Limosilactobacillus fermentum CECT5716 has become one of the most promising probiotics and it has been described to possess potential beneficial effects on inflammatory processes and immunological alterations.
- probiotic
- Limosilactobacillus fermentum CECT5716
- immunomodulation
- dysbiosis
- mechanisms of action
- gastrointestinal diseases
- microbiota
1. Introduction
The joint Food and Agriculture Organization (FAO) and World Health Organization (WHO) defined probiotics as “live microorganisms which when consumed in adequate amounts, confer a health effect on the host” [1]. Different characteristics are usually required to consider a microorganism as a probiotic, including: (i) must be taxonomically characterized, (ii) able to survive to the human intestinal environmental conditions, (iii) alive in sufficient numbers in the product at an efficacious dose throughout shelf life, (iv) supported by at least one positive human clinical trial conducted according to generally accepted scientific standards, and (v) safe for the intended use. Concerning the latter, most of the probiotics are categorized by Food and Drug Administration (FDA) as Generally Recognized as Safe (GRAS).
The microorganisms mostly considered as probiotics belong to the Lactobacillus and Bifidobacterium genera, but also to other lactic acid bacteria, such as Lactococcus spp. and Streptococcus thermophilus. Other probiotic strains include the genera Bacillus, Escherichia (E. coli Nissle 1917), and Propionibacterium or yeasts like Saccharomyces boulardii.
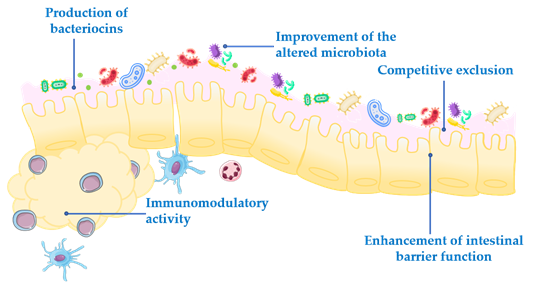
Probiotics exert their health-associated activities through some of the following general actions (Figure 1):
- Competitive exclusion of pathogenic microorganisms. This occurs when one species of bacteria competes for receptor sites in the intestinal tract more actively than other species [2].
Competitive exclusion of pathogenic microorganisms. This occurs when one species of bacteria competes for receptor sites in the intestinal tract more actively than other species [2].
- Enhancement of intestinal barrier function. The intestinal barrier function plays an important role in the absorption of nutrients from food and, at the same time, prevents the access of potentially harmful bacteria to the human body [3]. When the gut barrier is disrupted, food antigens and pathogenic microorganisms can develop intestinal disorders, mainly associated with a local inflammatory response [4]. It has been proposed that probiotics maintain the epithelial barrier function, through increased expression of junction proteins or mucins, and promote intestinal epithelial cell activation in response to bacterial infection [5,6].
Enhancement of intestinal barrier function. The intestinal barrier function plays an important role in the absorption of nutrients from food and, at the same time, prevents the access of potentially harmful bacteria to the human body [3]. When the gut barrier is disrupted, food antigens and pathogenic microorganisms can develop intestinal disorders, mainly associated with a local inflammatory response [4]. It has been proposed that probiotics maintain the epithelial barrier function, through increased expression of junction proteins or mucins, and promote intestinal epithelial cell activation in response to bacterial infection [5,6].
- Production of bacteriocins. These are antimicrobial peptides that prevent the proliferation of selected pathogens [7].
Production of bacteriocins. These are antimicrobial peptides that prevent the proliferation of selected pathogens [7].
- Improvement of the altered microbiota composition. In normal conditions, the gut is colonized by a large number of microorganisms in balance, to provide energy and nutrition, maintain the intestinal immune homeostasis and protect the intestinal structure [8]. This balance is altered in many diseases, leading to a situation known as dysbiosis [9].
Improvement of the altered microbiota composition. In normal conditions, the gut is colonized by a large number of microorganisms in balance, to provide energy and nutrition, maintain the intestinal immune homeostasis and protect the intestinal structure [8]. This balance is altered in many diseases, leading to a situation known as dysbiosis [9].
- Modulation of the immune response. Immunomodulation can be achieved by several mechanisms, including the modulation in the expression and/or production of anti- and pro-inflammatory cytokines [10] or increased production of immunoglobulins (Ig) [11].
Modulation of the immune response. Immunomodulation can be achieved by several mechanisms, including the modulation in the expression and/or production of anti- and pro-inflammatory cytokines [10] or increased production of immunoglobulins (Ig) [11].
In addition to these general actions exerted by the probiotics, it has been reported that other effects can also participate, which may be species- or even strain specific. However, the exact underlying mechanisms of action for each probiotic are still unclear, and the efficacy following their administration is quite different depending on the probiotic strain. Therefore, a deeper understanding of the mechanisms involved in the beneficial effects exerted by probiotics is especially relevant, and it should be considered that they must be characterized for each specific probiotic.
