
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcel Parvu | + 2849 word(s) | 2849 | 2021-04-01 05:23:05 | | | |
| 2 | Vivi Li | Meta information modification | 2849 | 2021-04-06 07:33:46 | | |
Video Upload Options
Morphological and anatomical traits of the Vinca leaf were examined using microscopy techniques. Outdoor Vinca minor and V. herbacea plants and greenhouse cultivated V. major and V. major var. variegata plants had interspecific variations. All Vinca species leaves are hypostomatic. However, except for V. minor leaf, few stomata were also present on the upper epidermis. V. minor leaf had the highest stomatal index and V. major had the lowest, while the distribution of trichomes on the upper epidermis was species-specific. Differentiated palisade and spongy parenchyma tissues were present in all Vinca species’ leaves. However, V. minor and V. herbacea leaves had a more organized anatomical aspect, compared to V. major and V. major var. variegata leaves. Additionally, as a novelty, the cellular to intercellular space ratio of the Vinca leaf’s mesophyll was revealed herein with the help of computational analysis.
1. Introduction
The Vinca genus belongs to the Apocynaceae family and comprises three species and one variety: V. major L. (bigleaf periwinkle), V. minor L. (lesser periwinkle), V. herbacea Walds. & Kit., (herbaceous periwinkle), and V. major L. var. variegata ‘Louden’ (greater periwinkle with white margins) [1]. Other databases complete the list of species with Vinca difformis Pourr., Vinca erecta Regel & Schmalh., and Vinca ispartensis Koyuncu & Eksi [2].
Vinca plants are intensely studied for their medical properties [3][4][5] due to the rich alkaloid content [6][7][8][9]. These natural products are produced and stored in the aerial parts of Vinca plants [6][10][11], serving as protection against herbivores and pathogens. [12][13]. V. minor is one of the most important medicinal species [14] and the sole source of vincamine in nature, one of the few alkaloids with beneficial effects on cells [3][15]. Quantitative and qualitative differences of the chemical composition in Vinca extracts are dependent on species and the high content of alkaloids, flavonoids, and phenolic compounds, are in correlation with different pharmacological effects [3][16][17][18][19].
In extract preparation, besides the plant species, many factors influence the variability and diversity of the chemical composition, such as the plant organ and tissue, harvesting period and environmental conditions, or extraction method and the solvent used [3][6][7][8]. Previous results confirmed that the chemical composition varies with the Vinca extract, obtained from different organs such as the leaves of V. minor [6][7][14][20]; flowers, leaves, and/or roots of Vinca sardoa [21]; or from other different species of Vinca [4][8].
Undoubtedly, the leaves are very important organs in this matter since other studies showed their direct connection with the alkaloid content. Plant natural products serve in defence mechanisms and are a mixture of substances that often include alkaloids. In Euphorbia characias the aqueous latex produced in the leaves is combined with polyphenols and alkaloids [22]. In Nicotiana stocktonii [23] and Thymus quinquecostatus [24], alkaloids are stored in the glandular trichomes found on the surface of the leaves. In Ficus carica, the alkaloids are synthesized in the epidermal cells and secreted on the surface of the leaves by the glandular trichomes [25][26].
Based on similar studies as the aforementioned, the leaves of Vinca species are often used for extract preparation due to the fast release of high alkaloid concentration [11][27], but to our knowledge, their morphology and anatomy are insufficiently studied highlighting the scope of this study. The chemical composition of Vinca extracts varies to a great extent due to plant photosynthesis [28][29], which is regulated by numerous environmental and internal factors. These factors include morphological and anatomical leaf traits, i.e., shape, size, and the number of stomata, type or number of trichomes, and the weight of epidermal cuticle [30][31].
2. Vinca Leaf Epidermises
The epidermises of leaves were studied by scanning electron microscopy (SEM) and details such as the presence or absence, type, shape, and distribution of trichomes and stomata were observed.
Pavement cells on the adaxial side of V. minor, V. major, and V. major var. variegata leaves had similar typical jigsaw puzzle shapes (Figure 1a,c,e), whereas pavement cells of V. herbacea were papillary shaped (Figure 1g). Except for V. minor, all species had stomata on the upper epidermis. Tector trichomes with various ornamentations were present on the adaxial leaf side of the leaves as well (Figure 1b,d,f,h).
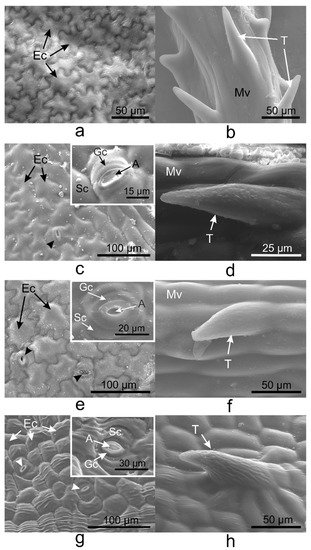
Figure 1. Scanning electron microscopy (SEM) micrographs showing the adaxial (upper) epidermis of the Vinca leaves with an overview of the differently shaped epidermal cells and insets with a detailed view of stomata, indicated by the arrowheads (a,c,e,g), and non-glandular trichomes observed on the midveins (b,d,f) and secondary veins (h). (a,b) V. minor, (c,d) V. major, (e,f) V. major var. variegata, (g,h) V. herbacea; A—aperture, Ec—epidermal cell, Gc—guard cell, Mv—midvein, Sc—subsidiary cell, T—trichome.
