Lactobacillus fermentum CECT5716: A novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. FASEB J. 2019, 33, 10005–10018.
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, A.; Castell, M.; Guardiola, F.; Perez-Cano, F.J.; Rodriguez-Lagunas, M.J. Lactobacillus fermentum CECT5716 supplementation in rats during pregnancy and lactation impacts maternal and offspring lipid profile, immune system and microbiota. Cells 2020, 9, 575.
- Melo, T.A.; Dos Santos, T.F.; Pereira, L.R.; Passos, H.M.; Rezende, R.P.; Romano, C.C. Functional profile evaluation of Lactobacillus fermentum tcuesc01: A new potential probiotic strain isolated during cocoa fermentation. Biomed. Res. Int. 2017, 2017, 5165916.
- Strompfova, V.; Kubasova, I.; Laukova, A. Health benefits observed after probiotic Lactobacillus fermentum CCM 7421 application in dogs. Appl. Microbiol. Biotechnol. 2017, 101, 6309–6319.
- Barone, M.V.; Zimmer, K.P. Endocytosis and transcytosis of gliadin peptides. Mol. Cell Pediatr. 2016, 3, 8.
- Garcia-Castillo, V.; Komatsu, R.; Clua, P.; Indo, Y.; Takagi, M.; Salva, S.; Islam, M.A.; Alvarez, S.; Takahashi, H.; Garcia-Cancino, A.; et al. Evaluation of the immunomodulatory activities of the probiotic strain Lactobacillus fermentum UCO-979C. Front. Immunol. 2019, 10, 1376.
- Kang, M.S.; Lim, H.S.; Oh, J.S.; Lim, Y.J.; Wuertz-Kozak, K.; Harro, J.M.; Shirtliff, M.E.; Achermann, Y. Antimicrobial activity of Lactobacillus salivarius and Lactobacillus fermentum against Staphylococcus aureus. Pathog. Dis. 2017, 75.
- Rossoni, R.D.; Dos Santos Velloso, M.; Figueiredo, L.M.A.; Martins, C.P.; Jorge, A.O.C.; Junqueira, J.C. Clinical strains of Lactobacillus reduce the filamentation of Candida albicans and protect Galleria mellonella against experimental candidiasis. Folia Microbiol. 2018, 63, 307–314.
- Garcia, A.; Saez, K.; Delgado, C.; Gonzalez, C.L. Low co-existence rates of Lactobacillus spp. and Helicobacter pylori detected in gastric biopsies from patients with gastrointestinal symptoms. Rev. Esp. Enferm. Dig. 2012, 104, 473–478.
- Lehri, B.; Seddon, A.M.; Karlyshev, A.V. Lactobacillus fermentum 3872 as a potential tool for combatting Campylobacter jejuni infections. Virulence 2017, 8, 1753–1760.
- Ghazvini, R.D.; Kouhsari, E.; Zibafar, E.; Hashemi, S.J.; Amini, A.; Niknejad, F. Antifungal activity and aflatoxin degradation of Bifidobacterium bifidum and Lactobacillus fermentum against toxigenic Aspergillus parasiticus. Open Microbiol. J. 2016, 10, 197–201.
- Fuochi, V.; Volti, G.L.; Furneri, P.M. Probiotic properties of Lactobacillus fermentum strains isolated from human oral samples and description of their antibacterial activity. Curr. Pharm. Biotechnol. 2017, 18, 138–149.
- Truusalu, K.; Kullisaar, T.; Hutt, P.; Mahlapuu, R.; Aunapuu, M.; Arend, A.; Zilmer, M.; Mikelsaar, R.H.; Mikelsaar, M. Immunological, antioxidative, and morphological response in combined treatment of ofloxacin and Lactobacillus fermentum ME-3 probiotic in Salmonella typhimurium murine model. APMIS 2010, 118, 864–872.
- Mikelsaar, M.; Zilmer, M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009, 21, 1–27.
- Jimenez, E.; Langa, S.; Martin, V.; Arroyo, R.; Martin, R.; Fernandez, L.; Rodriguez, J.M. Complete genome sequence of Lactobacillus fermentum CECT 5716, a probiotic strain isolated from human milk. J. Bacteriol. 2010, 192, 4800.