The aim of this review is to provide an overview of the current applications of the probiotic Limosilactobacillus fermentum CECT5716 and its potential use in different conditions, based on its specific mechanisms reported in different preclinical and clinical studies.
2. Limosilactobacillus fermentum
Lactic acid bacteria (LAB) belong to the phylum Firmicutes, class Bacilli, order Lactobacillales. They are considered to play important roles in food production, nutritional supplementation, agriculture, as well as in veterinary and human medicine [12]. LAB are Gram-positive bacteria, generally without catalase activity [13] that are able to produce lactic acid as the main end-product after carbohydrate fermentation. Different genera are considered in this LAB group: Aerococcus, Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, Oenococcus, Pediococcus, Streptococcus, Tetragenococcus, Vagococcus, and Weissella [14]. LAB are present in different ecological niches, and many studies have established differences when considering their genetic and physiology [15]. The genus Lactobacillus is certainly the most studied genus in the LAB group, and more than 240 species have been reported to be included [16], which can be found at different localizations in the human body, including the gastrointestinal tract, as well as the urinary and genital systems. As commented above, this genus is considered one of the most important representative groups of probiotics [17], and particularly, Lactobacillus fermentum has become one of the most promising probiotics. In fact, it is used as a standard reference species in comparative studies with other probiotics, due to their beneficial health properties [17].
Recently, whole genome studies have been performed, and the taxonomy of Lactobacillaceae has been newly evaluated. Thus, the previous name, Lactobacillus fermentum, has been changed by Limosilactobacillus fermentum [18].
- fermentum is a species with many strains isolated from different environments, including fermenting plant materials [19], dairy products [20], bread [21], naturally fermented sausages [22], breast milk [23], saliva [24], and human feces [25]. Remarkably, several L. fermentum strains have been described to possess promising beneficial effects both in preclinical studies (in vitro and in vivo models) and in human trials, which, in fact, have resulted in the development of different probiotic preparations for medical application and food preservation processes [26]. Actually, it has been suggested a potential role of L. fermentum in inflammatory-related diseases including intestinal inflammation [27], respiratory tract infections [28] and hepatic injury [29] (Table 1 and Table 2).
fermentum is a species with many strains isolated from different environments, including fermenting plant materials [19], dairy products [20], bread [21], naturally fermented sausages [22], breast milk [23], saliva [24], and human feces [25]. Remarkably, several L. fermentum strains have been described to possess promising beneficial effects both in preclinical studies (in vitro and in vivo models) and in human trials, which, in fact, have resulted in the development of different probiotic preparations for medical application and food preservation processes [26]. Actually, it has been suggested a potential role of L. fermentum in inflammatory-related diseases including intestinal inflammation [27], respiratory tract infections [28] and hepatic injury [29] (Table 1 and Table 2).
As expected, the mechanisms involved in the proposed beneficial effects reported for these probiotics include the general mechanisms described above [30,31]. Among these, the immunomodulatory properties have been proposed to have a key role in many strains of L. fermentum, since they are able to interact with immune cells, like macrophages and dendritic cells, as well as to regulate the synthesis and release of different cytokines [32,33]. In addition, L. fermentum strains have been proposed to exert bacteriostatic effects against a variety of pathogenic bacteria and fungi, including Staphylococcus aureus [34], Candida albicans [35], Helicobacter pylori [36], Campylobacter jejuni [37], and Aspergillus parasiticus [38], derived from their ability to produce organic acids (primarily lactic and acetic acids) and/or antimicrobial peptides [26,39]. Furthermore, it has been reported that some L. fermentum strains possess a complete glutathione-associated system, including the synthesis, transport, uptake, and redox cycling of this antioxidant peptide [40,41], thus providing protection against oxidative stress (Table 1 and Table 2).
Table 1. Effects of different L. fermentum strains in preclinical studies.
|
Strain/Origin |
Properties |
Mechanism of Action |
Model |
Reference |
|
L. fermentum UCO-979C/human gastric tissue |
Anti-inflammatory effect
|
↑ IL-10 production |
THP-1 cell line |
[42] |
|
L. fermentum SRK414/ unknown (Food Microbiology Laboratory, Korea University (Seoul, South Korea)) |
↓ TNF-α and IL-1β expression in LPS-stimulated inflamed |
HT-29 cell line |
[43] |
|
|
L. fermentum KBL374 and L. fermentum KBL375/feces of healthy Koreans |
↓ Leukocyte infiltration and Disease Activity Index |
DSS-induced colitis in C57BL/6N mice |
[25] |
|
|
L. fermentum MG901 and L. fermentum MG989/healthy woman’s vagina |
Urogenital and intestinal anti-infective activity |
Inhibit the Candida albicans growth |
HT-29 cell line |
[44] |
|
L. fermentum 3872/milk of healthy women |
Inhibition of Campylobacter jejuni growth and attachment to collagen I |
Collagen I coated plates |
[37] |
|
|
L. fermentum L23/vaginal smears of healthy woman |
Growth inhibition of Gardnerella vaginalis |
Vaginal infected BALB/C mice with Gardnerella vaginalis |
[45] |
|
|
L. fermentum LF31/unknown (Milonet, Bromatech s.r.l., Milan, Italy) |
Antioxidant capacity |
Antioxidant capacity detectable with the oxigenic radical absorbance capacity (ORAC) assay |
HT-29 cell line |
[46] |
|
L. fermentum JX306/Chinese traditional fermented vegetable |
↓ Malondialdehyde (MDA) levels ↑ Activity of glutathione peroxidase (GSH-Px) |
D-galactose-induced aging KM mice |
[47] |
Table 2. Effects of different L. fermentum strains in clinical trails.