On the abaxial leaf side, all Vinca species presented typical puzzle-shaped pavement cells, while stomata were randomly distributed on the entire surface, except for the midvein (Figure 2).
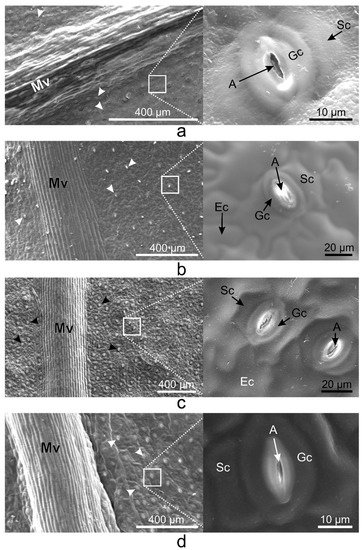
Figure 2. SEM micrographs showing the abaxial (lower) epidermis of the Vinca leaves with an overview along the midvein and detailed view of the stomata. (a) V. minor, (b) V. major, (c) V. major var. variegata, (d) V. herbacea; A—aperture, Ec—epidermal cell, Gc—guard cell, Mv—midvein, Sc—subsidiary cell, arrowheads—stomata.
3. Vinca Trichomes and Stomata Characteristics
Unicellular conical trichomes were observed on the margins of the adaxial side of V. major, V. major var. variegata, and V. herbacea leaves (Figure 3), while V. minor had trichomes only on the midvein. The trichomes had the same orientation in all Vinca species, i.e., from the petiole to the apex (from back to front).
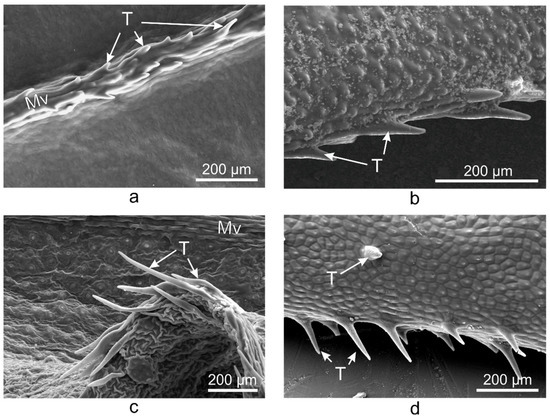
Figure 3. SEM micrographs showing the trichomes present on the midvein of V. minor leaf (a) and the marginal trichomes on the leaves of V. major (b), V. major var. variegata (c), and V. herbacea (d), all oriented towards the apex of the leaf; Mv—midvein, T—trichome.
The morphological parameters of the stomata and trichomes, and their distribution were calculated for each side of the leaves (Table 1).
Table 1. Morphological parameters measured on the adaxial and abaxial sides of the Vinca leaves.
| Species | SD * | SI (%) * | SL (µm) | SPI (%) | TD * | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ad | Ab | Ad | Ab | Ad | Ab | Ad | Ab | Ad | |
| V. minor | 0 | 223 | 0 | 25.3 | 0 | 9.57 ± 0.6 | 0 | 2.04 | 31 |
| V. major | 10 | 49 | 4.5 | 23.2 | 14.84 ± 1.1 | 19.75 ± 0.4 | 0.22 | 1.91 | 14 |
| V. major var. variegata | 12 | 68 | 4.2 | 20.9 | 12.33 ± 1 | 12.24 ± 1.4 | 0.18 | 1.01 | 25 |
| V. herbacea | 16 | 60 | 4.4 | 25 | 12.56 ± 1 | 13.05 ± 1.2 | 0.25 | 1.02 | 23 |
Ad = adaxial (upper) side, Ab = abaxial (lower) side, SD = Stomatal density, SI (%) = stomatal index, SL = Stomatal pore length, SPI (%) = Stomatal pore index, TD = Trichomes’ density; the criteria marked with * are measured in a one mm2 surface area; SL was measured three times on a minimum of six independent stomata ± standard error of the mean (s.e.m.).
Trichomes have protective roles in plants and their number is usually regulated by the same factors that affect the number of stomata [32]. As the Vinca trichomes are non-glandular, their main role remains as protectors against water loss, and their density is inversely correlated (Figure 4) with the stomatal index (r = −0.7).
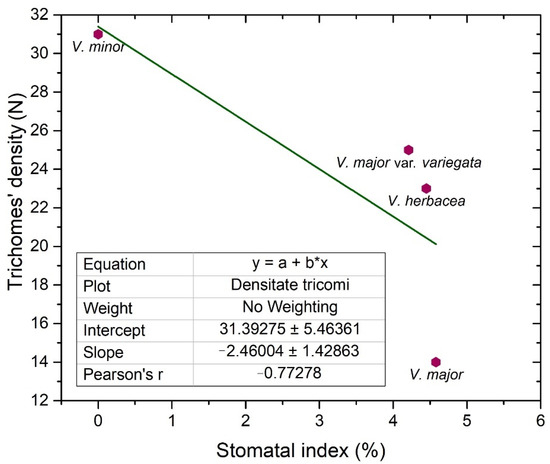
Figure 4. Linear fit of the trichomes’ density and stomatal index on the adaxial side of the examined Vinca leaves.
4. Vinca Mesophyll
The semithin sections (Figure 5) helped to determine the anatomical features of the Vinca leaves (Table 2). The mesophyll of V. minor and V. herbacea had a similar structure, with obvious delimitation between the palisade and spongy parenchyma (Figure 5a,d). In V. major and V. major var. variegata, the palisade parenchyma had a lax aspect and large intercellular spaces (Figure 5b,c).
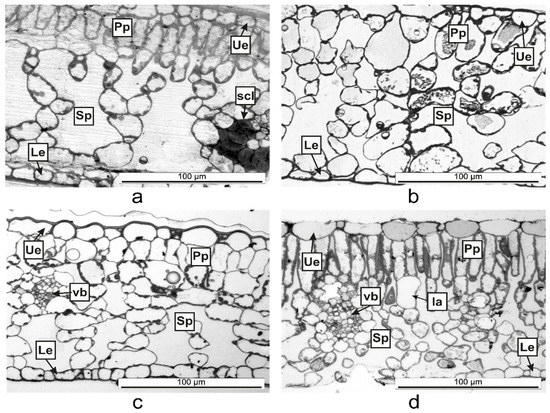
Figure 5. Semithin cross-sections of Vinca leaves showing the organization of the mesophyll between the upper epidermis (Ue) and lower epidermis (Le) as determined through light microscopy. (a) V. minor, (b) V. major, (c) V. major var. variegata, (d) V. herbacea; Ia—idioblast, Pp = palisade parenchyma, scl—sclerenchyma, Sp = spongy parenchyma, vb = vascular bundle.
Table 2. The anatomical features measured on the Vinca leaves.
| Species | LT (µm) | PTT (µm) | STT (µm) | CTR (%) | SR (%) |
|---|---|---|---|---|---|
| V. minor | 85.47 ± 2.6 | 12.58 ± 0.7 | 52 ± 0.5 | 14.72 | 60.84 |
| V. major | 136.85 ± 1.4 | 0 | 124 ± 0.5 | 0 | 90.61 |
| V. major var. variegata | 97.09 ± 1.5 | 14.57 ± 4.4 | 67.29 ± 1.7 | 15.01 | 69.31 |
| V. herbacea | 133.38 ± 2.2 | 36.66 ± 2.2 | 68.42 ± 3.7 | 27.48 | 51.3 |
LT = Leaf thickness, PTT = Palisade tissue thickness, STT = Spongy tissue thickness, CTR (%) = Cell tense ratio, SR (%) = Spongy tissue ratio; at least three independent measurements were conducted for each parameter measured on the leaf; for LT and STT n = 3, ± s.e.m.; for PTT n = 6, ± s.e.m.
A computational estimation of the intercellular space in the mesophyll was conducted (Figure 6) revealing a high percentage (over 60%) of airspaces for the studied Vinca species.
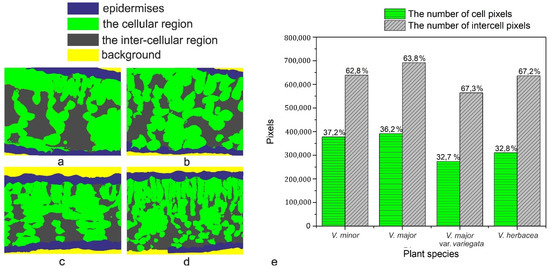
Figure 6. Computational estimation of the intercellular space of the mesophyll. (a) V. minor, (b) V. major, (c) V. major var. variegata, (d) V. herbacea, (e) graphical representation of the estimated number of cell pixels and inter-cell pixels as calculated using the Python script.
5. Discussion
There are quantitative and qualitative differences regarding the chemical composition of Vinca extracts, and previous studies showed that the morphological and anatomical traits of the leaves (shape and surface, cuticle, stomata, trichome, epidermis, etc.) are responsible for the chemical composition variability in the extracts [33][34][35][36]. These morphological and anatomical aspects of the Vinca leaf had been investigated in this study as well. The leaf surface area and dry leaf mass are directly correlated for vines species (Vinca major, Trachelospermum jasminoides, Hedera nepalensis var. sinensis) [37] and for Arabidopsis thaliana [38]. This aspect is important in extract preparation for maximal exploitation of the plants.