|
Strain/Origin |
Main Properties |
Physiological Conditions |
References |
|
L. fermentum RC-14/female urogenital tract |
−37% of improvement in vaginal flora - ↑ lactobacilli population - ↓ yeast levels |
Healthy women |
[48] |
|
L. fermentum VRI-003 PCC/unknown (®Probiomics Ltd., Eveleigh, NSW, Australia) |
- ↑ lactobacilli population - ↓ in the severity of gastrointestinal and respiratory illness in sportive males. |
Competitive athletes |
[49] |
|
- ↓ Severity Scoring of Atopic Dermatitis (SCORAD) index. - ↑ the cases of mild atopic dermatitis. |
Children with atopic dermatitis |
[50] |
|
|
Combination of L. fermentum LF10 and L. acidophilus La02/vaginal swabs of healthy women or from direct brushing of gut mucosa of healthy humans |
↓ 72% of clinical recurrences vaginal infections of vulvovaginal candidiasis |
Women with recurrent vulvovaginal candidiasis |
[51] |
|
Combination of L. fermentum LF15 and L. plantarum LP01/feces of healthy humans or vaginal swabs of healthy female subjects |
Reduction in the Nugent score and restoration (58%) of the vaginal microbiota of women |
Women diagnosed with bacterial vaginosis |
[52] |
|
L. fermentum ME-3/healthy Human intestinal tract |
- ↑ lactobacilli in feces -Improvement the blood Total Antioxidative Activity and Total Antioxidative Status |
Healthy humans |
[53] |
|
Combination of L. fermentum ME-3 and a food supplement/healthy human intestinal tract |
-Improvement cardiovascular and diabetes risk profile. - ↓ total cholesterol |
Clinically asymptomatic humans |
[54] |
|
Combination of L. fermentum ME-3, L. paracasei 8700:2 and Bifidobacterium longum 46/healthy human intestinal tract and human feces |
- ↑ the blood Total Antioxidative Status - ↓ oxidized/reduced glutathione ratio |
Adult volunteers without gastric symptoms |
[55] |
|
Combination of L. fermentum LN99, L. gasseri LN40, L. casei subsp. rhamnosus LN113 and Pediococcus acidilactici LN23)/vaginal flora of healthy women |
-Vaginal colonization of lactobacilli - ↓ recurrences of bacterial vaginosis and vulvovaginal candidiasis |
Women diagnosed and treated for vulvovaginal candidiasis and bacterial vaginosis |
[56] |
However, of all strains identified from L. fermentum, L. fermentum CECT5716 is one of the probiotics with more potential. Different studies have reported its possible beneficial effects in different pathologies [57,58]. However, the precise mechanisms underlying remain unknown. Therefore, it is still necessary to conduct more investigations to identify its mechanisms of action and possible interactions with the host. Here, we will summarize and provide updated information on its effect on host health, mechanisms, and therapeutic insights.
2.1. Limosilactobacillus fermentum CECT5716
fermentum CECT5716 is a probiotic strain initially isolated from the human breast milk of healthy mothers, and for over 15 years, it has been included in nutrition supplements and fermented milk products [23]. The application of whole-genome shotgun strategies provided the identification of its genome using L. fermentum IFO 3956 as reference. Both strains are highly similar, with the exception of 16 protein encoding genes that are not present in IFO 3965 [59]. Thus, the genome of L. fermentum CECT5716 is composed of 2100449 bp and contains 1109 protein encoding genes, 54 tRNA encoding genes, and 20 rRNA encoding genes. It is a circular chromosome with a CG content of 51.49%, with no plasmid, and includes putative enzymes with an important role in the metabolism of purines (allantoinase, guanosine monophosphate (GMP) oxidoreductase, GMP synthase), amino acids (serine-pyruvate transaminase, 3 glutamate synthases), lipids (acyltransferase), and carbohydrates (mannose-6-phosphate isomerase) (GenBank/EMBL under accession no. CP002033) [59].
(References would be added automatically after the entry is online)