Additionally, the morphological characters of all plant organs are used for accurate identification of species [14][33][34][35]. The leaf shape varies within species and populations, and even within the same individual plants; therefore, it affects photosynthesis, water balance, temperature control, and the interactions with other organisms [39].
On the same surface of the Vinca leaf within different species, the epidermal cells are distinct, and here was shown that they could vary on both sides even for the same species (Figure 1, Figure 2 and Figure 3). The Vinca leaf epidermis has a single layer of cells composed of pavement cells, stomatal complexes, and trichomes (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5), that serves as a protective barrier to environmental factors [40]. On the adaxial side, Vinca leaves had puzzle-shaped epidermal cells, except for V. herbacea that had papillary epidermal cells. Similar results were previously reported for V. minor and V. herbacea only [41], and there are several theories that try to explain this type of organization of the pavement cells [42][43][44].
These specialized epidermal cells differ intra- and interspecific, depending on various extrinsic and intrinsic parameters, e.g., the leaf age, dimension, insertion position on the stem, combined with CO2 atmospheric concentration, irradiance, humidity, and other environmental factors (pollution, pathogens, etc.) [44][45][46]. Therefore, the current study was conducted under minimum variation of these parameters. The outdoor plants (V. minor and V. herbacea) grew in a shaded area and had the same light exposition (46°45′51” N; 23°34′47” E). The indoor plants (V. major and V. major var. variegata) were exposed only to sunlight with the same circadian cycles as the outdoor plants and the humidity and CO2 levels were regulated so that they could resemble the natural conditions. All the above-mentioned criteria play vital roles for the medicinal taxa [47][48].
Also, a connection between the cell walls of the leaves’ epidermis and the natural products in plants had been previously established [6]. In most plant species, the epidermis has specialized roles in the biosynthesis and accumulation of a wide range of natural products, including alkaloids [40], terpenes, and flavonoids [40][49], indicating that the variances observed here could be indicative of the chemical variation of the plant extracts.
The Vinca leaf epidermal cells are covered by a cuticle (Figure 5, Figure 7 and Figure 8) with complex chemical composition and have different thicknesses on the upper and lower epidermises as previously indicated [50][51][52]. In terms of electron-dense cutin layers, the V. minor, V. major var. variegata and V. herbacea leaves are similar to the leaves of Pyrus communis and Populus bolleana [50], but were never described for the Vinca species. The cuticular waxes play significant roles in Vinca species due to their involvement in the defense mechanisms against pathogens, temperature variations, salinity, or excessive ultraviolet radiations [52].
Glandular and secretory trichomes are responsible for biosynthesis, secretion and/or accumulation of phytochemicals useful for defense [40]. In the leaves of Vinca species analyzed herein, only tector (protective) trichomes had been identified, with the main role in defense mechanisms against water loss. The number of trichomes increases while the stomatal index decreases, and this way, the transpiration process is balanced [53], explaining our results where the stomatal index was found to be inversely proportional to trichomes’ density. For V. minor, the trichomes were placed only on the midvein, while the other species had trichomes on the margins and on secondary veins. Similar results had been previously reported as well for V. minor and V. herbacea [41][54].
Stomata are important structures involved in exchange processes of the plant with the environment, balancing the CO2 influx during photosynthesis and water vapor efflux during transpiration [28][33][35][40][55]. Stomata exhibit a diverse range of shapes, sizes, and numbers across different plant species [53]. Leaf stomata in plants from the same genus or from different plants of the same species are variable [56][57]. Significant negative correlation was documented between stomatal density and stomatal shape parameters (stomatal area, stomatal perimeter, stomatal long axis, stomatal short axis) in other plant species as well [58].
The stomatal index is the recommended parameter to be measured since it reports the stomata density in relation to the number and size of the pavement cells, in each area [28][46]. The stomatal index for the adaxial side (around 4%), is evidently lower than the one registered for the abaxial side (20–30%); therefore, the studied Vinca species are all hypostomatic.
Our results showed that the overall thickness of the Vinca leaf differs with the species and this influences the photosynthetic rates as well [59]. Besides this, the stomatal index has different values for each Vinca species, and the chemical composition could vary as well. This is because a higher specific stomatal index is related to a higher net rate of photosynthesis, and consequently, the plant could enhance the carbon fixation [28][32]. For example, several sugars were shown to affect the growth and development of V. minor and C. roseus leaves [14]. As carbon is present in many sugars and alkaloids, this could also influence the chemical composition in the long run [28].
Vinca leaf mesophyll has different thicknesses, a parameter which is species depended [36]. The Vinca leaves contain different numbers of columnar palisade cells. It was confirmed that sunlight-grown plants have more columnar palisade cells than those of shade-grown plants [29][34][35]. Similar to our results where the indoor plants had a less differentiated palisade tissue than the outdoor plants, other studies showed that the palisade tissue cells in V. major var. variegata leaves were single-layered for the plants located at roof level and double-layered for ground-level plants [35]. Another study demonstrated that depending on light exposition, the leaf of V. minor had a double-layered palisade tissue and V. herbacea leaf, a single-layered one [41]. The shape of palisade cells and the movement of chloroplast in accordance with the light conditions, are essential for efficient leaf photosynthesis, by cell development regulation with the help of phototropin [29]. The number and shape of palisade cells, which compose the photosynthesis unit area, are different for each Vinca leaf analyzed herein, therefore the photosynthesis process varies along with the anatomical traits [29] and this might influence the chemical composition as well if the plants are to be considered for extract preparation.
The thickness of the spongy parenchyma in the Vinca leaf varies with the species and contains several layers of ovoid, oblong, or circular shaped cells and the mesophyll airspace [34][35][41]. The cells of the spongy parenchyma store important nutrients for the plant [60]; therefore, its thickness would be directly related to the abundance of natural compounds [61]. Other studies described 7 to 8 layers of cells in the spongy tissues of V. minor and V. herbacea [34][41], while for V. major var. variegata, the number of layers decreased to 6–7 for plants grown at ground level [35]. The results presented herein showed that the plants grown outdoor had 7 to 8 layers, while the plants grown indoor, had 5 to 6 layers of cells in the spongy parenchyma—results which are consistent with other findings—and this indicates that growth conditions are important parameters that should be considered before extract preparation.
The programmatic method used for computation helped to determine the ratio between intercellular spaces and cells in the mesophyll of the Vinca species. First, it must be stressed that the main objective was the accuracy of the mathematical computations with respect to the ground truth. Initially, various methods were employed for the semi-automatic segmentation of the regions in the semithin sections, using state-of-the-art methods such as those based on computer vision [62][63] or machine learning approaches [64][65]. The employed methods obtained the same accuracy levels as those found in the literature.
However, the visual assessment of the results leads to the conclusion that since the end goal was to compute a percentage as close to reality as possible through an automated method of visual segmentation and labeling of the cellular and inter-cellular regions, significant errors in the computational process occurred. In other words, an accuracy level that is acceptable from a computer science perspective may, however, damage the biological scientific accuracy of the research. V. minor had the lowest mesophyll airspace (62.8%), followed by V. major (63.8%) and very close to each other were V. major var. variegata and V. herbacea (67.3% and 67.2%, respectively). According to recent studies, there is a connection between the mesophyll airspace and functional stomata [36][66], implying that larger airspaces are connected to a higher stomatal index. Since the Vinca species are hypostomatic, the high percentage for intercellular spaces is justified in relation to the high stomatal index observed.
The ultrastructural analysis of the leaves offered a more detailed view of the anatomy and confirmed some of the hypotheses regarding the spongy parenchyma. Lipids are used as substitutes for energy production and their presence as big droplets in the V. major and V. major var. variegata leaf could indicate a compensatory mechanism adopted by the plant because of the greenhouse storage conditions [67]. As indicated in other studies, alkaloids are harmful to the plant and are therefore stored or synthesized in small amounts [61] and only when needed, and the chemical composition variability of Vinca plant extracts could be reflected by different amounts of lipid droplets identified in leaf spongy parenchyma [67]. Overall, the methods used to determine the morphological and anatomical aspects of the Vinca leaves are correlated with each other and can be used to analyze the leaves of other species as well. This is an important step since Vinca species are recognized for their medicinal properties, and a brief morphological and anatomical description of a batch could help determine if the plants are suited for extraction.
References
- United States Department of Agriculture. PLANTS Database. Available online: (accessed on 21 January 2021).
- Plants of the World Online. Available online: (accessed on 21 January 2021).
- Barrales-Cureño, H.J.; Reyes, C.R.; García, I.V.; Valdez, L.G.L.; De Jesús, A.G.; Cortés Ruíz, J.A.; Sánchez Herrera, L.M.; Calderón Caballero, M.C.; Magallón, J.A.S.; Espinoza Perez, J.; et al. Alkaloids of pharmacological importance in Catharanthus roseus. In Alkaloids—Their Importance in Nature and Human Life; Kurek, J., Ed.; Intech Open Ltd.: London, UK, 2019; Volume 1, p. 18.
- Cheng, G.-G.; Zhao, Y.-L.; Zhang, Y.; Lunga, P.-K.; Hu, D.-B.; Li, Y.; Gu, J.; Song, C.-W.; Sun, W.-B.; Liu, Y.-P.; et al. Indole alkaloids from cultivated Vinca major. Tetrahedron 2014, 70, 8723–8729.
- Sukhdev, S.; Shamsher, K.S.; Indu, K. Antilipase activity guided fractionation of Vinca major. J. King. Saud. Univ. Sci. 2017, 30, 433–439.
- Abouzeid, S.; Hijazin, T.; Lewerenz, L.; Hansch, R.; Selmar, D. The genuine localization of indole alkaloids in Vinca minor and Catharanthus roseus. Phytochemistry 2019, 168, 112110.
- Bahadori, F.; Topçu, G.; Boğa, M.; Türkekul, A.; Kolak, U.; Kartal, M. Indole alkaloids from Vinca major and V. minor growing in Turkey. Nat. Prod. Commun. 2012, 7, 731–734.
- Boga, M.; Kolak, U.; Topcu, G.; Bahadori, F.; Kartal, M.; Farnsworth, N.R. Two new indole alkaloids from Vinca herbacea L. Phytochem. Lett. 2011, 4, 399–403.
- Liu, J.; Liu, Y.; Pan, Y.-j.; Zu, Y.-G.; Tang, Z.-H. Determination of alkaloids in Catharanthus roseus and Vinca minor by high-performance liquid chromatography—Tandem mass spectrometry. Anal. Lett. 2015, 49, 1143–1153.
- Andrade, E.A.; Folquitto, D.G.; Luz, L.E.C.; Paludo, K.S.; Farago, P.V.; Budel, J.M. Anatomy and histochemistry of leaves and stems of Sapium glandulosum. Revista Brasileira de Farmacognosia 2017, 27, 282–289.
- Verma, P.; Mathur, A.K.; Shanker, K. Enhanced vincamine production in selected tryptophan-overproducing shoots of Vinca minor. Plant Cell Tissue Organ Cult. 2012, 111, 239–245.
- De-la-Cruz, I.M.; Cruz, L.L.; Martínez-García, L.; Valverde, P.L.; Flores-Ortiz, C.M.; Hernández-Portilla, L.B.; Núñez-Farfán, J. Evolutionary response to herbivory: Population differentiation in microsatellite loci, tropane alkaloids and leaf trichome density in Datura stramonium. Arthropod Plant Interact. 2019, 14, 21–30.
- Liu, X.; Vrieling, K.; Klinkhamer, P.G.L. Interactions between plant metabolites affect herbivores: A study with pyrrolizidine alkaloids and chlorogenic acid. Front. Plant Sci. 2017, 8, 903.
- Chen, Q.; Lu, X.; Guo, X.; Guo, Q.; Li, D. Metabolomics characterization of two apocynaceae plants, Catharanthus roseus and Vinca minor, using GC-MS and LC-MS methods in combination. Molecules 2017, 22, 997.
- Verma, P.; Khan, S.A.; Masood, N.; Manika, N.; Sharma, A.; Verma, N.; Luqman, S.; Mathur, A.K. Differential rubisco content and photosynthetic efficiency of rol gene integrated Vinca minor transgenic plant: Correlating factors associated with morpho-anatomical changes, gene expression and alkaloid productivity. J. Plant Physiol. 2017, 219, 12–21.
- Islam, B.; Lustberg, M.; Staff, N.P.; Kolb, N.; Alberti, P.; Argyriou, A.A. Vinca alkaloids, thalidomide and eribulin-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Soc. 2019, 24 (Suppl. 2), S63–S73.
- Liao, F.-Y.; Xie, Y.; Jiang, H. The effect of water stress on the physiology of Vinca major ‘variegata’. Appl. Mech. Mater. 2013, 409, 782–787.
- Cheng, G.-G.; Zhao, H.-Y.; Liu, L.; Zhao, Y.-L.; Song, C.-W.; Gu, J.; Sun, W.-B.; Liu, Y.-P.; Luo, X.-D. Non-alkaloid constituents of Vinca major. Chin. J. Nat. Med. 2016, 14, 56–60.
- Stagos, D. Antioxidant activity of polyphenolic plant extracts. Antioxidants 2020, 9, 19.
- Liu, Z.; Wu, H.-L.; Li, Y.; Gu, H.-W.; Yin, X.-L.; Xie, L.-X.; Yu, R.-Q. Rapid and simultaneous determination of five Vinca alkaloids in Catharanthus roseus and human serum using trilinear component modeling of liquid chromatography–diode array detection data. J. Chromat. B 2016, 1026, 114–123.
- Foddai, M.; Maldini, M.; Addis, R.; Petretto, G.L.; Chessa, M.; Pintore, G. Profiling of the bioactive compounds in flowers, leaves and roots of Vinca sardoa. Nat. Prod. Commun. 2017, 12, 933–936.
- Christodoulakis, N.S.; Mamoucha, S.; Termentzi, A.; Fokialakis, N. Leaf structure and histochemistry of the hardy evergreen Euphorbia characias L. (Mediterranean spurge). Flora 2015, 210, 13–18.
- Zador, E.; Jones, D. The biosynthesis of a novel nicotine alkaloid in the trichomes of Nicotiana stocktonii. Plant Physiol. 1986, 82, 479–484.
- Jing, H.; Liu, J.; Liu, H.; Xin, H. Histochemical investigation and kinds of alkaloids in leaves of different developmental stages in Thymus quinquecostatus. Sci. World J. 2014, 2014, 839548.
- Giordano, C.; Maleci, L.; Agati, G.; Petruccelli, R. Ficus carica L. leaf anatomy: Trichomes and solid inclusions. Ann. Appl. Biol. 2019, 176, 47–54.
- Mamoucha, S.; Fokialakis, N.; Christodoulakis, N.S. Leaf structure and histochemistry of Ficus carica (Moraceae), the fig tree. Flora 2016, 218, 24–34.
- Boyadzhiev, L.; Yordanov, B. Pertraction of indole alkaloids from Vinca minor L. Sep. Sci. Technol. 2004, 39, 1321–1329.
- Segev, R.; Nannapaneni, R.; Sindurakar, P.; Kim, H.; Read, H.; Lijek, S. The effect of the stomatal index on the net rate of photosynthesis in the leaves of Spinacia oleracea, Vinca minor, Rhododendron spp., Epipremnum aureum, and Hedera spp. J. Emerg. Investig. 2015, 20, 2018.
- Gotoh, E.; Suetsugu, N.; Higa, T.; Matsushita, T.; Tsukaya, H.; Wada, M. Palisade cell shape affects the light-induced chloroplast movements and leaf photosynthesis. Sci. Rep. 2018, 8, 1472.
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernandez-Marin, B.; Hernandez, A.; Garcia-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280.
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Environ. 2018, 6, 64.
- Opriş, O.; Ciorîţă, A.; Soran, M.-L.; Lung, I.; Copolovici, D.; Copolovici, L. Evaluation of the photosynthetic parameters, emission of volatile organic compounds and ultrastructure of common green leafy vegetables after exposure to non-steroidal anti-inflammatory drugs (NSAIDs). Ecotoxicology 2019, 28, 631–642.
- Csiky, J.; Purger, D. Herbaceous periwinkle, Vinca herbacea Waldst. et Kit. 1799 (Apocynaceae), a new species of the Croatian flora. Acta Bot. Croat. 2013, 72, 399–406.
- Ochirova, K.S.; Ovanova, E.A.; Dordzhieva, V.I. Vinca minor L. leaf anatomical structure. J. Pharm. Sci. Res. 2018, 10, 2528–2530.
- Petra, S.A.; Georgescu, M.I.; Manescu, C.R.; Toma, F.; Badea, M.L.; Dobrescu, E.; Popa, V.I. Leaves anatomical and physiological adaptations of Vinca major ‘Variegata’ and Hedera helix L. to specific roof garden conditions. Not. Bot. Horti Agrobot. 2020, 47, 318–328.
- Samiyarsih, S.; Nettyani, N.; Dian, P. Variability of Catharanthus roseus based on morphological and anatomical characters, and chlorophyll contents. Biodiv. J. Biol. Div. 2019, 20, 2986–2993.
- Shi, P.; Li, Y.; Hui, C.; Ratkowsky, D.A.; Yu, X.; Niinemets, Ü. Does the law of diminishing returns in leaf scaling apply to vines?—Evidence from 12 species of climbing plants. Glob. Ecol. Conserv. 2020, 21, e00830.
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167.
- Kidner, C.A.; Umbreen, S. Why is Leaf Shape so Variable? Int. J. Plant Dev. Biol. 2010, 4, 64–75.
- Murata, J.; Roepke, J.; Gordon, H.; De Luca, V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 2008, 20, 524–542.
- Gagua, N.; Mchedlidze, K.; Vachnadze, V.; Bakuridze, A. Structural peculiarities of the vegetative organs of the species of Vinca. PHCOG J. 2012, 4, 49–55.
- Sapala, A.; Runions, A.; Routier-Kierzkowska, A.-L.; Gupta, M.D.; Hong, L.; Hofhuis, H.; Verger, S.; Mosca, G.; Li, C.-B.; Hay, A.; et al. Why plants make puzzle cells, and how their shape emerges. eLife 2018, 7, e32794.
- Sampathkumar, A.; Krupinski, P.; Wightman, R.; Milani, P.; Berquand, A.; Boudaoud, A.; Hamant, O.; Jönsson, H.; Meyerowitz, E.M. Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 2014, 3, e01967.
- Vofely, R.V.; Gallagher, J.; Pisano, G.D.; Bartlett, M.; Braybrook, S.A. Of puzzles and pavements: A quantitative exploration of leaf epidermal cell shape. New Phytol. 2018, 221, 540–552.
- Royer, D.L. Stomatal density and stomatal index as indicators of paleoatmospheric CO2 concentration. Rev. Palaeobot. Palynol. 2001, 114, 1–28.
- Ticha, I. Ontogeny of leaf morphology and anatomy. In Photosynthesis During Leaf Development; Šestăk, Z., Ed.; Springer: Drodrecht, The Netherlands, 1985; Volume 11.
- Venkateshwar, C.; Rao, S.G.; Kumar, R.S. Epidermal study of medicinal plants with special reference to identification, adulteration and authentification of crude leaf drugs. Ann. Phytomed. 2013, 2, 115–125.
- El-Fiki, M.A.; El-Taher, A.M.; EL-Gendy, A.G.; Lila, M.I. Morphological and anatomical studies on some taxa of family Apocynaceae. Al Azhar J. Agric. Res. 2019, 44, 136–147.
- Roepke, J.; Salim, V.; Wu, M.; Thamm, A.M.; Murata, J.; Ploss, K.; Boland, W.; De Luca, V. Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc. Natl. Acad. Sci. USA 2010, 107, 15287–15292.
- Guzman, P.; Fernandez, V.; Garcia, M.L.; Khayet, M.; Fernandez, A.; Gil, L. Localization of polysaccharides in isolated and intact cuticles of eucalypt, poplar and pear leaves by enzyme-gold labelling. Plant Physiol. Biochem. 2014, 76, 1–6.
- Fernandez, V.; Guzman-Delgado, P.; Graca, J.; Santos, S.; Gil, L. Cuticle Structure in Relation to Chemical Composition: Re-assessing the Prevailing Model. Front. Plant Sci. 2016, 7, 427.
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and Evolutionary Mechanisms of Cuticular Wax for Plant Drought Tolerance. Front. Plant Sci. 2017, 8, 621.
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of Stomatal Density and Morphology on Water-Use Efficiency in a Changing World. Front. Plant Sci. 2019, 10, 225.
- Fjell, I. Anatomy of the xeromorphic leaves of Allamanda neriifolia, Thevetia peruviana and Vinca minor (Apocynaceae). Nord. J. Bot. 1983, 3, 383–392.
- Zhu, J.; Yu, Q.; Xu, C.; Li, J.; Qin, G. Rapid estimation of stomatal density and stomatal area of plant leaves based on object-oriented classification and its ecological trade-off strategy analysis. Forests 2018, 9, 616.
- Beghin, T.; Cope, J.S.; Remagnino, P.; Barman, S. Shape and Texture Based Plant Leaf Classification. In Advanced Concepts for Intelligent Vision Systems; Blanc-Talon, J., Bone, D., Philips, W., Popescu, D., Scheunders, P., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6475.
- Jia, C.; Zhang, L.; Wei, X.; Yu, J.; Li, M. Phenotypic Polymorphism of Litsea mollis Hemsl in West Sichuan Province. For. Res. 2015, 28, 844–850.
- Hong, T.; Lin, H.; He, D. Characteristics and correlations of leaf stomata in different Aleurites montana provenances. PLoS ONE 2018, 13, e0208899.
- Huang, W.; Ratkowsky, D.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf Fresh Weight Versus Dry Weight: Which is Better for Describing the Scaling Relationship between Leaf Biomass and Leaf Area for Broad-Leaved Plants? Forests 2019, 10, 256.
- O’Connor, S.E. Alkaloid biosynthesis. In Wiley Encyclopedia of Chemical Biology; Begley, T.P., Ed.; John Wiley & Sons, Inc: Hobokend, NJ, USA, 2008; Volume 1, pp. 17–33.
- Ziegler, J.; Facchini, P.J. Alkaloid biosynthesis: Metabolism and trafficking. Annu. Rev. Plant Biol. 2008, 59, 735–769.
- Kulwa, F.; Li, C.; Zhao, X.; Cai, B.; Xu, N.; Qi, S.; Chen, S.; Teng, Y. A State-of-the-art Survey for Microorganism Image Segmentation Methods and Future Potential. IEEE Access 2019, 7, 100243–100269.
- Minaee, S.; Boykov, Y.; Porikli, F.; Plaza, A.; Kehtarnavaz, N.; Terzopoulos, D. Image Segmentation Using Deep Learning: A Survey. arXiv 2020, arXiv:2001.05566.
- Xue, Y.; Ray, N. Cell Detection in microscopy images with deep convolutional neural network and compressed sensing. arXiv 2017, arXiv:1708.03307.
- Xue, Y.; Bigras, G.; Hugh, J.; Ray, N. Training Convolutional Neural Networks and Compressed Sensing End-to-End for Microscopy Cell Detection. IEEE Trans. Med. Imag. 2019, 38, 2632–2641.
- Lundgren, M.R.; Mathers, A.; Baillie, A.L.; Dunn, J.; Wilson, M.J.; Hunt, L.; Pajor, R.; Fradera-Soler, M.; Rolfe, S.; Osborne, C.P.; et al. Mesophyll porosity is modulated by the presence of functional stomata. Nat. Commun. 2019, 10, 2825.
- Fan, J.; Yu, L.; Xu, C. Dual role for autophagy in lipid metabolism in Arabidopsis. Plant Cell 2019, 31, 1598–1613.





